Abstract
Background. Aseptic abscesses (AA) are sterile lesions that represent an extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD). Though Canada has the highest prevalence of IBD in the world, reports of IBD-associated AA are absent in Canada. This may represent a different IBD phenotype or underrecognition and underreporting. Purpose. To explore AA as a possible EIM of IBD and evaluate clinical and investigative findings among patients with IBD-associated AA. Methods. Retrospective chart and literature reviews were performed to find cases of IBD-associated AA at our institution and in the literature. Results. We identified 2 cases of IBD-associated AA in our institution. Both patients had ulcerative colitis and presented with fever, abdominal pain, and weight loss. Radiological workup and aspiration showed sterile splenic abscesses. The AA were unresponsive to antibiotics. One patient improved on corticosteroids and one underwent splenectomy. We retrieved 37 cases of IBD-associated AA from the literature. All patients showed no evidence of infection, failed to resolve with antibiotics, and, if attempted, improved on corticosteroids. Conclusions. Our cases are the first reported in Canada. They support literature which suggests AA as an EIM of IBD and may help increase recognition and reporting of this phenomenon.
1. Introduction
Aseptic abscesses (AA) are a rare and serious extraintestinal manifestation (EIM) of inflammatory bowel disease (IBD). Despite having the highest incidence and prevalence of IBD in the world, there are no reports of this entity in Canada [1–4]. In this study, we describe two local cases and review the literature on AA with underlying IBD.
IBD-associated AA is a rare condition with fewer than 50 cases reported, largely in the European literature [5]. AA are deep abscesses with neutrophilic features associated with negative cultures and serologic tests, failure of antibiotic therapy, and improvement on corticosteroids. Due to the scarcity of reported cases and absence of observational and experimental studies, clinicians face challenges in diagnosing and managing patients. This report evaluates two cases of IBD-associated AA for clinical and investigative findings to add to the sparse Canadian literature.
2. Methods
2.1. Chart Review
This retrospective single center study was approved by our Institutional Review Board with a waiver for informed consent. Our radiology information system (syngo, Siemens Medical Solutions USA, Inc., Malvern, PA) was searched using Montage Search and Analytics (Montage Healthcare Solutions, Philadelphia, PA) for CT examinations performed between January 2005 and July 2016. The terms “splenic abscess,” “aseptic abscess,” “inflammatory bowel disease,” “ulcerative colitis,” and “Crohn's disease” were used. The Soarian Clinicals database (Cerner Corporation, Kansas City, MO) was used to find additional clinical data on identified patients. Diagnoses of AA were made based on (1) history of IBD; (2) imaging of focal enclosed lesions with the typical appearance of abscesses; (3) failure of antibiotic therapy; and (4) negative blood and aspirate. Patient charts were analyzed for demographics, symptoms, medical comorbidities, disease diagnosis and progression, and management.
2.2. Literature Review
For the literature review, an electronic search of the online databases MEDLINE, EMBASE, and CINAHL as well as the reference lists of retrieved articles was performed to identify potential articles published in print or online before July 14, 2016. Search terms included “Inflammatory bowel disease”, “aseptic splenic abscess”, “aseptic abscess”, and “extraintestinal”. Abstracts of all publications found through the above search strategy were screened for relevancy. Full-text articles, if available, were retrieved. If a translation for a non-English article was not available, authors were contacted for full-text translations. If they did not respond, these articles were excluded from the literature review.
3. Results
3.1. Case Reports
We describe two cases of IBD-associated AA of patients who presented to St. Michael's Hospital in Toronto. The clinical characteristics of our two cases are summarized in Table 1.
Table 1.
Summary of IBD associated AA cases from the literature database and our search at St. Michael's Hospital.
