Abstract
Precursor messenger RNA (Pre-mRNA) splicing is an essential biological process in eukaryotic cells. Genetic mutations in many spliceosome genes confer human eye diseases. Mutations in the pre-mRNA splicing factor, RP9 (also known as PAP1), predispose autosomal dominant retinitis pigmentosa (adRP) with an early onset and severe vision loss. However, underlying molecular mechanisms of the RP9 mutation causing photoreceptor degeneration remains fully unknown. Here, we utilize the CRISPR/Cas9 system to generate both the Rp9 gene knockout (KO) and point mutation knock in (KI) (Rp9, c.A386T, P.H129L) which is analogous to the reported one in the retinitis pigmentosa patients (RP9, c.A410T, P.H137L) in 661 W retinal photoreceptor cells in vitro. We found that proliferation and migration were significantly decreased in the mutated cells. Gene expression profiling by RNA-Seq demonstrated that RP associated genes, Fscn2 and Bbs2, were down-regulated in the mutated cells. Furthermore, pre-mRNA splicing of the Fscn2 gene was markedly affected. Our findings reveal a functional relationship between the ubiquitously expressing RP9 and the disease-specific gene, thereafter provide a new insight of disease mechanism in RP9-related retinitis pigmentosa.
Retinitis pigmentosa (RP [MIM 268000]) is a group of retinal degenerative disorders with high heritability and heterogeneity, affecting approximately 1 in 4,000 in dividuals1,2 and it is becoming the leading cause of irreversible midway blindness worldwide. In RP, progressive decline of retinal function leads to night blindness, peripheral vision loss, eventually completely loss of vision in advanced stage3. To date, at least 27 genes have been identified to associate with the adRP4,5. Among them, many genes are preferentially or exclusively expressed in the neuro-retina and/or retinal pigment epithelium. Notably, a group of spliceosome genes, including PRPF36, PRPF87, PRPF318, RP99, SNRNP20010, PRPF611, and PRPF412, are ubiquitously expressed and involved in the pre-mRNA splicing process.
Pre-mRNA is crucial for the posttranscriptional regulation in mammalian gene expression. Pre-mRNA splicing reaction occurs in spliceosome which is composed of 5 small nuclear ribonucleoproteins (snRNPs), U1, U2, U4/U6, U5, and a large number of accessory protein factors13. The U4/U6-U5 tri-snRNP is a dynamic complex, of which structural rearrangements are critical for assembly as well as catalytic activation in the spliceosome. RP9 has been proven to affect the assembly and/or disassembly of tri-snRNP11. Graziotto et al. and Farkas et al. demonstrated that PRPF3, PRPF8 and PRPF31 mutations could cause dysfunction of RPE14,15. Interestingly, the RP9 caused adRP is classified as the type 216, which refers to parallel deterioration of both rod and cone function in the early stage of the disease9. However, underlying molecular mechanisms of the RP9 mutation causing photoreceptor degeneration remains fully unknown due to lack of appropriate disease model in vitro or in vivo.
Gene editing technology has been dramatically developed to target any genes. Clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated nuclease 9 (Cas9)17,18, are the ideal way to generate in vitro mutational model. In CRISPR/Cas9 system, a short guide RNA (gRNA) contains about a 20 nt sequence and is capable of recognizing the targeted site followed by a protospacer adjacent motif (PAM) which recruits Cas9 to the targeted genome and induces the formation of site-specific double-stranded breaks (DSBs). The DSBs can be repaired by non-homologous end joining (NHEJ), leaving random insertions and deletions (indels), or by homology-directed repair (HDR), resulting in precise genome editing. Previous studies have shown that this system could modify genome editing in eukaryotic cells with high efficiency19,20,21,22,23,24.
Herein, we successfully generated Rp9 gene knockout (Rp9-KO) and point mutation knock in (Rp9-KI) (Rp9, c.A386T, P.H129L) which was analogous to the reported one in the retinitis pigmentosa patients (Rp9, c.A410T, P.H137L) in 661 W retinal photoreceptor cells by using CRISPR/Cas9-mediated approach. Based on this cell model, we observed significant change in cell property and down regulation of RP associated genes, Fscn2 and Bbs2. Furthermore, we elucidated that pre-mRNA splicing of the Fscn2 gene was remarkably reduced in the mutated cells. Our study for the first time revealed a functional relationship between Rp9, the general splicing factor, and FSCN2, the photoreceptor-specific gene, and provided a new insight of disease mechanism in Rp9-causing retinitis pigmentosa.
