Abstract
Mixed phenotypic acute leukemia (MPAL) is a rare clinical entity. MPAL associated with myeloidsarcoma (MS) is still rarer with only three cases mentioned in English literature. MS has been described in myriads of location, most commonly in skin, gums and lymph nodes. Although theoritically possible, it is very rare to find MS involving the thyroid gland. The diagnosis of MS can be elusive, very often masquerades and mislabeled as lymphoma. A high index of clinical suspicion coupled with PET/CT findings along with morphological clues and thorough peripheral blood, and bone marrow evaluation is mandatory for arriving at the definitive diagnosis. We report the case of a 58-year-old female presenting with thyroid swelling that was subsequently diagnosed to be MS of the thyroid with underlying MPAL (mixed myeloid/B-cell) only after 18F-FDG PET/CT, which revealed an unusual abnormal pattern of multifocal intense FDG uptake in the thyroid gland.
Keywords: Mixed myeloid/B-cell, mixed phenotypic acute leukemia, myeloid sarcoma, thyroid, FDG PET/CT
Introduction
Granulocytic sarcoma, originally described as chloroma by Rappaport in 1967, is an uncommon, localized tumor resulting from infiltration by the leukemic cells at anatomical sites other than the bone marrow. The World Health Organization (WHO) in 2008 renamed it myeloid sarcoma (MS).[1] MS has been described in myriads of location, commonly in skin, gums, lymph nodes, and less often in bones, paranasal sinuses, larynx, orbit, breast, pleura, meninges, and central nervous system.[2,3,4] Although theoretically possible, MS involving the thyroid gland has never been reported in the English literature. MS is traditionally associated with acute myeloid leukemia (AML) orchronic myeloid leukemia (CML) in blast crisis.[5] Mixed phenotypic acute leukemia (MPAL) presenting as MS is exceedingly rare with only three cases described till date.[6,7,8] Our case of MS is unique not only in its unusual location of thyroid, but its also a rare underlying morphology of MPAL.
Case presentation
A 58-year-old female presented with history of fever, weight loss, pelvic pain, and malaise for four months. On enquiry, the patient gave history of pelvic radiotherapy at another institution for a lytic lesion, whose source was hitherto unidentified. CT guided FNAC had been previously done from the lytic lesion, which revealed atypical cells. At our institution, general physical examination revealed a midline neck swelling that moved with deglutition, alerting the possibility of thyroid pathology. However, the patient was clinically and biochemically euthyroid. The peripheral blood examination showed pancytopenia with hemoglobin, total leucocyte and platelet count to the tune of 8.8 gm/dl, 1100 per µL and 30000 per µL, respectively. Other biochemical investigations including liver, renal function tests, serum calcium, uric acid, electrolyte levels were all within normal limits. An 18F-FDG PET/CT was done to localise the site of primary malignancy. It revealed enlargement of both lobes of thyroid with multiple foci of intense FDG uptake corresponding to hypodense nodules in both lobes. Also noted were FDG avid lesions in skull, cervical, dorso-lumbar vertebrae, sternum, ribs, bilateral pelvic bones, humerus, and femur. [Figure 1A and B].
Figure 1A and B.
