Abstract
Tumor stroma-secreted growth factors, cytokines, and reactive oxygen species (ROS) influence tumor development from early stages to the metastasis phase. Previous studies have demonstrated downregulation of ROS-producing extracellular superoxide dismutase (SOD3) in thyroid cancer cell lines although according to recent data, the expression of SOD3 at physiological levels stimulates normal and cancer cell proliferation. Therefore, to analyze the expression of SOD3 in tumor stroma, we characterized stromal cells from the thyroid. We report mutually exclusive desmoplasia and inflammation in papillary and follicular thyroid cancers and the presence of multipotent mesenchymal stem/stromal cells (MSCs) in non-carcinogenic thyroids and papillary thyroid cancer (PTC). The phenotypic and differentiation characteristics of Thyroid MSCs and PTC MSCs were comparable with bone marrow MSCs. A molecular level analysis showed increased FIBROBLAST ACTIVATING PROTEIN, COLLAGEN 1 TYPE A1, TENASCIN, and SOD3 expression in PTC MSCs compared to Thyroid MSCs, suggesting the presence of MSCs with a fibrotic fingerprint in papillary thyroid cancer tumors and the autocrine-paracrine conversion of SOD3 expression, which was enhanced by cancer cells. Stromal SOD3 had a stimulatory effect on cancer cell growth and an inhibitory effect on cancer cell migration, thus indicating that SOD3 might be a novel player in thyroid tumor stroma.
In solid tumors, paracrine factors secreted from the stroma regulate cancer cell growth and migration1,2,3,4,5,6,7,8,9. Reactive oxygen species (ROS), a well-known paracrine factor, contribute to stromal myofibroblast maturation10, thus emphasizing the effect of ROS in tumorigenesis. Extracellular superoxide dismutase (SOD3) has anti-oxidative, anti-inflammatory, anti-apoptotic, and growth promoting characteristics, exhibiting the most potent therapeutic responses and growth regulatory characteristics in cardiovascular and cancer models11,12,13,14,15,16,17,18,19,20,21,22. The expression of SOD3 is increased in a benign thyroid tumor goiter model and gradually downregulated in cell lines that model advanced papillary and anaplastic thyroid cancers correlating with the level of oncogene activation23,24. Of note, downregulation of growth stimulating SOD3 in epithelial cancer cells is controversial, particularly in light of recent data demonstrating SOD3-driven immortalization and even the transformation of murine primary cells, hence suggesting abrogation of the growth advantage in cancer cells23,24,25,26,27,28.
In the current study, we describe mesenchymal stem cells (MSCs) isolated from non-carcinogenic thyroids (Thyroid MSCs) and papillary thyroid cancer (PTC MSCs), the latter showing desmoplastic characteristics. Importantly, a redox gene expression analysis showed downregulation of SOD3 in papillary thyroid cancer TPC1 cells compared to Nthy control cells and upregulation in PTC MCS compared to Thyroid MSCs, hence suggesting autocrine-paracrine conversion of SOD3 mRNA expression. A functional analysis of stromal secreted SOD3 corroborated previously published data20,26 showing increased cancer cell proliferation and decreased cell migration in co-culture. Therefore, our data suggest that the growth-promoting characteristics of SOD3 are not limited to the initial benign growth phase of tumorigenesis but are sustained to the end phase of tumor development.
Results
Histological analysis of papillary thyroid cancer and follicular thyroid cancer stroma sections
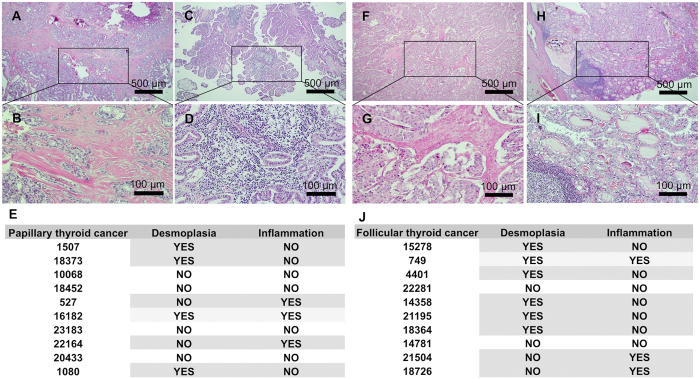
In thyroid cancers, desmoplastic stromal reactions, which correlate to lymph node metastasis, are a relatively common early phenomenon present in up to 80% of medullary thyroid cancers29. Characterization of papillary (PTC) and follicular (FTC) thyroid cancers 12 out of 20 cases (60%) demonstrated fibrosis or mononuclear cell infiltration. In PTC 40% of tumors showed desmoplastic regions and 30% inflammatory regions, whereas 40% of PTC tumors showed no detectable changes in stroma. In one case (10%) the stroma contained both desmoplastic and inflammatory regions. Interestingly, 50% of the cases suggested mutual exclusion between fibrosis and inflammation (Fig. 1A–D and F–I). The analysis of FTC showed desmoplasia or mononuclear cell infiltration in 8 out of 10 cases (80%). In seven cases (70%) there was mutual exclusion between fibrosis and inflammation: in five cases (50%) there was desmoplasia without inflammation and in two cases (20%) there was increased inflammation without fibrosis. In two cases (20%) there was no desmoplasia or inflammation, and in one case (10%) FTC stroma showed both desmoplasia and increased mononuclear cell content (Fig. 1E,J).
Figure 1.
Representative histological images of hematoxylin-eosin staining of sections from papillary (A–D) and follicular (F–I) thyroid cancer. (A,B) Papillary thyroid cancer regions with desmoplastic stroma. (C,D) Papillary thyroid cancer regions with mononuclear cell infiltration. (E) Table showing papillary thyroid cancer patient numbers and the corresponding desmoplasia and/or inflammation. (F,G) Follicular thyroid cancer regions with desmoplastic stroma. (C,D) Follicular thyroid cancer regions with mononuclear cell infiltration. (E) Table showing follicular thyroid cancer patient numbers and the corresponding desmoplasia and/or inflammation. Calibration bars: 500 μm (A,C,F,H); 100 μm (B,D,G,I).