| Study/patient ID | Age/sex | IBD phenotype (CD/UC/IC) | Age of IBD diagnosis/temporal relation to diagnosis of AA | IBD flare during AA | Symptoms | Location of AA | Other IBD EIM | Antibiotic treatment | Corticosteroids | Additional immunotherapy | Surgical procedures | Maintenance therapy after diagnosis of AA | Number of relapses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| André et al. 2007 [5]∗/1–21 | Mean 24.4 (range 10–54)/12 F & 9 M | 17CD, 3UC, 1IC | Average age of IBD onset = 23.7 (range 12–38)/before (n = 7), concomitant (n = 7), after (n = 7) | Yes (n = 8), no (n = 13) | Fever (n = 19), abdominal pain (n = 17), weight loss (n = 10), diarrhea (n = 5) | Spleen alone (n = 6) or spleen and lymph nodes (n = 4); pharynx and cervical muscles (n = 1); liver (n = 1); liver and lymph nodes (n = 3); kidney (n = 1), and lymph nodes alone (n = 4) | Arthritis (x4), myalgia (x5), ND (x2), aphthous ulcer (x7) | Yes (n = 21) | Yes (n = 21) | Total (n = 10), cyclophosphamide (n = 3), azathioprine (n = 7), methotrexate (n = 1), infliximab (n = 2) | Splenectomy (n = 9) | Information not provided | Mean = 1.38 (n = 12), range 0–5 |
|
| |||||||||||||
| Lamport et al. [6]/22 | 38/F | CD | 34/before | No | Leg weakness | Bilateral psoas muscle and epidural | — | Yes | Yes | — | — | — | 1 |
|
| |||||||||||||
| Actis et al. [7]/23 | 30/M | CD | 30/after | Yes | Fever, diarrhea, abdominal pain | Spleen, abdominal lymph nodes | Panniculitis, polyneuropathy | Yes | Yes | Azathioprine | Splenectomy and lymph node excision | Prednisone (stopped after 4th relapse), azathioprine (after last relapse) | 4 |
|
| |||||||||||||
| Tirpitz et al. [8]/24 | 80/F | UC | 72/before | Yes | Abdominal pain, abdominal swelling, bloody diarrhea | Bi-temporal upper eyelids | Pyoderma gangrenosum | Yes | Yes | — | — | Prednisone, sulfasalazine | 0 |
|
| |||||||||||||
| Murata et al. [9]/25 | 18/F | UC | 15/before | No | Fever | Sternum | Arthritis | Yes | Yes | — | I&D | — | 4 |
|
| |||||||||||||
| Hara et al. [10]/26 | 39/M | UC | 34/before | No | Painful facial lesions, fever, nonbloody diarrhea | SC scalp, face, right inner canthus, submaxilla, and chest | Arthritis | Yes | Yes | — | I&D | Sulfasalazine (after relapse) | 1 |
|
| |||||||||||||
| Coat et al. [11]/27 | 31/F | CD | 28/before | Yes | Fever, back pain | Spleen | — | Yes | Yes | Azathioprine | Splenectomy | — | 1 |
|
| |||||||||||||
| Kinjo et al. [12]/28 | 34/F | UC | 31/before | Yes | Fever, bloody stool | SC sternum | Arthritis | No | Yes | — | I&D | — | 1 |
|
| |||||||||||||
| Holstein et al. [13]/29 | 26/M | CD | 24/before | No | Fever, diarrhea, weight loss, weakness, loss of appetite | Liver, spleen | HLA B27 sacroiliitis, Arthritis | Yes | Yes | Azathioprine | Splenectomy | Azathioprine (after 3rd relapse) | 3 |
|
| |||||||||||||
| Li et al. [14]/30 | 39/F | UC | 26/before | Yes | Fever, bloody diarrhea, abdominal pain | SC left forearm | Arthritis | Yes | Yes | — | I&D | — | 0 |
|
| |||||||||||||
| Coyne [15]/31 | 34/M | CD | 33/before | No | Abdominal pain | Spleen | — | No | No | — | Splenectomy | — | 0 |
|
| |||||||||||||
| Renna et al. [16]/32 | 20/F | CD | 20/concomitant | Yes | Fever, abdominal pain, nonbloody diarrhea, weight loss | Spleen | — | Yes | Yes | — | Splenectomy | Prednisone (before, during, and after relapse) | 1 |
