Abstract
Ga-68 labeled prostate-specific membrane antigen (PSMA) whole body PET/CT scan is a novel upcoming modality for the evaluation of prostate cancer. We present three cases of prostate cancer showing rare sites of metastases like brain, penis, and liver detected on Ga-68 PSMA PET/CT scan thus emphasizing its role in lesion detection and staging.
Keywords: Ga-68, metastases, prostate cancer, PSMA
Introduction
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that has considerable overexpression on most prostate cancer cells. This antigen has gained increased interest as a target molecule for imaging and therapy.[1] Whole body Ga-68 labeled PSMA [Glu-NH-CO-NH-Lys-(Ahx)-[Ga-68(HBED-CC)] PET/CT scan is a novel and a promising radio ligand having a high sensitivity in detecting lesions of prostate cancer with a high tumor to background contrast, even at low-serum PSA- Prostate specific antigen levels.[2]
Brain, liver, and penis are some of the organs which are uncommonly involved in carcinoma of prostate. There are very few case reports till date which revealed detection of these sites on body Ga-68 PSMA PET/CT scans. This is the first paper to report the detection of three rare sites of metastases on whole body Ga-68 PSMA PET/CT scans.
Case 1
This 45-year-old male was diagnosed with adenocarcinoma prostate 18 months ago with initial PSA of 98 ng/mL and Gleason's score of 4+5. He underwent baseline work up with MRI of pelvis which revealed disease in prostate gland with infiltration of contiguous pelvic organs and skeletal lesions in pelvis. He underwent external beam radiotherapy to pelvis and was started on GnRH analog therapy and bicalutamide. He presented 1 year later with evidence of biochemical disease progression with high PSA doubling kinetics, despite being on hormonal therapy. The patient was otherwise asymptomatic except occasional pain in the pelvis. Ga-68 PSMA PET/CT whole body scan done for further evaluation at our department revealed PSMA avid nodular lesions involving the entire prostate gland with infiltration of bilateral seminal vesicles, enlarged pelvic lymph nodes, sclerotic skeletal metastases, and extensive pulmonary metastases. In addition, atleast four Ga-68 PSMA avid enhancing intracranial space occupying lesions were noted on the brain sequences, in left frontal and left fronto-parietal regions [Figure 1]. The largest lesion was in the left frontal lobe, measured 1.4 × 1.4 cm in size with SUV Max: 4.0 and demonstrated significant perilesional edema with resultant midline shift of approximately 4 mm. An MRI scan with MR spectroscopy was also done for the further evaluation of these brain lesions which corroborated with our findings of metastases. The patient then underwent stereotactic radiosurgery for the brain metastases at our hospital.
Figure 1.
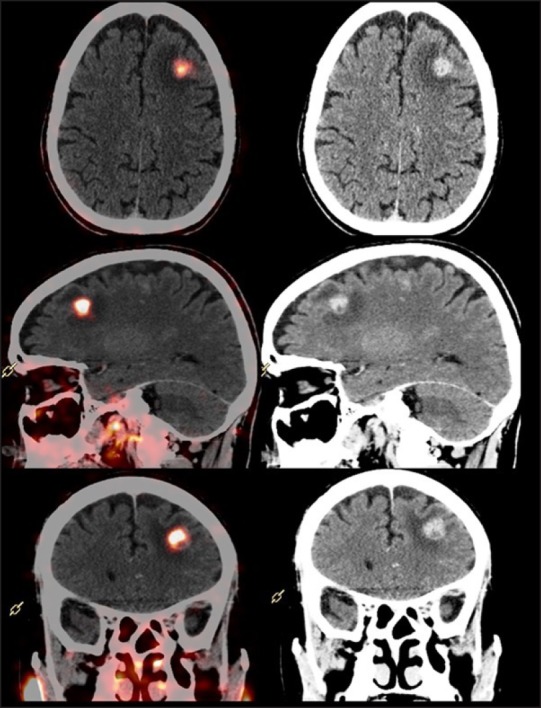
Ga-68 prostate-specific membrane antigen positron emission tomography-computed tomography scan: PET/CT fused and CT sections trans-axial, sagittal, and coronal, showing brain metastases in left fronto-parietal region on Ga-68 PSMA PET/CT scan.