Results
Validation of cone photoreceptor-specific markers in 661 W Cells
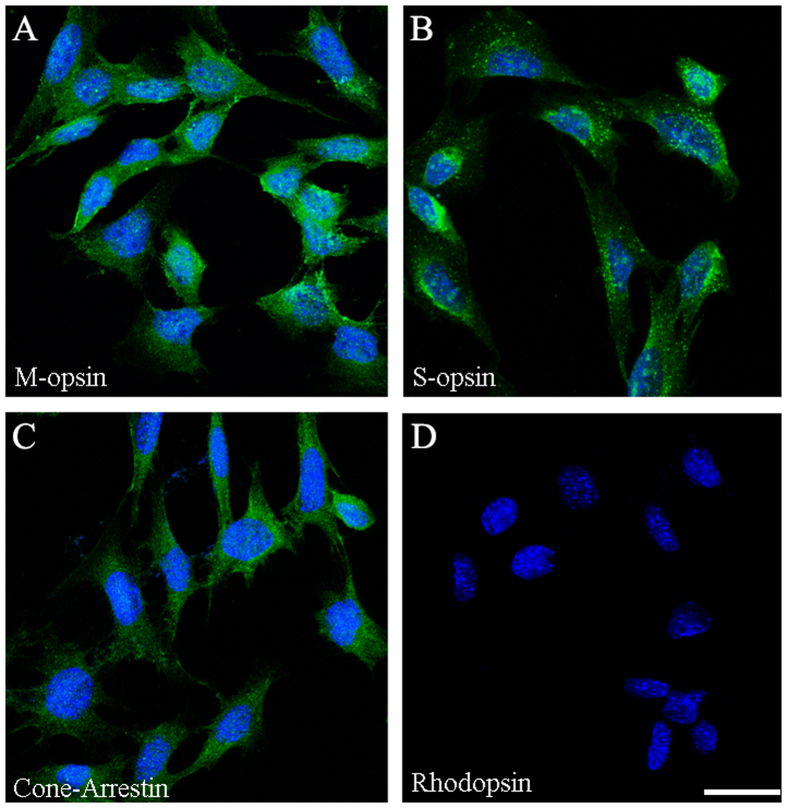
To test 661 W cell line whether is rod photoreceptor or cone photoreceptor, cells were immunostaining for rhodopsin, blue opsin, red or green opsin and cone arrestin. The results showed that 661 W cells were positive with the cone photoreceptor-specific markers, red or green opsin (Fig. 1A), blue opsin (Fig. 1B) and cone arrestin (Fig. 1C), but negative with rod photoreceptor-specific marker, rhodopsin (Fig. 1D). These results verified that 661 W cells were cone photoreceptor originated.
Figure 1. Immunocytochemical staining of 661 W cells.
Cells grew on glass cover slips and were fixed with cold 4% PFA, immunolabeled with primary antibodies against red/green opsin (A), blue opsin (B), cone arrestin (C) and rhodopsin (D). DAPI (blue) was used to detect the nuclei. Scale bar: 25 μm.
Site-specific DNA cleavage executed by Cas9/sgRNA in 661 W cells
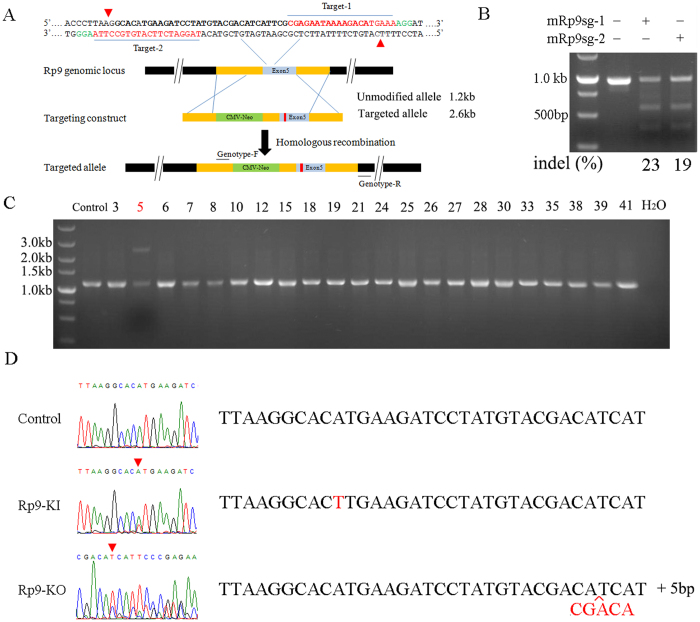
To test whether Cas9/sgRNA could cleave targeted regions of Rp9, we designed two sgRNAs targeting different regions of Rp9 gene (Fig. 2A). Each Cas9/sgRNA was transfected into 661 W cells and a T7EI assay was performed to determine the cutting efficiency of each sgRNA. The results indicated that sgRNA-1 and sgRNA-2 designed to target the Rp9 gene were highly active, inducing mutations at frequencies of 23% for sgRNA-1 and 19% for sgRNA-2 (Fig. 2B). Furthermore, no mutation was detected using the T7EI assay when cells were transfected with Cas9 plasmid alone. Taken together, these results suggested that sgRNA-1 and sgRNA-2 efficiently targeted the Rp9 and led to NHEJ-mediated indels at target sites.
Figure 2. Genome editing via the type II CRISPR system in 661 W cells.
(A) Schematic illustrating Cas9n double nicking the mouse Rp9 locus and strategy of Cas9n pairs- mediated HDR. mRp9sg-1 and mRp9sg-2 sequences are shown in red. Representative cleavage sites are shown by a red triangle for 661 W cells transfected with Cas9n pairs matching target-1 and 2. The targeting vector includes homology arms (HA) flanking a CMV-Neomycin element and Rp9 point mutation. (B) SURVEYOR assay for Cas9 and sgRNA-mediated indels. 661 W cells were transfected by empty vectors or vectors expressing mRp9sg-1 or mRp9sg-2 to test cutting efficiency in the endogenous Rp9 locus. The DNA in the specific cells mentioned above were extracted and performed by PCR and T7EI assays. The percentage of indels was quantified by the ImageJ software. (C) PCR genotyping. As shown, 1 clone (marked with red number) out of 22 randomly selected clones had the expected Rp9 mutation insertion. (D) Representative Sanger sequencing results of the PCR amplicons from 22 clones showing point mutation and insertion (red).