|
| |||||||||||||
| Zakout et al. [17]/33 | 29/F | CD | 29/concomitant | Yes | Fever, abdominal pain, pain in right shoulder radiating from abdomen, right pleuritic chest pain, nonbloody diarrhea | Liver | — | Yes | No | Azathioprine | — | Azathioprine, sulfasalazine | 0 |
|
| |||||||||||||
| Yilmaz et al. [18]/34 | 34/F | UC | 22/before | Yes | Fever, nasal ache, difficulty with breathing | Nasal septum | — | Yes | Yes | — | I&D | — | 0 |
|
| |||||||||||||
| Brooks and Ghaffari [19]/35 | 19/F | CD | 19/concomitant | Yes | Abdominal pain | Spleen | Pustular skin lesions | Yes | Yes | Azathioprine | — | Azathioprine | 0 |
|
| |||||||||||||
| Sakharpe et al. [20]/36 | 48/F | CD | Unknown/before | No | Fever, weakness, loss of appetite, coughing, chest pain | Liver | — | No | Yes | — | — | — | 0 |
|
| |||||||||||||
| Boschetti et al. [21]/37 | 40/F | CD | Unknown/after | No | Fever, abdominal pain | Spleen, pancreas | Sweet's syndrome | Yes | Yes | Adalimumab | Laparoscopic biopsy of mesenteric lymph nodes | Adalimumab | 0 |
|
| |||||||||||||
| Bollegala et al./case 1 | 33/M | UC | 18/before | Yes | Abdominal pain, fever, weight loss, sweat | Spleen | Pyoderma gangrenosum | Yes | Yes | Infliximab | — | Prednisone & 5-ASA (before relapse), infliximab (after relapse) |
1 |
|
| |||||||||||||
| Bollegala et al./case 2 | 27/F | UC | 26/before | No | Abdominal pain, chest pain radiating to left shoulder, fever, weight loss | Spleen | Sweet's syndrome, oral ulcers, arthritis | No | No | Infliximab | Splenectomy | Infliximab | 0 |
AA = aseptic abscess, CD = Crohn's disease, EIM = extraintestinal manifestation, F = female, IC = indeterminate colitis, IBD = inflammatory bowel disease, I&D = incision and drainage, M = male, ND = neutrophilic dermatosis, SC = subcutaneous, UC = ulcerative colitis.
∗Case series summary data available.
3.1.1. Case One
A 33-year-old man with a history of ulcerative colitis (UC) and prior subtotal colectomy and ileal pouch-anal anastomosis (IPAA) presented to hospital with a six-day history of fevers, nonradiating left-upper quadrant abdominal pain worse with movement, a 15 lbs weight loss, and genital skin lesions with the typical appearance of pyoderma gangrenosum. His bowel movements were unchanged from 8–10 nonbloody stools per day. Past medical history was significant for corticosteroid-refractory pancolonic UC managed with subtotal colectomy and IPAA at age 18. Surgical pathology on the colectomy specimen confirmed ulcerative pancolitis. In the subsequent 15 years, he was treated for three episodes of pouchitis where each responded well to three weeks of ciprofloxacin and metronidazole
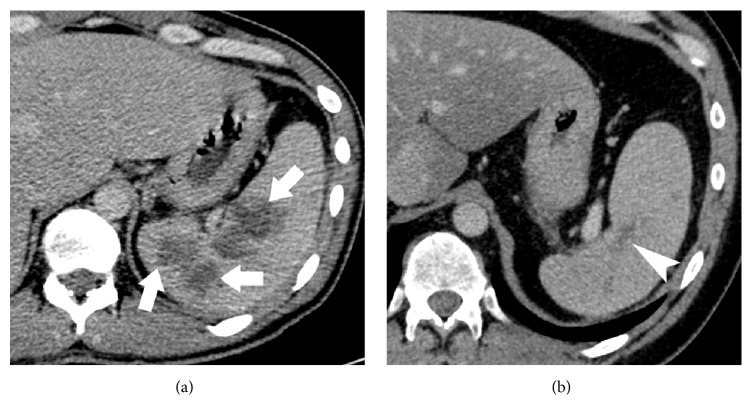
On presentation, laboratory testing showed an elevated white blood cell (WBC) count of 18.0 × 103 cells/μL with a neutrophil count of 16.4 × 103 cells/μL, C-reactive protein (CRP) of 114.9 mg/L, and erythrocyte sedimentation rate (ESR) of 51 mm/hr. An abdominal CT scan revealed multiple mostly confluent hypodense lesions in the spleen, the largest being 7.0 × 3.2 × 5.2 cm (Figure 1(a)), concerning for abscesses. Aspiration of the largest abscess revealed pus. The abscesses were drained and he was started on ceftriaxone and metronidazole.
Figure 1.