Mesenchymal stem cells from thyroid and papillary thyroid cancer
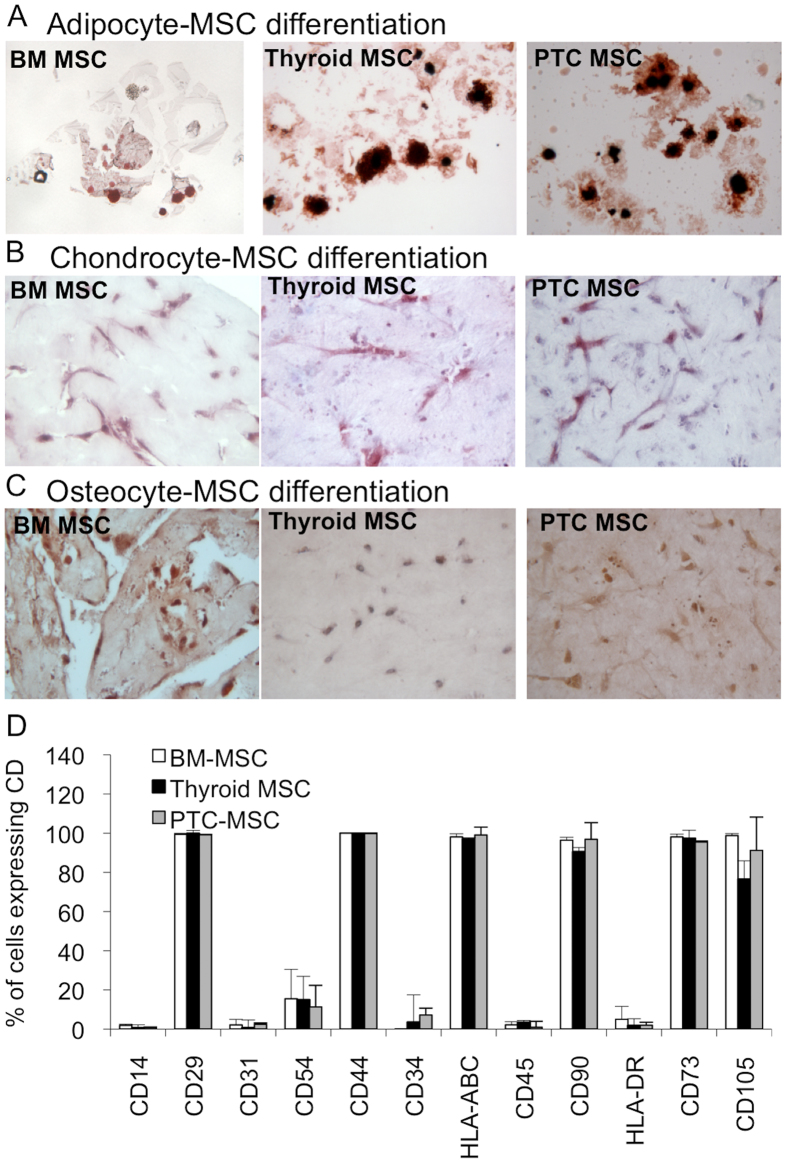
Most of the tissues have been suggested to contain multipotent mesenchymal stem/progenitor cells that support tissue renewal and function as a source of cytokines and growth factors30. To study the presence of MSCs in papillary thyroid cancer and a non-carcinogenic thyroid tissue counterpart, we isolated plastic adherent mesenchymal cells and characterized their phenotype. To test the stemness of the isolated cells, adipocyte, chondrocyte, and osteocyte lineage differentiation assays were performed to define the multipotency of the isolated cells. We observed similar multipotency among the bone marrow derived MSCs (BM MSC), the Thyroid MSCs, and the PTC MSCs, indicating the presence of mesenchymal stem cells in the adult thyroid tissues and papillary thyroid cancer tumor tissues (Fig. 2A–C). The analysis of the expression of cell surface cluster of differentiation (CD) molecules used to identify MSCs by flow cytometer suggested no differences among the BM MSCs, Thyroid MSCs, and PTC MSCs (Fig. 2D), corroborating the differentiation data and suggesting the presence of mesenchymal stem cells similar to BM MSCs in thyroid and thyroid cancers.
Figure 2. Phenotypic assays for isolated BM MSCs, Thyroid MSCs, and PTC MSCs.
(A) Adipocyte, (B) chondrocyte, and (C) osteocyte differentiation assays suggested no differences between BM-derived and thyroid-derived MSCs. (D) Flow cytometer analysis supported the differentiation data showing identical of cluster of differentiation (CD) expression in BM MSC, Thyroid MSC, and PTC MSC cell surfaces.
PTC MSCs have fibrotic characteristics
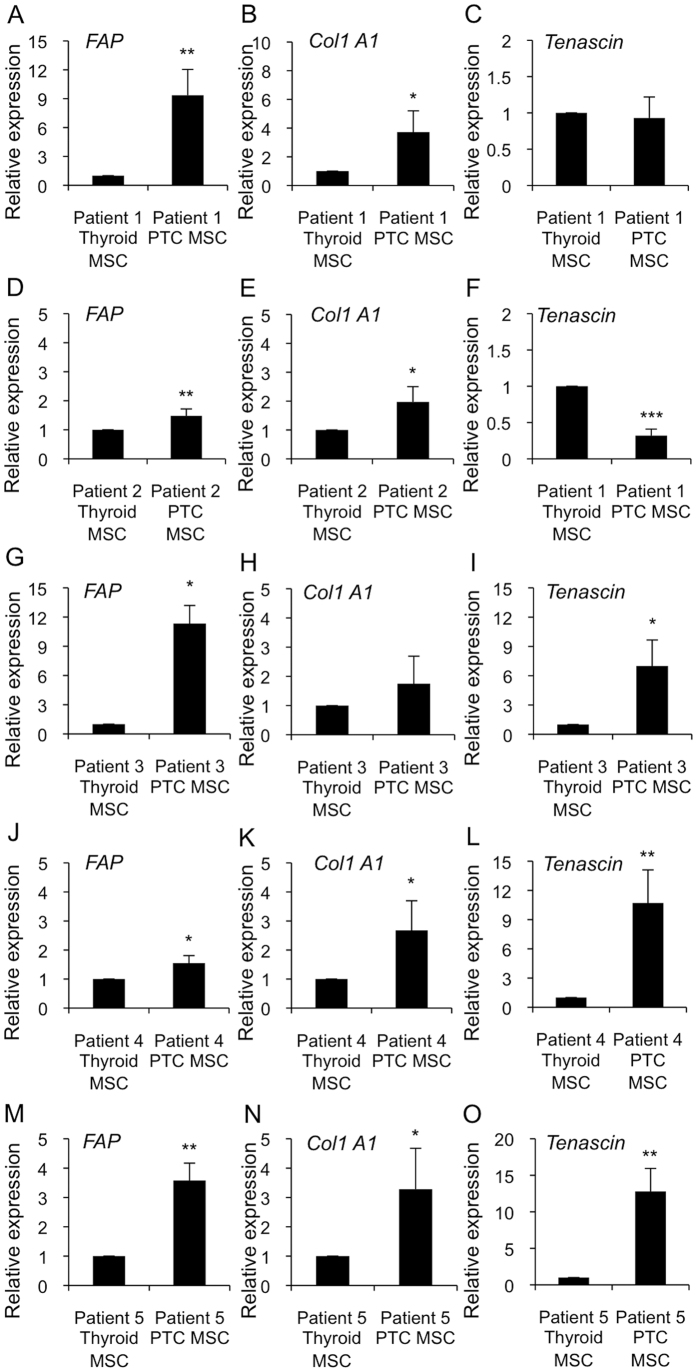
Because our data showed the presence of fibrosis in papillary thyroid cancer, we studied desmoplastic molecular marker expression from Thyroid MSCs and PTC MSCs. Strikingly, in all five patients, PTC MSCs had higher FIBROBLAST ACTIVATING PROTEIN (FAP) mRNA expression compared to Thyroid MSCs. Similarly, PTC MSCs had significantly higher COLLAGEN 1 TYPE A1 (Col1 A1) and TENASCIN mRNA expression levels as compared to Thyroid MSCs in four out of five and in three out of five patients, respectively. Although we observed variation in fibrotic marker expression in the patient 1 (TENASCIN expression was at the level of controls), in the patient 2 (TENASCIN expression lower than in the controls), and in the patient 3 (Col1 A1 expression was at the level of controls) our data could suggest that mesenchymal stem cells may function as an origin for myofibroblasts (Fig. 3A–O).