Generation of Rp9-KI and Rp9-KO in 661 W cells
Encouraged by the high efficiency of Cas9/sgRNAs, we explored whether these Cas9n pairs could catalyze site-specific DNA cleavage and HDR in 661 W cells. 661 W cells were transfected with donor vector and Cas9n pairs targeting the exon 5 of Rp9 gene. 3days later, transfected cells were selected for further 7 days with 1.0 mg/ml G418. After selection, cells were digested and 500 cells were transferred to a 10-cm dish. 2 weeks later, clones were picked out and propagated for another week. After propagation, 22 clones were randomly selected for genotyping. The genomic DNA of 22 clones was analyzed by PCR amplification with specific primers showed as Supplemental Table 1. Figure 2C illustrated that clone 5 contained two different amplification products whereas other clones contained only one amplification product. Sequencing result of the upper bands of clone 5 revealed the point mutation, c.A386T (Fig. 2D). The result demonstrated that the clone 5 was a heterozygous Rp9-KI cell. Also, we sequenced the other 21 clones, the results showed that five clones were heterozygous Rp9-KO cells which only one allele was mutated with nucleotide indels thus causing a frameshift mutation. Thus Clone 12 would represent for Rp9-KO (Fig. 2D).
Decreased proliferation and migration by Rp9-KI and Rp9-KO
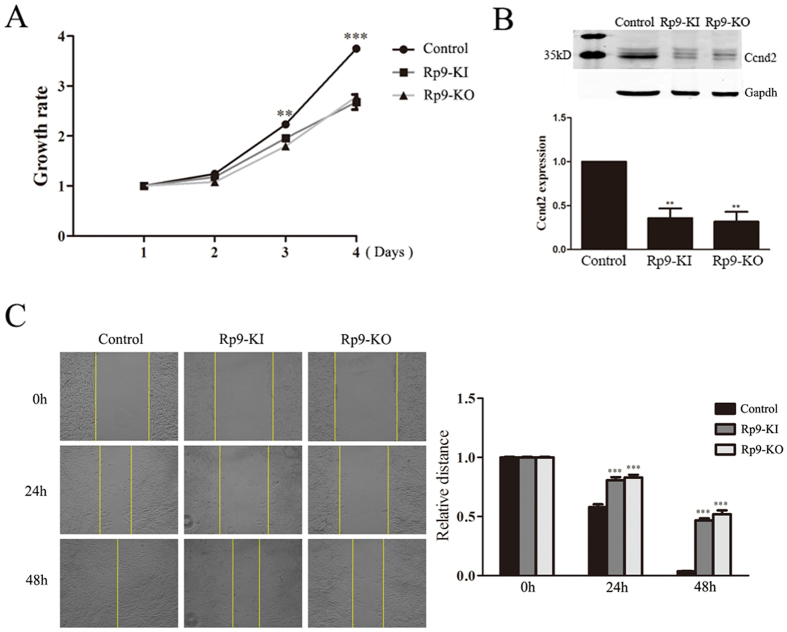
We sought to detect whether these Rp9 mutations had any biological effects on 661 W cells. Rp9-KI and Rp9-KO showed inhibition of cell growth as compared with control based on the MTT assay (Fig. 3A). A reduction in growth rate was detected at day 3. At day 4, the growth rate decreased 27.6% in Rp9-KI and 25.6% in Rp9-KO cells compared with control group (n = 3, P < 0.001, Fig. 3A). Western blot analysis confirmed that proliferation-related gene Ccnd2 expression was remarkably reduced in Rp9-KI and Rp9-KO cells (Fig. 3B). We then performed in vitro scratch assay to examine whether Rp9 mutations were involved in the regulation migration of 661 W cells. A dramatic migration reduction was observed in Rp9-KI and Rp9-KO cells (Fig. 3C).
Figure 3. Rp9 gene mutation inhibited the proliferation and migration of 661 W cells.
(A) MTT cell proliferation assay was performed at indicated days. Data at each time point were expressed as mean ± SEM based on results obtained from triplicates. **P < 0.01, ***P < 0.001. (B) Rp9 gene mutation down-regulated Ccnd2 expression. 661 W, Rp9-KI and Rp9-KO cell lysate were prepared and used for Western blot analysis. Gapdh was used as an internal loading control. The band intensity was measured with ImageJ software, the fold change was normalized to the level of 661 W group. Data are representative of three independent experiments. **P < 0.01. (C) In vitro scratch assays were performed to evaluate the migration potential of 661 W, Rp9-KI and Rp9-KO cells.
RNA-seq and differential expression analysis of Rp9-KI and Rp9-KO cells
Three libraries were generated from 661 W, Rp9-KI and Rp9-KO groups and summaries of RNA-Seq analyses are shown in Supplement Table 3. About 42.66 (661 W), 38.56 (Rp9-KI), and 39.83 (Rp9-KO) million clean reads were obtained for each transcriptome. The Q30 scores (the average quality value) were above 93%. The RNA-Seq raw reads have been submitted to the NCBI SRA database (accession number: SRR5131155, SRR5131263, and SRR5131264).