Axial contrast enhanced CT images demonstrating (a) multiple splenic abscess (thick arrows) with (b) resolution and residual scarring (thin arrow) following treatment with steroids.
The patient failed to improve. Cultures of blood and the aspirate remained sterile even after 7-day incubation. All antibiotics were then stopped. Pouchoscopy revealed inflammation in the IPAA with no inflammation in the pouch or proximal small bowel. A repeat abdominal CT scan showed progression of the splenic lesions. The patient was started on IV corticosteroids. His abdominal pain, fevers, and genital skin lesions improved and he was discharged home on a tapering course of oral steroids and rectal aminosalicylates. He was admitted one year later with a one-month history of left-sided flank pain, sweats, fevers, and abdominal pain with no change in bowel habits or weight loss. An abdominal and pelvic CT scan showed multiple focal ill-defined hypoattenuating lesions scattered throughout the spleen with the largest measuring 4.2 cm in diameter. Aspiration of an abscess proved sterile. Pouchoscopy showed active inflammation.
The patient was commenced on IV steroids and transitioned to an oral tapered dose of prednisone. Additionally, he was initiated on 5 mg/kg of infliximab every eight weeks, increased to 10 mg/kg due to lack of response. His abdominal pain and fever ceased and a repeat abdominal CT scan six months later revealed resolution of the multiple splenic abscesses (Figure 1(b)).
3.1.2. Case Two
A 27-year-old woman presented to hospital with fever, abdominal pain, chest pain, and weight loss. Past medical history was of UC diagnosed at age 26, treated with prednisone and azathioprine, for which she was nonadherent and ceased therapy. At the time of presentation, she described four days of left-upper quadrant abdominal pain and pleuritic chest pain radiating to her left shoulder. There was fever, oral ulcers, a 10 lbs weight loss, and arthritis of the hands, elbows, knees, and ankles. She had an elevated WBC count (21.1 × 103 cells/μL), neutrophil count (16.0 × 103 cells/μL), CRP (112 mg/L), and ESR (88 mm/hr). Abdominal CT scans revealed an enlarging heterogeneous abscess measuring 3.7 × 4.0 × 2.4 cm initially and 4.2 × 3.6 × 2.6 cm 3 days later (Figure 2).
Figure 2.
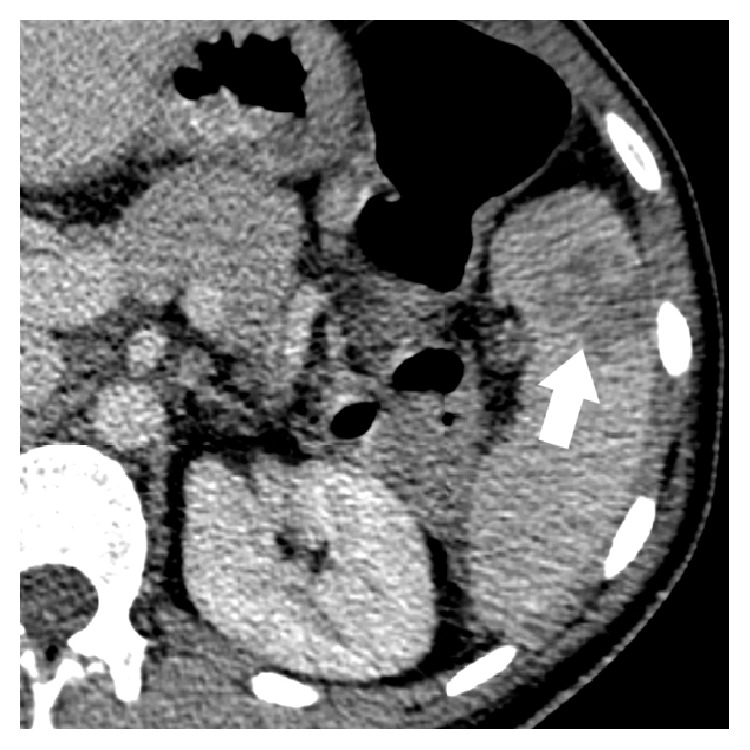
Axial contrast enhanced CT image demonstrating a heterogeneous splenic abscess (thick arrow).