Figure 3. Expression analysis of FAP, Col1A1, and TENASCIN desmoplastic markers in Thyroid MSCs and PTC MSCs.
(A–O) The qRT-PCR data demonstrated significantly increased desmoplastic marker expression in all five patient-derived PTC MSC samples compared to their non-carcinogenic thyroid counterparts. (A–C) Patient 1, (D–F) Patients 2, (G–I) Patient 3, (J–L) Patient 4, and (M–O) Patient 5. The p-values (*p < 0.05, **p < 0.01, ***p < 0.001) were determined by two-tail independent samples t-tests.
SOD3 expression is reduced in epithelial cancer cells and is increased in MSCs with fibrotic characteristics
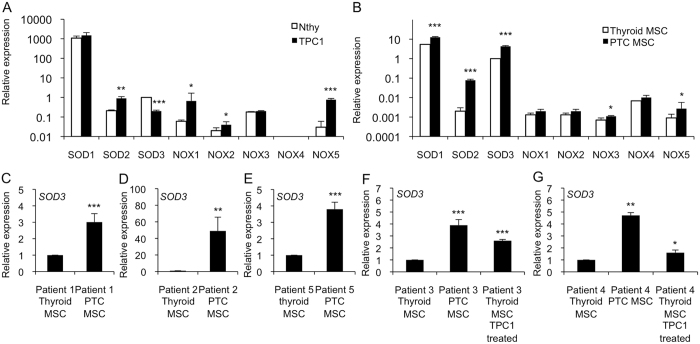
Thyroid cancer progression and fibrosis correlate with increased reactive oxygen species (ROS) production and increased redox enzyme expression23,31. Hence, we investigated the gene expression of the superoxide dismutase SOD1-3 family and the NOX1-5 family from human immortalized Nthy thyroid cells modeling a normal thyroid and from papillary thyroid cancer patient-derived TPC1 cells. Comparable to our previous data26, we observed increased SOD2, NOX1, NOX2, and NOX5 mRNA expression in papillary thyroid cancer cells compared to Nthy cell line. Importantly, we found decreased expression of SOD3 recurrently with recent works23,26 and an absence of NOX4 expression (Fig. 4A).
Figure 4. Gene expression of SOD1-3 and NOX1-5 in thyroid cells.
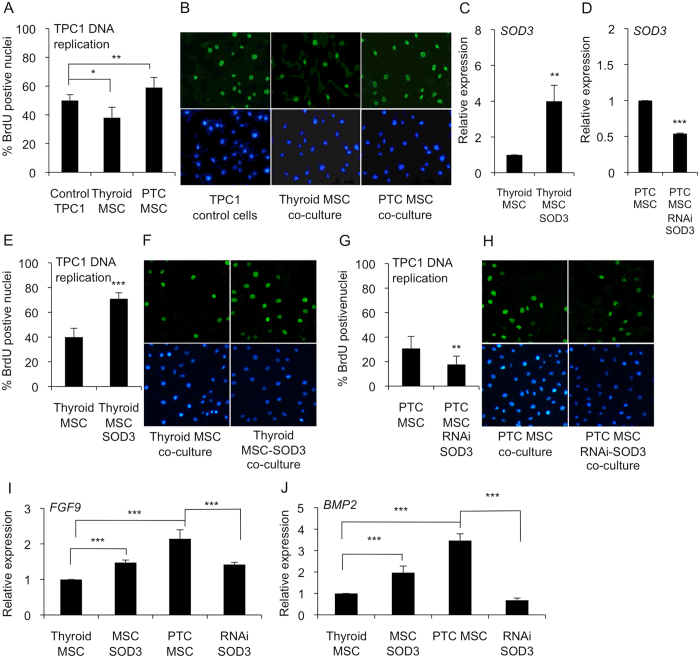
We used Nthy cells modeling normal thyroid epithelia, TPC1 cells modeling papillary thyroid cancer, Thyroid MSCs, and PTC MSCs. (A) SOD1, SOD2, NOX1-3 and NOX5 showed increased expression levels in TPC1 cells compared to in the Nthy cell line, whereas SOD3 expression was downregulated in the TPC1 cells. NOX4 expression was not detectable. (B) SOD1, SOD2, NOX1-3, and NOX5 expression levels in the PTC MSCs were increased or remained at the same level as in the Thyroid MSCs. SOD3 expression was significantly higher in the PTC MSCs compared to in the Thyroid MSCs. There was high NOX4 expression in both the Thyroid MSCs and the PTC MSCs. (C–G) Increased SOD3 mRNA synthesis was confirmed in each of the five patients. (F,G) Expression analysis using TPC1-condensed medium suggested a cancer-derived paracrine effect in SOD3 activation. The p-values (*p < 0.05, **p < 0.01, ***p < 0.001) were determined by two-tail independent samples t-tests.
The redox gene expression patterns of Thyroid MSCs compared to PTC MSCs were similar to the expressions observed in thyroid epithelial Nthy and TPC1 cells, with the exceptions of SOD3 and NOX4. The NOX4 gene was undetectable in the epithelial Nthy and TPC1 cells, whereas Thyroid MSCs and PTC MSCs both had high NOX4 expression levels. Analysis of SOD3 mRNA demonstrated increased expression in PTC MSCs compared to in Thyroid MSCs, thus suggesting autocrine-paracrine conversion of SOD3 mRNA synthesis (Fig. 4B). To confirm the increased SOD3 levels in the PTC MSCs, we analyzed the mRNA expression of the enzyme from each patient and determined the stimulatory effect of TPC1 cancer cell condensed medium on SOD3 mRNA levels. Interestingly, the mRNA production was significantly increased in all five samples of patient-derived PTC MSCs compared to their Thyroid MSC counterparts (Fig. 4C–G), which was shown to be enhanced by TPC1 paracrine action (Fig. 4F,G).