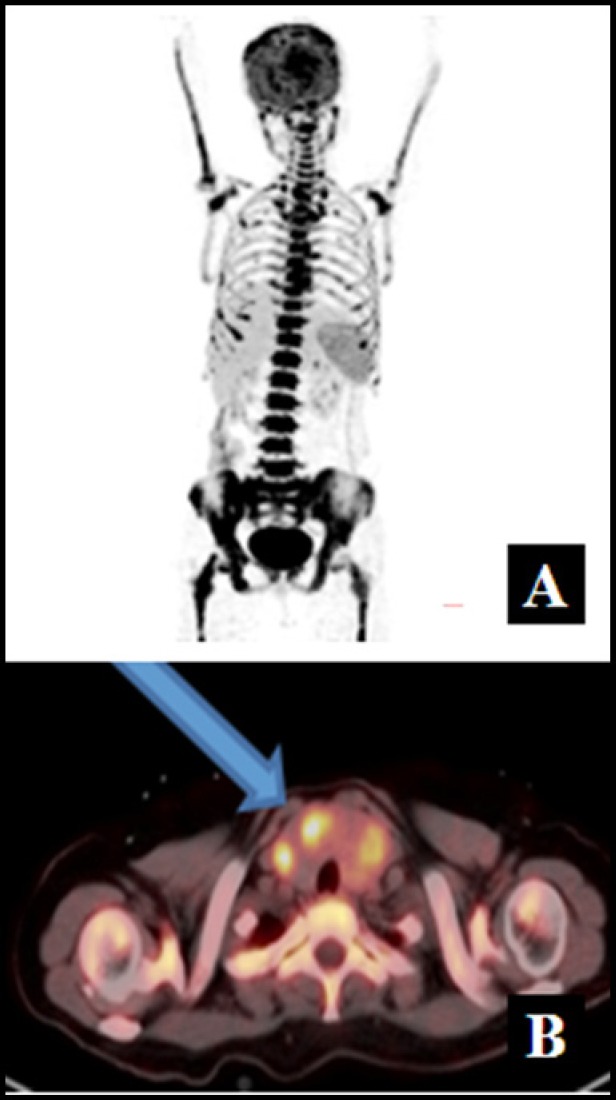
Maximum Intensity Projection (MIP) image of FDG PET/CT scan showing intense uptake in axial as well as appendicular skeleton, which appears focal and patchy at some sites B, Fused 18 F-FDG PET/CT transaxial image showing intense tracer uptake in multiple thyroid nodules in an enlarged thyroid gland.
Based on the PET findings, fine needle aspiration smears, which were obtained from the thyroid swelling by using a 23 G needle, were stained with papanicolaou and May-Grünwald Giemsa stains. The smears were cellular and comprised of dispersed population of atypical lymphoid cells amidst groups of benign thyroid follicular cells. The atypical cells were intermediate to large in size with scant to moderate amount of cytoplasm and nuclei having fine chromatin with two to three prominent nucleoli. A cytological impression of high grade non Hodgkin lymphoma (NHL) involving the thyroid gland was offered. [Figure 2A and B].
Figure 2A and B.
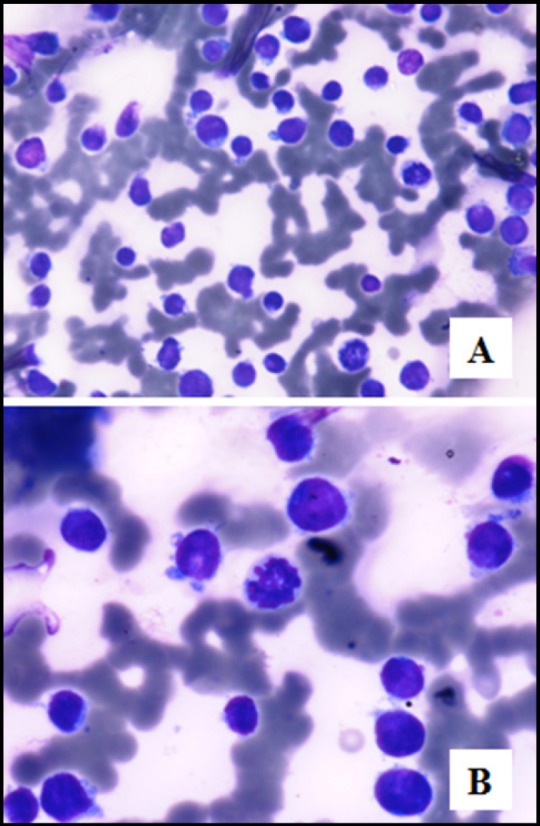
Cellular thyroid aspirate comprising intermediate to large sized atypical lymphoid cells with scant cytoplasm and fine chromatin (Giemsa stain A, 20x B, 40x)
The patient underwent bone marrow aspiration and biopsy along with biopsy of the thyroid gland in the same setting. The marrow was hypercellular with normal hematopoietic elements replaced by sheets of intermediate to large sized blasts. On flow cytometry, these blasts were immunopositive for leucocyte common antigen (LCA), CD34 (marker of blast), HLA DR, cytoplasmic myeloperoxidase (MPO) (myeloid marker), CD 79a, and CD19 (marker of B-lymphocyte); while were negative for CD13, CD 33, CD117, CD 14, CD 64, and cytoplasmic CD 3. The bone marrow biopsy showed similar morphological and immunophenotypic features. European Group of Immunological Markers for Leukemias (EGIL) score used for MPAL was 5. [Figure 3A-F] The thyroid biopsy showed infiltration of the normal thyroid follicular cells by neoplastic cells with identical morphology. These cells were immunopositive for LCA, CD 79a, CD 20 (marker of B-lymphocyte), CD10 (common ALL leucocyte antigen or CALLA), CD34, Tdt (marker of lymphoblast), and CD 43 (myeloid marker); while negative for MPO, CD 3, CD 68, and cyclin D1 confirming infiltration of the thyroid by MPAL. [Figure 4A-D]
Figure 3A-F.