Aspiration of the splenic collections revealed pus. Cultures of blood and aspirate were negative. The patient declined further endoscopic staging of her UC. She underwent splenectomy and the resected spleen contained multiple aseptic abscesses. Histology revealed areas of neutrophil-dominant necrotic tissue.
The patient presented two months later with fever, arthralgia, oral ulcers, and tender papules on her buttocks, arms, and legs. Biopsy of the lesions revealed neutrophilic dermatosis (ND). Based on her clinical and histological findings, she was diagnosed with Sweet's syndrome and started on prednisone 60 mg PO daily. Her dermatologic and rheumatologic symptoms rapidly resolved. She was initiated and remains on infliximab 5 mg/kg every six weeks, continuing to do well on this regimen.
3.2. Literature Review of Aseptic Abscess in Inflammatory Bowel Disease
A total of 37 cases of IBD-associated AA were found in our literature search of MEDLINE, EMBASE, and CINAHL and through hand-searching. These articles were published from 1994 [6] to 2016 [20, 21]. The clinical characteristics of these cases can be found in Table 1. Of the 37 total patients, approximately 65% (n = 24) were female and 35% (n = 13) were male. The mean ages of patients with AA and IBD at the time of diagnosis were 28.9 years (range 10–80 years) and 26.1 years (range 12–72 years), respectively.
IBD preceded the diagnosis of AA in 48.6% of patients (n = 18), was concomitant in 24.3% (n = 9), and appeared subsequently in 27.0% (n = 10). With respect to IBD type, 73.0% of patients had underlying CD (n = 27), 24.3% (n = 9) had UC, and 2.7% (n = 1) had indeterminate colitis. During their diagnosis of AA, 45.9% patients (n = 17) had an IBD flare, while 54.1% (n = 20) did not. Regarding initial symptoms, 83.8% of patients presented with fever (n = 31), 67.6% with abdominal pain (n = 25), 32.4% with diarrhea (n = 12), and 32.4% with weight loss (n = 12). The most common AA locations were the spleen (n = 23) and liver (n = 5), with 32.4% of patients found to have lymph node involvement (n = 12). 10.8% of patients had ND (n = 4).
Regarding treatment, 91.9% of patients received antibiotics (n = 34), to which they were all unresponsive. Conversely, 94.6% received and responded to corticosteroid therapy (n = 35). 40.5% of patients (n = 15) received additional immunosuppressive therapy of azathioprine (n = 12), cyclophosphamide (n = 3), methotrexate (n = 1), infliximab (n = 2), and adalimumab (n = 1). Additionally, 37.8% of patients underwent splenectomy (n = 14), 13.5% had incision and drainage of abscesses (n = 5), and 5.4% had lymph node excision (n = 2). Relapses were reported in 54.1% of patients (n = 20), with the average number of relapses among all 37 cases being 1.22 (range: 0–5). The implementation (or absence) of maintenance therapy was reported in 45.9% of cases (n = 17), with 47.1% of these patients placed on maintenance therapy (n = 8/17), including low-dose prednisone (n = 3), azathioprine (n = 4), sulfasalazine (n = 3), and adalimumab (n = 1). Among the patients receiving maintenance therapy, 5 relapses occurred while on prednisone maintenance therapy. Alternatively, no relapses occurred while patients were on azathioprine, sulfasalazine, or adalimumab.
4. Discussion
We present two cases of aseptic abscesses, a rare EIM of IBD. Our patients were aged 33 and 27 years old, respectively, and both had underlying UC. Both patients presented with fever, abdominal pain, and weight loss and had splenic lesions which proved sterile. Patient 1 did not respond to antibiotics, improved on corticosteroids, and was maintained with infliximab. Patient 2 received a splenectomy and subsequently was maintained with infliximab.
4.1. Clinical Diagnosis
There are no accepted guidelines for the diagnosis of AA. However, Andre and colleagues created a set of common characteristics based on a case series with literature review: (1) deep abscesses with neutrophilic features; (2) negative serologic tests for bacteria and fungi and cultures of blood and aspirate; (3) when administered, failure of broad-spectrum antibiotic therapy including antituberculosis therapy; (4) rapid clinical improvement on corticosteroid therapy with or without additional immunosuppressant therapy and subsequent radiologic evidence of abscess resolution [5]. Our patients were assessed using this definition of AA, with patient 1 meeting all criteria and patient 2 meeting all but the fourth criterion (i.e., corticosteroid therapy), as corticosteroids were not attempted on this patient. Additionally, when applying these criteria to patients from the literature, we found multiple reports of superficial AA in IBD. Sterile abscesses were reported on the upper eyelids, scalp, face, chest, forearm, and nasal septum [8, 10, 11, 13, 18]. These findings may warrant expansion of criterion 1 to include superficial AA.