Case 2
This 59 year case of carcinoma prostate (poorly differentiated adenocarcinoma, Gleason's score 4+4, initial PSA 5.6 ng/mL) diagnosed with organ confined disease 7 years ago, presented to us with complaint of pain during micturition. He had undergone radiotherapy to the pelvis, and was currently on antiandrogen hormonal therapy. His recent serum PSA level was 9.8 ng/mL. He underwent Ga-68 PSMA PET/CT scan at our department which revealed an ill-defined Ga-68 PSMA avid (SUV max—12.1) nodular thickening along the ventral aspect of penis involving the base and shaft and abutting the corpus spongiosum muscle [Figure 2]. Also a nodular lesion was noted in the right posterior peripheral zone of prostate gland with intense Ga-68 PSMA expression, likely representing local residual/recurrent active disease focus. The penile lesion was reported as suspicious for metastatic disease and was further subjected to ultrasonography and biopsy. The histopathology of metastatic adenocarcinoma confirmed our findings.
Figure 2.
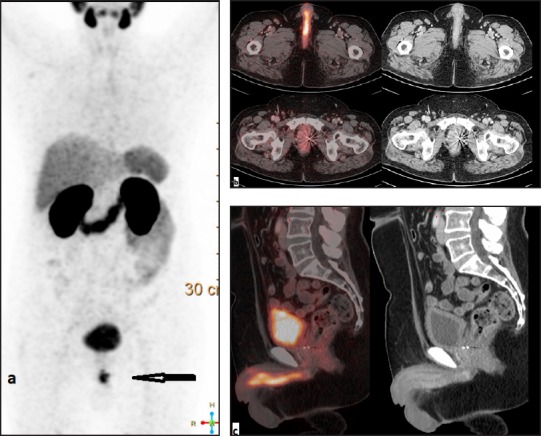
Ga-68 prostate-specific membrane antigen positron emission tomography-computed tomography scan, (a) maximum intensity projection; (b) trans-axial sections of PET/CT fused and computed tomography; (c) sagittal sections of PET/CT fused and computed tomography. Images show prostate-specific membrane antigen avid nodular lesion along the ventral aspect of penis involving the base and shaft.
Case 3
This 78-year-old male patient, a case of adenocarcinoma prostate (Gleason's score 4+3, initial PSA 20.6 ng/mL) was detected with metastatic disease at diagnosis 2 years ago. He was on docetaxel-based chemotherapy with zoledronic acid. He presented for evaluation of rising PSA level. Whole body Ga-68 PSMA PET CT scan was done which showed at least four hypodense lesions showing increased uptake of Ga-68 PSMA involving both lobes of liver, the largest in segment IV measuring 1.8 × 1.8 cm in size with SUV Max of 11 [Figure 3]. Along with the hepatic metastases, PSMA avid lesions were noted in both lobes of the prostate gland involving bilateral seminal vesicles with pelvic lymphadenopathy and extensive intensely Ga-68 PSMA avid sclerotic skeletal metastases. An ultrasound guided liver biopsy was done which confirmed metastatic lesions. In view of extensive metastatic disease with PSMA radioligand expression, the patient was planned for Lutetium-177 labeled PSMA therapy.
Figure 3.