Paracrine MSC secreted SOD3 increases epithelial cancer cell growth
According to previous data, BM MSCs could reduce cancer cell proliferation via the paracrine system, thereby inhibiting tumorigenesis32. On the contrary, tumor-associated stromal cells, such as tumor-associated fibroblasts and mesenchymal stem cells, have been shown to promote cancer cell proliferation and tumor growth33,34. Therefore, we tested the growth stimulating properties of Thyroid MSCs and PTC MSCs on the papillary thyroid cancer TPC1 cell line. In line with previous reports, a BrdU DNA incorporation assay for TPC1 papillary thyroid cancer cells cultured together with Thyroid MSCs suggested decreased DNA replication, whereas cancer cells grown in the presence of PTC MSCs showed significantly increased BrdU incorporation (Fig. 5A,B).
Figure 5. Paracrine effect of SOD3 on TPC1 cancer cell growth.
(A,B) TPC1 cell growth in Thyroid MSC and PTC MSC co-cultures suggested significantly decreased TPC1 DNA replication in the Thyroid MSC culture and significantly increased DNA replication in the PTC MSC co-culture. (C) SOD3 expression in Thyroid MSCs and Thyroid MSCs transduced with SOD3 lentivirus. (D) SOD3 expression in PTC MSCs and PTC MSCs transduced with RNAi SOD3 lentivirus. (E,F) TPC1 DNA replication in Thyroid MSCs and Thyroid MSCs transduced with SOD3 lentivirus suggested increased SOD3-driven TPC1 growth. (G,H) RNA interference of SOD3 in PTC MSCs suggested decreased TPC1 DNA replication correlated with decreased SOD3 expression. (I,J) FGF9 and BMP2 gene expression analysis suggested increased SOD3-driven expression in Thyroid MSCs transduced with SOD3 lentivirus and decreased growth factor expression in PTC MSCs transduced with RNAi SOD3 lentivirus compared to controls. The p-values (*p < 0.05, **p < 0.01, ***p < 0.001) were determined by two-tail independent samples t-tests.
To probe the paracrine effect of SOD3 expression on cancer cell growth, we over-expressed SOD3 in Thyroid MSCs (Thyroid MSC SOD3) and silenced the gene expression by SOD3 RNAi in PTC MSCs (PTC MSC SOD3 RNAi) (Fig. 5C,D). Co-culture of TPC1 cells with Thyroid MSC SOD3 suggested significantly (p < 0.01) increased DNA replication of TPC1 cells compared to the parental Thyroid MSCs (Fig. 5E,F). Correspondingly, RNAi SOD3 treatment of PTC MSCs decreased (p < 0.001) TPC1 DNA replication compared to parental PTC MSCs (Fig. 5G,H), thus demonstrating that PTC MSCs may support cancer cell growth by secreting SOD3.
SOD3 has been demonstrated to regulate the activation of a large number of growth-related signaling molecules14,27. We then studied the growth factor gene expression in Thyroid MSC SOD3 and PTC MSC SOD3 RNAi cells. The analysis revealed correlations among SOD3, FIBROBLAST GROWTH FACTOR 9 (FGF9), and BONE MORPHOGENIC PROTEIN 2 (BMP2) expression (Fig. 5I,J).
Paracrine effect of SOD3 on epithelial cancer cell migration
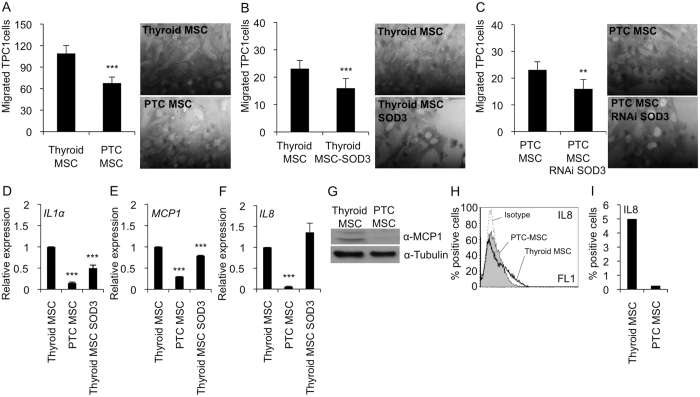
Desmoplastic thyroid tumors frequently metastasize to the lymph nodes29, suggesting increased intratumoral cancer cell migration toward the lymphatic vasculature. In the current work, the Matrigel migration analysis demonstrated significantly (p < 0.001) increased TPC1 migration levels towards Thyroid MSCs compared to PTC MSCs (Fig. 6A), which may indicate a lower affinity of TPC1 cells to tumor stroma. The analysis of the effect of SOD3 on TPC1 cell migration showing significantly (p < 0.01) reduced migration toward SOD3 over-expressing Thyroid MSCs (Thyroid MSC SOD3) (Fig. 6B) could suggest regulation of local cancer cell migration by paracrine secretion of the enzyme. However, silencing of SOD3 in PTC MSCs (PTC MSC SOD3 RNAi) failed to show increased cancer cell migration (Fig. 6C), indicating that SOD3 might have a different function in fibrotic MSCs or that the activation process of PTC MSCs modifies the ROS balance, hence altering the effect of single redox genes.
Figure 6. Effect of SOD3 on TPC1 cancer cell migration.
(A) TPC1 cancer cells migrated significantly faster towards Thyroid MSCs than PTC MSCs. Representative high-power fields from Matrigel shown. (B) Increased SOD3 expression in Thyroid MSCs significantly decreased TPC1 cell migration. Representative high-power fields from Matrigel are shown. (C) Reduced SOD3 expression reduced TPC1 cell migration toward PTC MSCs. Representative high-power fields from Matrigel are shown. (D) IL1α expression was significantly decreased in the PTC MSCs compared to the Thyroid MSCs. SOD3 over-expression in Thyroid MSCs significantly reduced IL1α mRNA synthesis compared to the Thyroid MSC control. (E) MCP1 mRNA expression was significantly decreased in PTC MSCs compared to Thyroid MSCs. SOD3 over-expression in the Thyroid MSCs significantly reduced MCP1 mRNA synthesis compared to the Thyroid MSC control. (F) IL8 mRNA expression was significantly decreased in the PTC MSCs compared to the Thyroid MSCs. SOD3 over-expression in the Thyroid MSCs did not affect IL8 expression. (G) MCP1 Western blot confirmed decreased MCP1 mRNA expression in the PTC MSCs compared to the Thyroid MSCs. (H) Flow cytometer analysis of IL8 expression confirmed decreased IL8 mRNA expression in the PTC MSCs compared to Thyroid MSCs. (I) Percentage of positive cells in the IL8 flow cytometer analysis. The p-values (*p < 0.05, **p < 0.01, ***p < 0.001) were determined by two-tail independent samples t-tests.