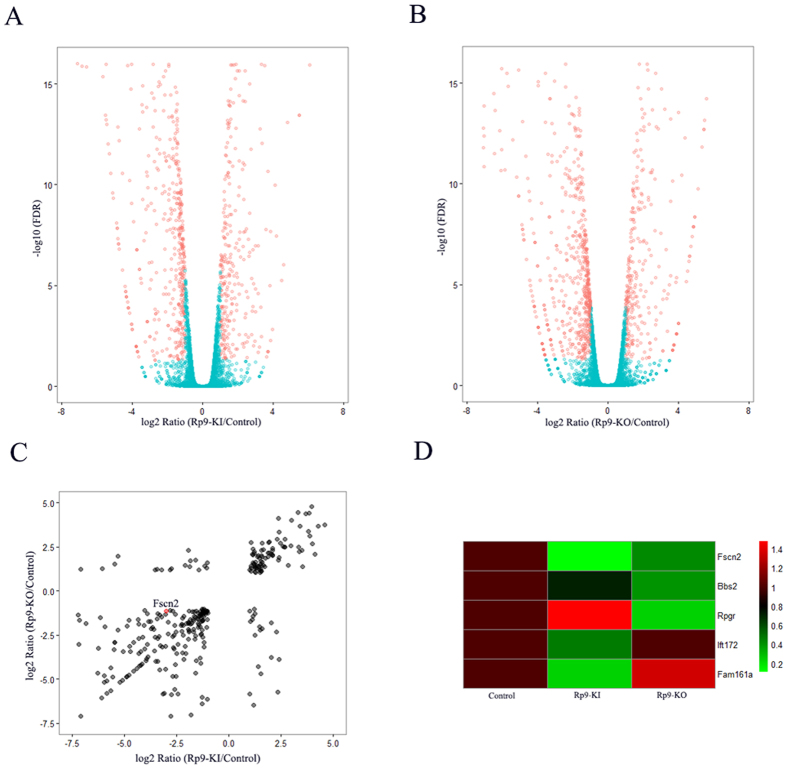
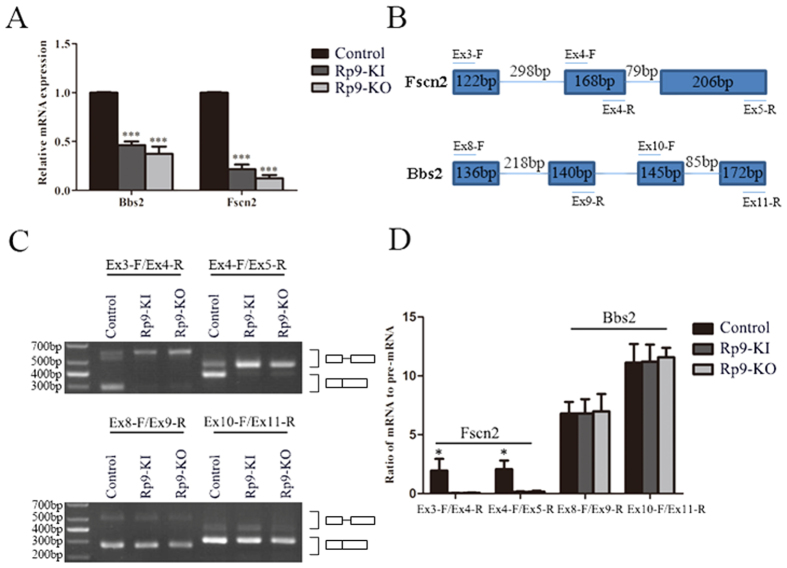
Through RNA-Seq, a total of 14006, 13885 and 13226 expressed genes were detected in 661 W, Rp9-KI and Rp9-KO cells respectively. Among them, 784 and 934 genes were differentially expressed in Rp9-KI and Rp9-KO cells compared with 661 W cells (Fig. 4A,B). Overlap of differentially expressed genes containing down-regulated RP associated gene, Fscn2, identified by Rp9-KI and Rp9-KO cells were presented (Fig. 4C). We used qRT-PCR to confirm the expression of Fscn2 and found it to be markedly down-regulated in Rp9-KI and Rp9-KO cells (Fig. 5A). We also used qRT-PCR to quantify other RP associated genes which were significant down-regulated in Rp9-KI or Rp9-KO cells (Fig. 4D) and found another gene, Bbs2, also significantly decreased in Rp9-KI and Rp9-KO cells (Fig. 5A).
Figure 4. RNA-seq analyses detected gene expression changes in Rp9 mutant cells.
(A) Volcano plot showing genes differentially expressed in Rp9-KI compared with control group. Red dots show the 784 genes with a |log2FC| > 1 and FDR < 0.05. (B) Volcano plot showing genes differentially expressed in Rp9-KO compared with control group. Red dots show the 934 genes with a |log2FC| > 1 and FDR < 0.05. (C) Overlap of differential gene expression betweenRp9-KI and Rp9-KO group. Red dots show the Fscn2 gene down-regulated in both groups. (D) Heatmaps present RP associated gene expression in Rp9-KI and Rp9-KO group.