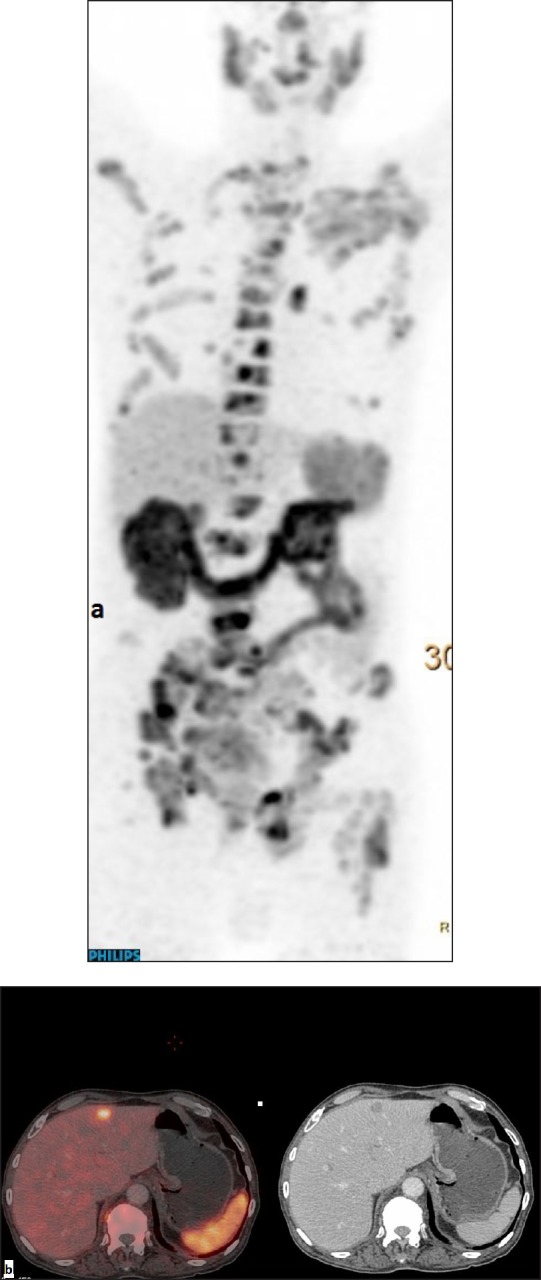
Ga-68 prostate-specific membrane antigen positron emission tomography-computed tomography scan: (a) maximum intensity projection and (b) trans-axial images of PET/CT fused and CT demonstrating hypodense lesion in segment IV of liver with abnormal uptake of Ga-68 PSMA.
Discussion
Prostate cancer is the second most common cancer and sixth leading cause of cancer death in men worldwide.[3] Early and accurate diagnosis is the key to perfect cancer management. To date, several small molecules have been developed and investigated as imaging probes, however recent data on novel PET tracer Ga-68 PSMA[Glu-NH-CO-NH-Lys-(Ahx)-[Ga-68(HBED-CC)] have demonstrated promising sensitivity and specificity with high contrast.[1] Two of the comparative studies done between Ga-68 PSMA PET/CT and F-18 fluoro-methylcholine PET/CT in patients with rising PSA in one and low PSA levels in the other, have demonstrated significantly higher lesion detection rate with PSMA over the choline tracer.[2,4] This may be because the choline metabolism is not increased in many cases whereas PSMA is overexpressed in most prostate cancers.
Prostate cancer mostly metastasizes to bone, lymph nodes, contiguous pelvic structures with brain, penis, and liver being the uncommon sites. Brain metastases are mostly seen in patients with widely disseminated bone and soft tissue disease[5] with an incidence of about 0.16%.[6] Metastases to the penis are also uncommon, with less than 400 cases reported in the literature till date.[7] Liver metastases are also seen in only about 4.29% of carcinoma prostate cases.[8] In our series, cases are presented with Ga-68 PSMA uptake in these structures thus showing its promising role in the detection of lesions at uncommon sites.
Conclusions
Ga-68 PSMA PET/CT scan allows detection of the metastatic sites, even the rare sites making it a powerful diagnostic tool for an assessment of extent of disease in patients of prostate cancer.
Financial support and sponsorship
Nil
Conflict of interest
There are no conflicts of interest.
References
- 1.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–35. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 2.Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-Fluoromethylcholine versus 68Ga-PSMA PET/CT in Prostate Cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 3.Jain S, Saxena S, Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–5. doi: 10.1016/j.mgene.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty PS, Kumar R, Tripathi M, Das CJ, Bal C. Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: A novel radiotracer for imaging of prostate carcinoma. Clin Nucl Med. 2015;40:328–9. doi: 10.1097/RLU.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 6.Hatzoqlou V, Patel GV, Morris MJ, Curtis K, Zhang Z, Shi W. Brain metastasis from prostate cancer: 11 year analysis in the MRI era with emphasis on imaging characteristics, incidence and prognosis. J Neuroimaging. 2014;24:161–6. doi: 10.1111/j.1552-6569.2012.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierro A, Cilla S, Digesu C, Morganti AG. Penile metastases of recurrent prostatic adenocarcinoma without PSA level increase: A case report. J Clin Imaging Sci. 2012;2:44. doi: 10.4103/2156-7514.99178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Li B, Zhang P, Yao Y, Chang J. Clinical Characteristics and prognostic factors of prostate cancer with liver metastases. Tumor Bio. 2014;35:595–601. doi: 10.1007/s13277-013-1083-6. [DOI] [PubMed] [Google Scholar]