Previous publications have proposed that inflammatory and cancer cell migration are regulated by the same chemoattractants35,36. We recently demonstrated SOD3-derived decreased expression of TNFα, IL1α, IL6, MIP2, and MCP-1 inflammatory cytokines in cardiovascular models with consequent inhibition of monocyte/macrophage migration17,20,37. The expression analysis of IL1α, MCP-1, and IL8 showed reduced cytokine expression in PTC MSCs compared to Thyroid MSCs (Fig. 6D–J). The data, however, suggest that only IL1α and MCP-1 are downregulated by SOD3 in stromal cells (Fig. 6D,E), whereas IL8 expression is independent of the enzyme (Fig. 6F). Western blot analysis for MCP-1 (Fig. 6G) and flow cytometer analysis for IL8 (Fig. 6H,I) supported the mRNA expression data showing decreased expression in PTC MSCs compared to Thyroid MSCs. Our current data thus support our previous observations demonstrating that SOD3 stimulates cell proliferation and inhibits cell migration17,18,20,23,24,25,26,27,28,37,38.
Discussion
Local vascular permeability with consequent extravasation of fibrinogen and plasminogen initiate the formation of fibrin gel deposits, the early form of tumor stroma, which attracts migrating fibroblasts, epithelial cancer cells and inflammatory cells. Importantly, continuous tumor growth creates a demand for continuous stroma expansion. Thus, tumor stroma has different developmental stages that affect cancer cells, regulating their proliferation and local migration. According to recent data, the development of stroma is connected to the progression of carcinogenesis, such as lymph node metastasis39, indicating that stroma responds to the microenvironmental needs of epithelial cancer cells through paracrine growth factor secretion and direct cell-to-cell contact40,41,42. Additionally, activated desmoplastic stroma, which is frequently observed in medullary thyroid cancers, is used as a clinical intraoperative diagnostic marker to characterize tumor progression and to predict lymph node metastasis29,39,43,44. In the present work, we observed a marked inverse correlation between desmoplasia and inflammatory cell infiltration in PTC and FTC: 12 out of 20 patients had increased desmoplasia or increased mononuclear cell infiltration, whereas only two out of 20 patients had simultaneous desmoplasia and inflammation (Fig. 1). Previous articles have suggested that aggressive cancers, such as pancreatic ductal adenocarcinoma characterized by an excessive stromal reaction, may have reduced intratumoral vascularization45 that could cause reduced mononuclear cell migration into desmoplastic tumors. However, in a recent work the immunohistochemical analysis of fibroblast activating protein α-positive medullary thyroid carcinomas showed no correlation between new vessel formation and stromal fibrosis29, thus indicating other mechanisms than the physiological fibrotic barrier in the inhibition of cell migration. Inflammation has also been suggested to precede fibrosis development: infiltrated inflammatory cells secrete profibrogenic cytokines that affect fibroblast activation, perivascular fibrosis development, and cellular migration46,47.
An interesting, although rare, characteristic of thyroid tumors is intratumoral heterotopic ossification48,49,50,51,52,53,54, which may suggest the presence of multipotent mesenchymal stem/stromal cells in thyroid tissue. Indeed, tumor-associated MSCs recently characterized in ovarian carcinoma and prostate cancer have similar phenotype and functionality as BM MSC but altered growth factor expression profile55,56. The current data, which demonstrate the presence of MSCs in non-carcinogenic thyroid and papillary thyroid cancer with a similar differentiation capacity and CD marker expression as BM MSCs (Fig. 2) strengthening the previous observations, could further indicate that MSCs maintain their stemness even in conditions where they are under constant external carcinogenic stimuli. However, a more accurate gene expression analysis of fibrotic markers in Thyroid MSCs and PTC MSCs (Fig. 3) showed differentiation stage-related differences that affected functional properties, such as proliferation (Fig. 5A) and migration (Fig. 6A), in neighboring cells. We demonstrated that PTC MSCs had increased expression of fibrotic markers (Fig. 3). Although MSC cultures in general are heterogeneous containing cells with various degrees of differentiation, the current data may suggest that tumor stroma MSCs exposed to cancer cell secretion could obtain similar phenotypic characteristics as cancer associated fibroblasts and therefore serve as an origin of cells for activated fibroblasts.
ROS have been demonstrated to contribute to carcinogenesis and myofibroblast differentiation. Interestingly, NOX4 expression, which is absent in thyroid cancer cells26 (Fig. 4), was observed in MSCs, which may suggest a paracrine role for the protein in the thyroid. Furthermore, SOD3 expression suggested autocrine-paracrine conversion from epithelial cancer cells to mesenchymal stromal cells (Fig. 4), which could shed light on the function of SOD3 in tumorigenesis. Previous data have shown increased SOD3 expression in an experimental rat thyroid goiter model23 with corresponding growth-supporting function at physiological expression levels14,18,25,26,28,57. A strong connection of SOD3 to growth regulation is caused by positive a feedback loop between the enzyme, small GTPase activation, and RAS-ERK1/2 signaling24,25,27,28. Moderately increased small GTPase RAS activation stimulates SOD3 mRNA synthesis, but at aberrant levels of RAS activity (>10-fold RAS activation level), there is a sudden decrease in SOD3 expression24. Therefore, we have hypothesized that SOD3 benefits the initial phase of tumorigenesis at low (<10-fold) RAS activation levels. Once SOD3 expression reaches non-physiological toxic levels, cancer cells are programmed to downregulate autocrine SOD3 production and instead stimulate stromal paracrine SOD3 secretion simultaneously with activation of stroma, as shown in Figs 3 and 4. Paracrine secretion of SOD3 then continues to support cancer cell growth (Fig. 5) and, correspondingly, decreases the affinity of cancer cells to tumor stroma by decreasing chemotactic cytokine expression (Fig. 6), allowing local intratumoral cancer cell migration.
In conclusion, based on the current data, PTC MSCs with a fibrotic fingerprint might function as a source of myofibroblasts. Importantly, clarification of the mechanisms of fibroblast activation could result in the discovery of novel drug targets that are specific to thyroid cancer58 or cancer stroma. ROS, although forming a challenging molecular ensemble, control growth- and migration-related signaling routes that potentially contain drug targets. The current results suggest the autocrine-paracrine conversion of SOD3 expression, corroborating the growth supportive nature of the enzyme throughout the carcinogenic process. Thus, the identification of SOD3-coordinated signal transduction in tumor stroma and epithelial cancer cells may reveal small drug target molecules that could be used in combination therapy for the treatment of thyroid cancer.