Figure 5. Rp9 gene mutation significantly affects Fscn2 pre-mRNA splicing.
(A) qRT- PCR analysis of Fscn2 and Bbs2 transcripts in 661 W, Rp9-KI and Rp9-KO cells. Relative expression levels of mRNA were normalized against Gapdh. ***P < 0.001. (B) Genomic structures of Fscn2 and Bbs2 genes and primers used for RT-PCR. (C) Effects of Rp9 gene mutation on the pre-mRNA splicing of Fscn2 intron 3, intron 4 and Bbs2 intron 8, intron 10. RT-PCR was used to detect the splicing products and corresponding pre-mRNA using specific primers as depicted in panel B. (D) Quantification of Fscn2 and Bbs2 splicing efficiency by measuring the ratio of mRNA to pre-mRNA using ImageJ from three independent experiments. *P < 0.05.
RP9 mutagenesis affects pre-mRNA splicing of photoreceptor gene FSCN2
Previous report showed that PRPF31 mutation inhibited splicing of retina-specific gene, RHO25. In order to confirm whether RP9 mutation inhibited retina-specific splicing substrate genes expression, we selected Fscn2 and Bbs2 for the further experiments. Fscn2 and Bbs2 pre-mRNA splicing were examined using RT-PCR with specific primers (Fig. 5B, Supplemental Table 1). As shown in the Fig. 5C, Fscn2 splicing was significantly inhibited in Rp9-KI and Rp9-KO compared with control group. Quantification of splicing efficiency obtained from three independent experiments was shown in Fig. 5D. However, Rp9-KI and Rp9-KO did not affect the Bbs2 splicing, because there was no significant change in the ratio of pre-mRNA in splicing products (Fig. 5D).
Discussion
We have demonstrated that RP9 patient-specific rod photoreceptors conferred degeneration in vitro by using patient-specific induced pluripotent stem cells26. However, underlying molecular mechanisms of the RP9 mutation causing photoreceptor degeneration remains unknown. Pre-mRNA splicing is crucial for the posttranscriptional regulation of gene expression, providing significant expansion of the functional proteome of eukaryotic organisms with limited genes27. Mutations that interfere with splicing play an important role in human eye disease28,29,30. Here we have shown that proliferation and migration significantly decrease in the Rp9 mutant 661 W photoreceptor cells. RP associated genes, Fscn2 and Bbs2, both are markedly down-regulated in the mutated cells. Further investigation indicated that pre-mRNA splicing of the Fscn2 gene was markedly reduced.
In this study, we successfully generated Rp9-KI (HDR) with an efficiency of 4.5% and Rp9-KO (Indels) with an efficiency of 22.7%, which was consistent with reports from other groups. For example, Mali et al. reported 10–25% indel rates in 293 T cells in 201320. Likewise, in mouse embryonic stem cells study, the efficiency of NHEJ-mediated knock-out was 28–50%, whereas the efficiency of HDR-mediated knock-in was below 10%23. Importantly, Rp9-KI and RP9-KO resulted in inhibition of cell proliferation and migration.
Previous work by Yuan et al. demonstrated that PRPF31 mutation could inhibit RHO splicing25. Through RNA sequencing, we confirmed the expression of Fscn2 markedly down-regulated in Rp9-KI and Rp9-KO cells. Fscn2, actin-bundling proteins, is a photoreceptor-specific protein of fascin family, which plays an important role in photoreceptor disk morphogenesis. A frame-shift mutation in FSCN2, 208delG, was reported in Japanese adRP patients31. A mouse model carrying a targeted disruption of Fscn2 showed progressive photoreceptor degeneration32.
RT-PCR results showed that the 661 W cell had normal pre-mRNA splicing of Fscn2, whereas Rp9-KI and Rp9-KO cells inhibited this splicing progress. Our results were consistent with those recently reported by Mordes et al., who found dominant-negative effect of PRPF31 mutation on FSCN2 pre-mRNA splicing33. However, Maita et al. reported that RP9 p.H137L mutation had no effect on E1A splicing34. This might suggest that Rp9 only inhibits a fraction of gene splicing. Our experiments revealed a functional link between ubiquitously expressed Rp9 gene and the expression of the retina-specific gene, Fscn2. Our results that Rp9-KI and Rp9-KO blocking pre-mRNA splicing of Fscn2 provided a new insight for the photoreceptor-specific phenotype of RP9 mutations. Defected Fscn2 gene products may contribute to photoreceptor cell death.
We also detected the RP associated gene, Bbs2, markedly down-regulated in Rp9-KI and Rp9-KO cells. Our result showed that Rp9-KI and Rp9-KO did not affect the Bbs2 splicing. It demonstrated that not all splicing events were equally sensitive to Rp9 mutation. Rp9 mutants may inhibit pre-mRNA splicing of a subset of photoreceptor genes, such as Fscn2 intron 3 and intron 4, but not other splicing events such as Bbs2 intron 8 and intron 10.
In summary, we have shown that Rp9 mutations obviously inhibit cell proliferation and migration. Furthermore, the splicing of adRP associated gene, Fscn2, is also significantly inhibited in Rp9 mutation cells. Our results on RP9 provide an explanation why the mutations in the ubiquitously expressed splicing factors can cause adRP.