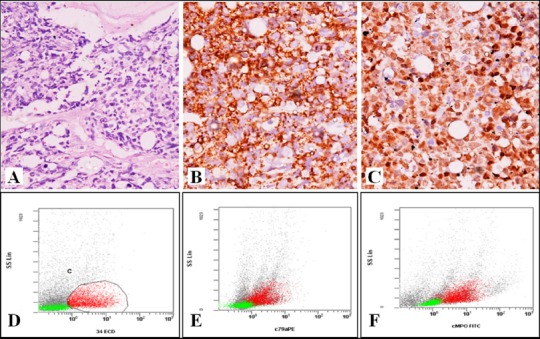
Bone marrow biopsy showing A, hypercellular marrow with near total replacement of hematopoietic cells by leukemic cells (H and E 200x) B, CD 20 C, Tdtimmunopositivity (200x) Flow cytometry showing leukemic cells positive for D, CD 34 E, CD79a F, cMPO
Figure 4A-D.
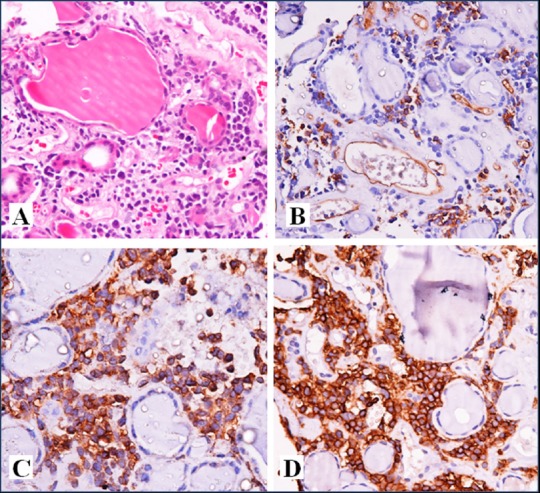
Thyroid Biopsy showing A, infiltration of thyroid parenchyma by leukemic cells (H and E, 200x) B, CD 34 C, CD 20 D, CD 43 immunopositivity (200x)
A final diagnosis of a mixed phenotypic acute leukemia (mixed myeloid/B-cell) with myeloid sarcoma, involving the thyroid gland, was rendered.
The chromosomal study using GTG Banding did not reveal any specific molecular alterations. However, it showed random loss of varied chromosomes in all the metaphases. The patient is receiving an ALL based chemotherapy Hoelzer's protocol) comprising of daunorubicin, vincristine, methylprednisolone, and L-asparaginase along with intrathecal methotrexate.