Methods
Histological analysis
For histological analyses, papillary and follicular thyroid carcinoma samples were fixed in formalin, embedded in paraffin, and cut into 4–5 μm sections with a microtome. Sections were de-waxed in xylene for 10 minutes, rehydrated by sequential immersion in decreasing ethanol concentrations. The sections were then stained with hematoxylin-eosin and analyzed using an optical microscope (Nikon ECLIPSE Ni, Nikon Instrument Europe B.V. Amsterdam, Netherlands). Two independent observers (F.C. and F.R.) examined the specimens.
Isolation and culture of stromal cells
Thyroid mesenchymal stem/stromal cells (MSCs) were isolated from non-malignant sites (Thyroid MSCs) and papillary thyroid cancer sites (PTC MSCs) of patients. De-identified thyroid tissue samples were obtained from patients who underwent thyroid surgery at the University of Wisconsin-Madison. The protocol was approved by the Health Sciences Institutional Review board (IRB) of the University and performed in accordance with the relevant guidelines and regulations59. Informed consent was obtained from all subjects according the requirements of University of Wisconsin-Madison protocol permission. Due to differences between individuals all analysis of the study were done comparing thyroid MSCs and PTC-MSCs of the same patient. Tumor tissue was washed several times with PBS, cut into small pieces, and then was plated in alpha MEM (Mediatech, Manassas, VA, USA) supplemented with 10% FBS (Hyclone, Logan, UT), non-essential amino acids (Mediatech) and L-alanine-l-glutamine (Life Technologies, Grand Island, NY, USA). After reaching 70–90% confluency, cells were harvested using TrypLE (Life Technologies) and passaged until reaching passage 4 before characterizing and using them in the study60. The MSCs were transduced with SOD3 and RNAi SOD3 lentivirus (MOI 10) (Dharmacon, Lafayette, Colorado, United States).
Differentiation assay
Cells seeded in dishes were stimulated with 10−6 M dexamethasone, 100 μg/ml 3-isobutyl-1-methylxanthine, 50 μM indomethacin, and 10 μg/ml insulin (Sigma) for adipogenic differentiation, with 1 ng/ml recombinant human transforming growth factor-β1 (TGF-β1) (Sigma) for chondrogenic differentiation and with 10−7 M dexamethasone, 10 mM β-glycerophosphate disodium, and 50 μg/ml ascorbic acid (Sigma) for osteogenic differentiation. Adipogenic differentiation was analyzed after 0.3% oil red O (Sigma) treatment, chondrogenic differentiation was analyzed after anti-human type II collagen MAb treatment, and osteogenic differentiation was analyzed after alizarin red S (Sigma) treatment60,61.
Flow cytometer assay
For the flow cytometry analysis, 100 000 cells were incubated in a 100 μl volume with antibodies on ice. The cells were washed twice with PBS, resuspended in 500 μl, filtrated, and analyzed.
Gene expression analysis
RNA was isolated using an RNeasy minikit (Qiagen, Hilden, Germany). The cDNA synthesis was performed by QuantiTect Reverse Transcription (Qiagen). SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) was used for qPCR. The primers are listed in the Supplementary Table T1.
Western blot analysis
The cells were homogenized in lysis buffer (50 mmol/L HEPES pH 7.5, 150 mmol/L NaCl, 10% glycerol, 1% Triton X-100, 1 mmol/L EGTA, 1.5 mmol/L MgCl2, 10 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 1 mmol/L Na3VO4, 10 μg approtinin/ml, and 10 μg leupeptin/ml) (Sigma). The antibodies used were αMCP1 (Abcam, Cambridge, UK) and α-tubulin (Cell Signaling, Danvers, MA, USA).
Growth analysis
To characterize DNA replication, 10 mM bromodeoxyuridine (BrdU) (Roche, Basel, Switzerland) was added to the growth medium for 15 min. Subsequently, the cells were washed three times with PBS, fixed in 3% paraformaldehyde (Sigma) for 20 minutes at −20 °C, washed three times with PBS, and stained using anti-BrdU antibody (Roche) primary antibody, FITC–conjugated secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), and Hoechst nuclear stain (Sigma).
Migration analysis
For the migration assay, 100 μl of Matrigel (Corning, Corning, NY, USA) at 1 mg/ml was added to the migration chamber (8 microns, BD, San Jose, CA, USA) and allowed to stabilize at room temperature for 30 minutes. The chambers were moved to MSC cultures into 12-well plates, 50 000 TPC1 cells were added to the Matrigel and allowed to migrate for 24 hours at 37 °C. After incubation, the Matrigel was removed from the chamber, and the migrated cells were fixed with 7% paraformaldehyde (Sigma), washed with PBS, and stained with Cristal violet (Sigma). The migrated cells were counted from the high-power microscope fields.
Statistical analyses
The experiments were repeated at least three times. All results are expressed as the mean ± SD. The p-values (*p < 0.05, **p < 0.01, ***p < 0.001) were determined by two-tail independent samples t-tests.
Additional Information
How to cite this article: Parascandolo, A. et al. Extracellular Superoxide Dismutase Expression in Papillary Thyroid Cancer Mesenchymal Stem/Stromal Cells Modulates Cancer Cell Growth and Migration. Sci. Rep. 7, 41416; doi: 10.1038/srep41416 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The authors are grateful to Professor Rosamarina Melillo and Dr. Nella Prevete for flow cytometer analysis. This work was supported by the Ministry of Health, SDN 5x1000 2012, and SDN Foundation RC2010-M-0001.
Footnotes
The authors declare no competing financial interests.
Author Contributions A.P., F.R., F.C., J.K., D.A.C., M.D.C., and M.O.L. planned and performed experiments, H.C. and P.H. isolated human tissues, G.M., M.S., M.D.C., and M.O.L. designed the work and wrote the manuscript.