Materials and Methods
Donor vectors and sgRNA
To construct donor templates, Rp9 fragments and CMV-Neo were amplified with Phanta Max Super-Fidelity DNA Polymerase (Vazyme Biotech Co.Ltd, Nanjing, China) using gene-specific primers (Supplemental Table 1). Rp9 fragments were cloned into pEASY-Blunt Simple Cloning Vector (pEASY-Rp9), and then CMV-Neo was cloned into SacI sites of pEASY-Rp9 (Supplemental Table 2). sgRNAs of mRp9 were ordered, annealed, phosphorylated and cloned into the BbsI-digested Cas9 nickase vector (pX335, Addgene plasmid ID: 42335) and Cas9 vector (pX330, Addgene plasmid ID: 42230).
Cell culture
Cone-derived cell line (661 W) originated from a transgenic mouse line with retinal tumor35 was cultured in DMEM medium (Gibco, Carlsbad, USA) supplemented with 10% heat-inactivated FBS (Gibco, Carlsbad, USA)and 100 μg/mL penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2. 661 W cells were plated into 6-well plates for transfection. After twenty four hours, cells were replaced with new complete medium and the DNA mixed with FuGENE HD Reagent (Roche, Basel, Switzerlands) in Opti-MEM (Gibco, Carlsbad, USA) according to the manufacturer’s manual. For G418 selection, 661 W cells transfected with px335-mRp9sg1, px335-mRp9sg2 (Cas9n pairs) and donor vector were selected with 1 mg/ml of G418.
Genomic DNA isolation, amplication and T7EI assay
To validate the mRp9 sgRNAs, the genomic DNA of each Cas9/gRNA-transfected cells was extracted using the Blood/Cultured cells DNA Kit (Simgen Biotech Co.Ltd, Hangzhou, China) following the manufacturer’s instruction. The regions containing thetarget sites were amplified by PCR using Phanta Max Super-Fidelity DNA Polymerase with gene-specific primers (Supplemental Table 1) under the following conditions: 95 °C for 3 min; 30 cycles (95 °C for 15 s, 58 °C for 15 s, 72 °C for 30 s) and 72 °C for 5 min.
The T7EI assay was performed according to the manufacturer’s instructions. In brief, 20 μl of PCR product was denatured and annealed by heating at 95 °C for 5 min and ramped down to 25 °C at 6 °C/min. Then, 5 μl annealed samples, 1.1 μl NEBuffer 2 (10x), 0.5 μl T7EI and 4.4 μl ddH2O were added together and incubated at 37 °C for 30 min. Cleaved DNA fragments were separated on 1.5% agarose gels and the DNA concentration of each band was quantified using the ImageJ software. Percent values of indels were calculated as described15. For genotyping PCR, genomic DNA was amplified using 2 × Super Taq PCR MasterMix (BioTeke Corporation, Beijing, China) with primers listed (Supplemental Table 1).
Immunocytochemistry
661 W cells were fixed in 4% paraformaldehyde (PFA) for 15 minutes. Cells were permeabilized and blocked in PBS containing 4% BSA and 0.5% Triton X-100 for 1 hour at room temperature, incubated overnight with primary antibody at 4 °C, and then subjected to immunohistochemistry as previously described36.
Primary antibodies were rabbit anti-opsin blue (1:500; chemicon, AB5407), rabbit anti-opsin red/green (1:500; chemicon, AB5405), rabbit anti-cone arrestin (1:500; Millipore, AB15282) and mouse anti-rhodopsin (1:10000; sigma, o4886).
Cell proliferation assay
A total of 5 × 103 661 W cells were seeded in 96-well plates with 100 μl DMEM containing 5% FBS and 100 μg/mL penicillin/streptomycin. Cell proliferation was assessed by MTT assay. MTT assay was carried out according to the method by Mosmann37. Briefly, 10 μl of MTT solution was added into each well and the cells were incubated for 4 hours. Then medium was discarded and supplied with 150 μl DMSO. Finally, Cell proliferation was assessed by measuring the absorbance at 490 nm using Spectra Max M5 (Molecular Devices, Sunnyvale, CA, USA).
In Vitro scratch assay
In Vitro Scratch Assay was performed as previously reported38. Simply, cells were scratched with a 200 μl pipet tip. Remove the debris by washing the cells once with 1 ml of DPBS and then replace with 2 ml DMEM supplemented with 2% FBS and 100 μg/mL penicillin/streptomycin. Photograph was taken immediately after scratching and at 24 or 48 hours after culture. The ability of migration was evaluated by comparing with the migration rate in the center of the gap.
Western blot analysis
Total protein extracts were prepared using cell lysis buffer containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 2 mM leupeptin, 1 mM pepstatin, 80 mM aprotinin). Protein contents were quantified by the Bradford reagent according to the manufacturer’s instructions. Equal amounts of proteins were separated by 12% SDS-PAGE and transferred to a nitrocellulose blotting membrane (PALL Corporation, Port Washington, USA). Membranes were blocked for 1 h in 1 × TBS containing 10% non-fat milk, 0.1% Tween 20 and incubated overnight with primary antibodies: rabbit anti-Ccnd2 (Santa Cruz Biotechnology, Santa Cruz, USA), mouse anti-Gapdh (KangChen Bio-tech Inc., Shanghai, China). Then membranes were incubated with IRDye® 680-conjugated goat anti- rabbit, IRDye 800CW-conjugated goat anti-mouse. Fluorophore-conjugated antibodies were detected using the Odyssey® Imager (LI-COR Biosciences Inc., Lincoln, USA).