Discussion
The MS afflicts 2-8% of patients with AML, wherein more than half of the cases are diagnosed after the onset of AML, and about 15 to 35% patients present either simultaneously or precede the diagnosis of AML. MS may also herald the relapse of AML after achieving complete remission.[5] It has been very rare where MS have been reported in association with acute promyelocytic leukemia, CML in blast crisis and myelodysplastic syndrome.[4,5,9]
MPAL is a rare clinical entity, accounting for approximately 5% of all acute leukemias. In 2008, WHO defined MPAL as “a single population of blasts that would meet criteria for B-acute lymphoblastic leukemia (B-ALL) or T- acute lymphoblastic leukemia (T-ALL). It also expresses MPO” or have “unequivocal evidences of monoblastic differentiation”. MPAL with the t (9;22) (q34;q11)/BCR-ABL1 and MPAL with t (v;11q23)/ MLL rearrangement have been recognized as two distinct categories, while the remaining cases are grouped under MPAL, NOS (not otherwise specified).[1] CD43 and lysozyme are the most sensitive markers overall; whereas MPO and CD117 are the most sensitive in highlighting myeloid differentiation.[10] However, the extramedullary tumor in the thyroid also exhibited mixed (myeloid/B-cell) immunophenotype, in the patient of MPAL. A complex interaction between chemokine/ chemokine receptor is generally regarded as the driver of leukemic blasts to extramedullary site.[11]
MS in association with MPAL have been reported in only three patients worldwide. The first case reported by Hossain et al[6] developed extramedullary disease in the cervical lymph node with biphenotypic (mixed myeloid/T-cell) immunophenotype during progression of CML to blast crisis phase. The second patient was diagnosed with myeloid sarcoma in the lateral pharyngeal wall following the autologous stem cell transplantation for MPAL. The extramedullary tumor, however, expressed only myeloid phenotype.[7] The third patient developed MS arising from the pleura following the allogenic stem cell transplantation for MPAL. Both the medullary and extramedullary disease at relapse exhibited mixed (myeloid/B-lymphoblastic) phenotype.[8]
The diagnosis of MS poses considerable diagnostic challenge especially when the diagnosis in the extramedullary site precedes the medullary disease. An incompletely worked up case often gets signed out as lymphoma since NHL and MS share certain morphologic and immunophenotypic features. MS morphologically may also be mistaken for other small round cell tumors namely Neuroblastoma, Rhabdomyosarcoma, Ewing sarcoma/primitive neuroectodermal tumor, and Medulloblastoma in the pediatric population. In solid organs involved by MS, the neoplastic cells may appear in clusters mimicking a carcinoma. A high index of suspicion coupled with an appropriate panel of immunohistochemistry is pivotal in establishing the diagnosis.[10]
The early reports on the utility of FDG PET/CT in MS suggested that it can be detected in the correct clinical context despite its nonspecific imaging findings. FDG PET/CT increases sensitivity in early detection, when it is clinically occult or before it is evident on conventional imaging. It also identifies the disease sites, which facilitates biopsy. FDG PET/CT allows staging of the disease, which can tailor treatment planning. FDG PET/CT may potentially serve as an assessment tool for treatment response monitoring but this requires further validation with larger studies.[11]
Stölzel F et al. FDG PET/CT scans in 10 patients with de novo and relapsedacute myeloid leukemiaand, histologically proven, extramedullarydisease. The scans were able to detect the knownextramedullarylesions in 9 out of 10 patients (90%). They showed that additional extramedullarysites were detected in 6 patients (60%). Since most of these patients relapsed within a short period of time after initiation of therapy or had refractory disease, they concluded thatdetectionofextramedullarydisease with FDG PET/CT might be helpful in the development of individual treatment algorithms for these high-risk patients.[12]