References
- Pastan I. & Willingham M. Cellular transformation and the ‘morphologic phenotype’ of transformed cells. Nature 274, 645–650, doi: 10.1038/274645a0 (1978). [DOI] [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of the controls for growth and differentiation in myeloid leukemia and the development of cancer. J Natl Cancer Inst 65, 675–679 (1980). [DOI] [PubMed] [Google Scholar]
- Ghesquiere B., Wong B. W., Kuchnio A. & Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature 511, 167–176, doi: 10.1038/nature13312 (2014). [DOI] [PubMed] [Google Scholar]
- Di Mitri D. & Alimonti A. Non-Cell-Autonomous Regulation of Cellular Senescence in Cancer. Trends Cell Biol 26, 215–226, doi: 10.1016/j.tcb.2015.10.005 (2016). [DOI] [PubMed] [Google Scholar]
- Berindan-Neagoe I., Monroig Pdel C., Pasculli B. & Calin G. A. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA: a cancer journal for clinicians 64, 311–336, doi: 10.3322/caac.21244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni E. & Chiarugi P. Redox circuitries driving Src regulation. Antioxid Redox Signal 20, 2011–2025, doi: 10.1089/ars.2013.5525 (2014). [DOI] [PubMed] [Google Scholar]
- Viswanathan A. N. & Schernhammer E. S. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett 281, 1–7, doi: 10.1016/j.canlet.2008.11.002 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza A. et al. Interplay between SOX9, beta-catenin and PPARgamma activation in colorectal cancer. Biochim Biophys Acta 1833, 1853–1865, doi: 10.1016/j.bbamcr.2013.04.004 (2013). [DOI] [PubMed] [Google Scholar]
- Mazzoccoli G., Laukkanen M. O., Vinciguerra M., Colangelo T. & Colantuoni V. A Timeless Link Between Circadian Patterns and Disease. Trends Mol Med 22, 68–81, doi: 10.1016/j.molmed.2015.11.007 (2016). [DOI] [PubMed] [Google Scholar]
- Toullec A. et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med 2, 211–230, doi: 10.1002/emmm.201000073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen M. O. et al. Rabbit extracellular superoxide dismutase: expression and effect on LDL oxidation. Gene 254, 173–179, doi: 10.1016/S0378-1119(00)00272-9 (2000). [DOI] [PubMed] [Google Scholar]
- Laukkanen M. O. et al. Gene transfer of extracellular superoxide dismutase to atherosclerotic mice. Antioxid Redox Signal 3, 397–402, doi: 10.1089/15230860152409040 (2001). [DOI] [PubMed] [Google Scholar]
- Sentman M. L. et al. Extracellular superoxide dismutase deficiency and atherosclerosis in mice. Arterioscler Thromb Vasc Biol 21, 1477–1482, doi: 10.1161/hq0901.094248 (2001). [DOI] [PubMed] [Google Scholar]
- Laatikainen L. E. et al. SOD3 decreases ischemic injury derived apoptosis through phosphorylation of Erk1/2, Akt, and FoxO3a. PLoS One 6, e24456, doi: 10.1371/journal.pone.0024456 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoquist P. O., Carlsson L., Jonason G., Marklund S. L. & Abrahamsson T. Cardioprotective effects of recombinant human extracellular-superoxide dismutase type C in rat isolated heart subjected to ischemia and reperfusion. J Cardiovasc Pharmacol 17, 678–683 (1991). [DOI] [PubMed] [Google Scholar]
- Li Q. et al. Gene therapy with extracellular superoxide dismutase protects conscious rabbits against myocardial infarction. Circulation 103, 1893–1898 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen M. O. et al. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation 106, 1999–2003, doi: 10.1161/01.CIR.0000031331.05368.9D (2002). [DOI] [PubMed] [Google Scholar]
- Laukkanen M. O. et al. EC-SOD gene therapy reduces paracetamol-induced liver damage in mice. J Gene Med 3, 321–325, doi: 10.1002/jgm.194 (2001). [DOI] [PubMed] [Google Scholar]
- Sentman M. L., Brannstrom T. & Marklund S. L. EC-SOD and the response to inflammatory reactions and aging in mouse lung. Free Radic Biol Med 32, 975–981, doi: 10.1016/S0891-5849(02)00790-6 (2002). [DOI] [PubMed] [Google Scholar]
- Laurila J. P., Laatikainen L. E., Castellone M. D. & Laukkanen M. O. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One 4, e5786, doi: 10.1371/journal.pone.0005786 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkka T. et al. Superoxide dismutase 3 limits collagen-induced arthritis in the absence of phagocyte oxidative burst. Mediators Inflamm 2012, 730469, doi: 10.1155/2012/730469 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A. K. et al. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic Biol Med 49, 1937–1946, doi: 10.1016/j.freeradbiomed.2010.09.022 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen L. E. et al. Extracellular superoxide dismutase is a thyroid differentiation marker down-regulated in cancer. Endocr Relat Cancer 17, 785–796, doi: 10.1677/ERC-10-0021 (2010). [DOI] [PubMed] [Google Scholar]
- Cammarota F., de Vita G., Salvatore M. & Laukkanen M. O. Ras oncogene-mediated progressive silencing of extracellular superoxide dismutase in tumorigenesis. Biomed Res Int 2015, 780409, doi: 10.1155/2015/780409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurila J. P. et al. Extracellular superoxide dismutase is a growth regulatory mediator of tissue injury recovery. Mol Ther 17, 448–454, doi: 10.1038/mt.2008.282 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone M. D. et al. Extracellular superoxide dismutase induces mouse embryonic fibroblast proliferative burst, growth arrest, immortalization, and consequent in vivo tumorigenesis. Antioxid Redox Signal 21, 1460–1474, doi: 10.1089/ars.2013.5475 (2014). [DOI] [PubMed] [Google Scholar]
- Laukkanen M. O., Cammarota F., Esposito T., Salvatore M. & Castellone M. D. Extracellular superoxide dismutase regulates the expression of small gtpase regulatory proteins GEFs, GAPs, and GDI. PLoS One 10, e0121441, doi: 10.1371/journal.pone.0121441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukkanen M. O. Extracellular Superoxide Dismutase: Growth Promoter or Tumor Suppressor? Oxid Med Cell Longev 2016, 3612589, doi: 10.1155/2016/3612589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koperek O. et al. Molecular characterization of the desmoplastic tumor stroma in medullary thyroid carcinoma. Int J Oncol 31, 59–67 (2007). [PubMed] [Google Scholar]
- da Silva Meirelles L., Chagastelles P. C. & Nardi N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119, 2204–2213, doi: 10.1242/jcs.02932 (2006). [DOI] [PubMed] [Google Scholar]
- Lambeth J. D. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43, 332–347, doi: 10.1016/j.freeradbiomed.2007.03.027 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R. et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia 21, 304–310, doi: 10.1038/sj.leu.2404489 (2007). [DOI] [PubMed] [Google Scholar]