RNA library construction and sequencing
RNA was extracted from the 661 W cells, Rp9-KI and Rp9-KO mutated cells using TRIzol reagent (Invitrogen, San Diego, USA). RNA concentration and purity were measured with NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, USA).
High-quality RNA was sent to Biomarker Technologies Corporation (Biomarker Technologies Corporation, Beijing, China) for cDNA libraries construction and sequencing. The RNA-Seq libraries were constructed according NEBNext UltraTM RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, USA) following manufacturer’s recommendations. Briefly, mRNA was purified by NEBNext Poly (A) mRNA Magnetic Isolation Module. The isolated mRNA was fragmented and used to synthesize the first cDNA. Second strand cDNA synthesis was generated using DNA Polymerase I and RNase H. The double-stranded cDNAs were purified by Agencourt AMPure XP system (Beckman Coulter, Brea, USA) and subjected to end repair and adapter ligation. The ligation products were enriched by PCR amplification and purified using Agencourt AMPure XP system. Sequencing reactions were carried out on the Illumina HiSeq 2500.
Transcriptome analysis and identification of differential gene expression
The raw reads were firstly processed through in-house perl scripts. Clean reads were obtained by removing reads containing adapter sequences, unknown nucleotides>5%, low quality reads. The clean reads were mapped to mouse genome (mm10) with TopHat239. Gene expression levels were estimated using fragments per kilobase of exon per million fragments mapped (FPKM).
Prior to differential gene expression analysis, the read counts of each sequenced library were adjusted by edgeR program package40. DEGseq41 was performed to evaluate differential expression between control, Rp9-KI and Rp9-KO groups. The false discovery rate (FDR) control method was applied to define the threshold of the P-value to compute the level of significance. Significantly differential expression was accepted as |log2FC| > 1 and FDR < 0.05.
RT-PCR
For reverse transcription, isolated RNA was treated with DNase I (Thermo Scientific, Rockford, USA) according to the manufacturer’s manual. The DNase I-treated RNA was transcribed into cDNA using MMLV Reverse Transcriptase (Promega, Madison, USA). The cDNA was subsequently used as template to perform RT-PCR and qRT-PCR.
RT-PCR was performed according to the following procedures: 12.5 μl 2×Super Taq PCR Master Mix, 0.5 μl cDNA, 11 μl ddH2O, 0.5 μl forward primer (10 μM), 0.5 μl reverse primer (10 μM) (Supplemental Table 1), reaction conditions including 95°C for 3 min; 40 cycles (95 °C for 15 s, 58 °C for 15 s, 72 °C for 30 s) and 72 °C for 5 min.
qRT-PCR was performed as described in the method of Fast start universal SYBR Green Master (Roche Molecular Biochemicals, Mannheim, Germany) with 7500 Real-Time PCR System. Gapdh expression was used for normalization. The specificity of PCR products was verified by melt curve analysis. Sequences of primers are shown (Supplemental Table 1).
Statistical analysis
Data were expressed as means ± SEM and analyzed by two-way analysis of variance (ANOVA) with GraphPad. Differences were considered to be statistically significant at a P value of 0.05 or less.
Additional Information
How to cite this article: Lv, J.-N. et al. Targeted RP9 ablation and mutagenesis in mouse photoreceptor cells by CRISPR-Cas9. Sci. Rep. 7, 43062; doi: 10.1038/srep43062 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank Drs. Chun-Yun Feng and Xiao-Yun Wang for their suggestions. This study was supported by the National Natural Science Foundation of China (81371059, 81522014, 81500741), Zhejiang Provincial Natural Science Foundation of China (LR13H120001, Q14B020018), National Key Basic Research Program (2013CB967502), Zhejiang Provincial Key Research and Development Program (2015C03029), the NHFPC Grant-in-Aid for Medical and Health Science (WKJ-ZJ-1417), Wenzhou Science and Technology Innovation Team Project (C20150004), Eye Hospital of Wenzhou Medical University General Program (YNKT201501).
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the experiments: Z.J. Performed the experiments: J.L., G.Z., X.C. Analyzed the data: J.L., H.C., K.W., L.X., X.L., X.Z. Contributed reagents/materials/analysis tools: Z.J., R.W. Wrote the paper: J.L., Z.J.