The prognosis of MPAL is generally perceived to be poor when compared to de novo AML or ALL. In 2009, Xu et al.[13] in their paper showed that MPAL patients, who received ALL-based chemotherapeutic regimen, achieved higher complete remission rates than those treated with AML chemotherapeutic protocol. Although there are no prospective trials assessing the impact of presence of MS in acute leukemia patients, MS is traditionally considered to herald poor clinical outcome.[14]
The diagnosis of MS often eludes the histopathologist, especially when it preceedes the diagnosis of acute leukemia and masquerades as lymphoma. A high index of suspicion irrespective of the site involved coupled with the clues from peripheral blood and thorough bone marrow evaluation is mandatory for arriving at the definitive diagnosis. Being a whole body imaging modality with capability to detect Glucose hypermetabolism, FDG PET/CT can have a critical role in such cases by identifying the sites for biopsy based on high uptake.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflict of interest
Acknowledgement
None
References
- 1.Weinberg OK, Seetharam M, Ren L, Alizadeh A, Arber DA. Mixed phenotype acute leukemia: A study of 61 cases using World Health Organization and European Group for the Immunological Classification of Leukaemias criteria. Am J Clin Pathol. 2014;142:803–8. doi: 10.1309/AJCPPVUPOTUVOIB5. [DOI] [PubMed] [Google Scholar]
- 2.Sen R, Singh S, Qury M S, Marwah S, Aggarwal G, Singla S. Chloroma of perianal region masquerading as perianal abscess. Indian J Dermatol. 2013;58:85. doi: 10.4103/0019-5154.105326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uner AH, Altundag K, Saglam A, Tekuzman G. Granulocytic sarcoma of the urinary bladder. Am J Hematol. 2004;75:262–3. doi: 10.1002/ajh.20022. [DOI] [PubMed] [Google Scholar]
- 4.Kim LY, Purkey MT, Patel MR, Ghosh A, Hartner L, Newman JG. Primary granulocytic sarcoma of larynx. Head Neck. 2014;37:E38–44. doi: 10.1002/hed.23805. [DOI] [PubMed] [Google Scholar]
- 5.Avni B, Koren-Michowitz M. Myeloid Sarcoma: Current Approach Therapeutic Options. Ther Adv in Hematol. 2011;2:309–16. doi: 10.1177/2040620711410774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain D, Weisberger J, Sreekantaiah C, Seiter K. Biphenotypic (mixed myeloid/T-cell) extramedullary myeloid Cell tumor. Leuk Lvmphoma. 1999;33:399–402. doi: 10.3109/10428199909058443. [DOI] [PubMed] [Google Scholar]
- 7.Alrumaih R, Saleem M, Velagapudi S, Dababo MA. Lateral pharyngeal wall myeloid sarcoma as a relapse of acute biphenotypic leukemia: a case report and review of the literature. J Med Case Rep. 2013;7:292. doi: 10.1186/1752-1947-7-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaya Z, Kocak U, Albayrak M, Gursel T, Akyurek N, Oktar SO. Granulocytic sarcoma after stem cell transplantation in a child with biphenotypic leukemia. Turk J Hematol. 2009;26:151–153. [PubMed] [Google Scholar]
- 9.Evans GD, Grimwade DJ. Extramedullary disease in acute promyelocytic leukemia. Leuk Lymphoma. 1999;33:219–29. doi: 10.3109/10428199909058422. [DOI] [PubMed] [Google Scholar]
- 10.Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007;2:42. doi: 10.1186/1746-1596-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EY, Anthony MP, Leung AY, Loong F, Khong PL. Utility of FDG PET/CT in the assessment of myeloid sarcoma. AJR Am J Roentgenol. 2012;198:1175–9. doi: 10.2214/AJR.11.7743. [DOI] [PubMed] [Google Scholar]
- 12.Stölzel F, Röllig C, Radke J, Mohr B, Platzbecker U, Bornhäuser M, et al. 18F-FDG PET/CT fordetection of extra medullary a cute myeloid leukemia. Haematologica. 2011;96:1552–6. doi: 10.3324/haematol.2011.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu XQ, Wang JM, Lü SQ, Chen L, Yang JM, Zhang WP, et al. Clinical and biological characteristics of adult biphenotypic acute leukemia in comparison with that of acute myeloid leukemia and acute lymphoblastic leukemia: a case series of a Chinese population. Haematologica. 2009;94:919–27. doi: 10.3324/haematol.2008.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan TY, Lin DT, Tien HF, Yang RS, Chen CY, Wu K. Prognostic factors of treatment outcomes in patients with granulocytic sarcoma. Acta Haematol. 2009;122:238–46. doi: 10.1159/000253592. [DOI] [PubMed] [Google Scholar]