- Guzzo M., Cantu G. & Di Palma S. Malignant myoepithelioma of the palate: report of case. J Oral Maxillofac Surg: official journal of the American Association of Oral and Maxillofacial Surgeons 52, 1080–1082, doi: 10.1016/0278-2391(94)90183-X (1994). [DOI] [PubMed] [Google Scholar]
- Cammarota F. & Laukkanen M. O. Mesenchymal Stem/Stromal Cells in Stromal Evolution and Cancer Progression. Stem Cells Int 2016, 4824573, doi: 10.1155/2016/4824573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johrer K., Janke K., Krugmann J., Fiegl M. & Greil R. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine up-regulation of MCP-1. Clin Cancer Res 10, 1901–1910, doi: 10.1158/1078-0432.CCR-1053-03 (2004). [DOI] [PubMed] [Google Scholar]
- Youngs S. J., Ali S. A., Taub D. D. & Rees R. C. Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 71, 257–266, doi: 10.1002/(SICI)1097-0215(19970410)71:2257::AID-IJC223.0.CO;2-D (1997). [DOI] [PubMed] [Google Scholar]
- Brasen J. H. et al. Extracellular superoxide dismutase accelerates endothelial recovery and inhibits in-stent restenosis in stented atherosclerotic Watanabe heritable hyperlipidemic rabbit aorta. J Am Coll Cardiol 50, 2249–2253, doi: 10.1016/j.jacc.2007.08.038 (2007). [DOI] [PubMed] [Google Scholar]
- Cammarota F. et al. Clinical relevance of thyroid cell models in redox research. Cancer Cell Int 15, 113, doi: 10.1186/s12935-015-0264-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koperek O., Asari R., Niederle B. & Kaserer K. Desmoplastic stromal reaction in papillary thyroid microcarcinoma. Histopathology 58, 919–924, doi: 10.1111/j.1365-2559.2011.03791.x (2011). [DOI] [PubMed] [Google Scholar]
- Miller F. R., McEachern D. & Miller B. E. Growth regulation of mouse mammary tumor cells in collagen gel cultures by diffusible factors produced by normal mammary gland epithelium and stromal fibroblasts. Cancer Res 49, 6091–6097 (1989). [PubMed] [Google Scholar]
- Wu X., Jin C., Wang F., Yu C. & McKeehan W. L. Stromal cell heterogeneity in fibroblast growth factor-mediated stromal-epithelial cell cross-talk in premalignant prostate tumors. Cancer Res 63, 4936–4944 (2003). [PubMed] [Google Scholar]
- Castellone M. D. et al. Brief report: Mesenchymal stromal cell atrophy in coculture increases aggressiveness of transformed cells. Stem Cells 31, 1218–1223, doi: 10.1002/stem.1361 (2013). [DOI] [PubMed] [Google Scholar]
- Koperek O., Akin E., Asari R., Niederle B. & Neuhold N. Expression of hypoxia-inducible factor 1 alpha in papillary thyroid carcinoma is associated with desmoplastic stromal reaction and lymph node metastasis. Virchows Arch 463, 795–802, doi: 10.1007/s00428-013-1484-3 (2013). [DOI] [PubMed] [Google Scholar]
- Scheuba C., Kaserer K., Kaczirek K., Asari R. & Niederle B. Desmoplastic stromal reaction in medullary thyroid cancer-an intraoperative “marker” for lymph node metastases. World J Surg 30, 853–859, doi: 10.1007/s00268-005-0391-4 (2006). [DOI] [PubMed] [Google Scholar]
- Olive K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461, doi: 10.1126/science.1171362 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjomsland V. et al. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol 2011, 212810, doi: 10.1155/2011/212810, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti A. & Michel J. B. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res, 41, 532–543, doi: 10.1016/S0008-6363(98)00305-8 (1999). [DOI] [PubMed] [Google Scholar]
- Takeda M., Mikami T., Numata Y., Okamoto M. & Okayasu I. Papillary thyroid carcinoma with heterotopic ossification is a special subtype with extensive progression. Am J Clin Pathol 139, 587–598, doi: 10.1309/AJCPQZQN50HKIAHA (2013). [DOI] [PubMed] [Google Scholar]
- Na K. Y. et al. Papillary thyroid carcinoma with bone formation. Pathol Res Pract 209, 14–18, doi: 10.1016/j.prp.2012.10.001 (2013). [DOI] [PubMed] [Google Scholar]
- Akbulut S. et al. Ectopic bone formation and extramedullary hematopoiesis in the thyroid gland: report of a case and literature review. Int Surg 96, 260–265 (2011). [DOI] [PubMed] [Google Scholar]
- Pontikides N., Botsios D., Kariki E., Vassiliadis K. & Krassas G. E. Extramedullary hemopoiesis in a thyroid nodule with extensive bone metaplasia and mature bone formation. Thyroid: official journal of the American Thyroid Association 13, 877–880, doi: 10.1089/105072503322401087 (2003). [DOI] [PubMed] [Google Scholar]
- Harsh M., Dimri P. & Nagarkar N. M. Osseous metaplasia and mature bone formation with extramedullary hematopoiesis in follicular adenoma of thyroid gland. Indian J Pathol Microbiol 52, 377–378, doi: 10.4103/0377-4929.54999 (2009). [DOI] [PubMed] [Google Scholar]
- Wasiljew B. K., Apostol J. V. & Rao M. S. Heterotopic ossification in a lymph node with metastatic follicular carcinoma of thyroid: a case report. J Surg Oncol 17, 45–48, doi: 10.1002/jso.2930170108 (1981). [DOI] [PubMed] [Google Scholar]
- Curry J. T. 3rd & Zallen R. D. Ossifying fibroma of the maxilla occurring with hyperthyroidism. Report of a case. Oral Surg Oral Med Oral Pathol 35, 28–34, doi: 10.1016/0030-4220(73)90090-X (1973). [DOI] [PubMed] [Google Scholar]
- McLean K. et al. Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 121, 3206–3219, doi: 10.1172/JCI45273 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W. et al. RUNX1 is essential for mesenchymal stem cell proliferation and myofibroblast differentiation. Proc Natl Acad Sci USA 111, 16389–16394, doi: 10.1073/pnas.1407097111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. A., Harrell J. C., Iwanaga R., Jedlicka P. & Ford H. L. Vascular endothelial growth factor C promotes breast cancer progression via a novel antioxidant mechanism that involves regulation of superoxide dismutase 3. Breast Cancer Res 16, 462, doi: 10.1186/s13058-014-0462-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantisani M. C. et al. A loss-of-function genetic screening identifies novel mediators of thyroid cancer cell viability. Oncotarget 7, 28510–28522, doi: 10.18632/oncotarget.8577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskula-Sztul R. et al. Tumor-suppressor role of Notch3 in medullary thyroid carcinoma revealed by genetic and pharmacological induction. Mol Cancer Ther 14, 499–512, doi: 10.1158/1535-7163.MCT-14-0073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. & Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37, 1445–1453, doi: 10.1016/j.exphem.2009.09.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D. A., Hematti P. & Kao W. J. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cells Transl Med 1, 740–749, doi: 10.5966/sctm.2012-0061 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.