References
- Hartong D. T., Berson E. L. & Dryja T. P. Retinitis pigmentosa. The Lancet 368, 1795–1809 (2006). [DOI] [PubMed] [Google Scholar]
- Berger W., Kloeckener-Gruissem B. & Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 29, 335–375 (2010). [DOI] [PubMed] [Google Scholar]
- Berson E. L. Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophth Vis Sci 34, 1659–1676 (1993). [PubMed] [Google Scholar]
- Ran X. et al.‘RetinoGenetics’: a comprehensive mutation database for genes related to inherited retinal degeneration. Database-Oxford 2014, 1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger S. P., Sullivan L. & Bowne S. R. Retinal information network. The University of Texas Health Science Center (2003). [Google Scholar]
- Chakarova C. F. et al. Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Hum Mol Genet 11, 87–92 (2002). [DOI] [PubMed] [Google Scholar]
- McKie A. B. et al. Mutations in the pre-mRNA splicing factor gene PRPC8 in autosomal dominant retinitis pigmentosa (RP13). Hum Mol Genet 10, 1555–1562 (2001). [DOI] [PubMed] [Google Scholar]
- Vithana E. N. et al. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13. 4 (RP11). Mol Cell 8, 375–381 (2001). [DOI] [PubMed] [Google Scholar]
- Keen T. J. et al. Mutations in a protein target of the Pim-1 kinase associated with the RP9 form of autosomal dominant retinitis pigmentosa. Eur J Hum Genet 10, 245–249 (2002). [DOI] [PubMed] [Google Scholar]
- Zhao C. et al. Autosomal-dominant retinitis pigmentosa caused by a mutation in SNRNP200, a gene required for unwinding of U4/U6 snRNAs. Am J Hum Genet 85, 617–627 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanackovic G., Ransijn A., Ayuso C., Harper S., Berson E. L. & Rivolta C. A missense mutation in PRPF6 causes impairment of pre-mRNA splicing and autosomal-dominant retinitis pigmentosa. Am J Hum Genet 88, 643–649 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. et al. PRPF4 mutations cause autosomal dominant retinitis pigmentosa. Hum Mol Genet 23, 2926–2939 (2014). [DOI] [PubMed] [Google Scholar]
- Wahl M. C., Will C. L. & Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell 136, 701–718 (2009). [DOI] [PubMed] [Google Scholar]
- Graziotto J. J. et al. Three gene-targeted mouse models of RNA splicing factor RP show late-onset RPE and retinal degeneration. Invest Ophthalmol Vis Sci 52, 190–198 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas M. H. et al. Mutations in pre-mRNA processing factors 3, 8, and 31 cause dysfunction of the retinal pigment epithelium. Am J Hum Genet 184, 2641–2652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp C. M., Jacobson S. G. & Faulkner D. J. Two types of visual dysfunction in autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci 29, 1235–1241 (1988). [PubMed] [Google Scholar]
- Cong L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N. et al. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res 23, 465–472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. et al. High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol 31, 822–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long C. et al. Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351, 400–403 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. L. et al. Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Rep 4, 143–154 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers B. et al. Direct production of mouse disease models by embryo microinjection of TALENs and oligodeoxynucleotides. Proc Natl Acad Sci USA 110, 3782–3787 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Kawada M., Havlioglu N., Tang H. & Wu J. Y. Mutations in PRPF31 inhibit pre-mRNA splicing of rhodopsin gene and cause apoptosis of retinal cells. J Neurosci 25, 748–757 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.-B. et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PloS one 6, e17084 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. & Rio D. C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu Rev Biochem 84, 291–323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B. et al. Systemic splicing factor deficiency causes tissue-specific defects: a zebrafish model for retinitis pigmentosa. Hum Mol Genet 20, 368–377 (2010). [DOI] [PubMed] [Google Scholar]
- Maubaret C. G. et al. Autosomal dominant retinitis pigmentosa with intrafamilial variability and incomplete penetrance in two families carrying mutations in PRPF8. Invest Ophth Vis Sci 52, 9304–9309 (2011). [DOI] [PubMed] [Google Scholar]
- Ajmal M. et al. A missense mutation in the splicing factor gene DHX38 is associated with early-onset retinitis pigmentosa with macular coloboma. J Med Genet 51, 444–448 (2014). [DOI] [PubMed] [Google Scholar]
- Wada Y., Abe T., Takeshita T., Sato H., Yanashima K. & Tamai M. Mutation of human retinal fascin gene (FSCN2) causes autosomal dominant retinitis pigmentosa. Invest Ophth Vis Sci 42, 2395–2400 (2001). [PubMed] [Google Scholar]
- Yokokura S. et al. Targeted disruption of FSCN2 gene induces retinopathy in mice. Invest Ophth Vis Sci 46, 2905–2915 (2005). [DOI] [PubMed] [Google Scholar]
- Mordes D., Yuan L., Xu L., Kawada M., Molday R. S. & Wu J. Y. Identification of photoreceptor genes affected by PRPF31 mutations associated with autosomal dominant retinitis pigmentosa. Neurobiol Dis 26, 291–300 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maita H., Kitaura H., Keen T. J., Inglehearn C. F., Ariga H. & Iguchi-Ariga S. M. PAP-1, the mutated gene underlying the RP9 form of dominant retinitis pigmentosa, is a splicing factor. Exp Cell Res 300, 283–296 (2004). [DOI] [PubMed] [Google Scholar]
- Al-Ubaidi M. R. et al. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol 119, 1681–1687 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V. et al. miRNAs 182 and 183 are necessary to maintain adult cone photoreceptor outer segments and visual function. Neuron 83, 586–600 (2014). [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65, 55–63 (1983). [DOI] [PubMed] [Google Scholar]
- Liang C.-C., Park A. Y. & Guan J.-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2, 329–333 (2007). [DOI] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R. & Salzberg S. L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biol 14, R36 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S. & Huber W. Differential expression analysis for sequence count data. Genome biol 11, R106 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.