4.2. Pathology
There is some ambiguity on how to best describe sterile lesions with underlying IBD. Superficial lesions have been described as neutrophil-dominant with surrounding granulomatous tissue [10, 11, 13]. Similarly, in our report, aspiration of patient 2's splenic abscess revealed areas of neutrophil-dominant necrosis. Lesions in previously published cases have also been described as rheumatoid nodules, though this assertion has been debated [12, 22]. With this lack of consensus in mind, the most accurate term to describe these lesions is “aseptic abscess” [5].
AA shares a similar pathological picture with ND, showing polymorphonuclear leukocyte infiltrates and sterile abscesses [23–25]. Both of our patients had ND: patient 1 developed pyoderma gangrenosum, and patient 2 developed Sweet's syndrome. In the literature, 10.8% of patients had ND (n = 4). Sweet's syndrome and pyoderma gangrenosum are recognized complications of IBD, though it is unclear if their incidence is higher in IBD patients with AA compared to those without AA [23, 25].
4.3. Association with Inflammatory Bowel Disease
AA has been documented as an EIM found before, during, and after the diagnosis of IBD [5]. Our patients had established IBD diagnoses before any clinical symptoms of AA appeared. Other cases in our institution may not have been found through the database search if the AA preceded IBD and thus was not classified as an EIM. This may indicate a need to perform colonoscopy on patients with AA and no underlying IBD, as 51.3% of published cases reported IBD diagnoses concomitant with or following AA diagnoses (n = 19).
With regard to types of IBD, both of our patients had UC compared to only 24.3% (n = 9) of patients reported in the literature. A recent case report by Boschetti et al. of AA described a diagnosis of multiple systemic abscess syndrome, though they called this a complication of CD and not IBD, removing patients with UC from consideration [21]. Despite the higher incidence of CD, compared to UC-associated AA, it is important for clinicians to realize that AA can be an EIM regardless of the type of underlying IBD.
The association between disease severity of IBD and the manifestation of AA is unclear. Among included studies, only one reported clinical or endoscopic measures of disease activity for IBD, such as the Crohn's Disease Activity Index (CDAI) or the Crohn's Disease Endoscopic Index of Severity (CDEIS) [11, 26, 27]. Though inflammatory biomarkers such as CRP and ESR were commonly presented, they cannot be used as surrogates IBD activity, as AA is an inflammatory process itself.
4.4. Treatment
AA has been treated with corticosteroid therapy, azathioprine, cyclophosphamide, methotrexate, infliximab, adalimumab, incision and drainage, and splenectomy. From the literature and from our own institution, there were 36 of 39 patients that received and responded to corticosteroid therapy, which indicates its effectiveness as the first-line for induction. Maintenance therapy should follow because relapse is common, as seen in over half of the patients in the literature review and in one of our two patients. Interestingly, no patients in the literature experienced relapse while on maintenance therapy of azathioprine, sulfasalazine, adalimumab, or infliximab, although the durability of this response is unknown [7, 8, 10, 13, 17, 19, 21]. There were no reported fatal outcomes.
5. Conclusions
The cases described in this study are the first to be reported in Canada. They highlight the importance of recognizing IBD-associated AA in at-risk patients. The lack of diagnostic guidelines can lead clinicians to forego potentially effective corticosteroid and immunosuppressant therapy in favour of surgical intervention, as in our patient 2 who received a splenectomy. Future research should be aimed towards rigorous multicentre prospective case-control studies with greater participant numbers to better understand the natural history of this condition, to form the foundation for diagnostic criteria and to help improve recognition, reporting, and management.
Abbreviations
- AA:
Aseptic abscesses
- EIM:
Extraintestinal manifestation
- IBD:
Inflammatory bowel disease
- CD:
Crohn's disease
- UC:
Ulcerative colitis
- IC:
Indeterminate colitis
- IV:
Intravenous
- WBC:
White blood cell
- ND:
Neutrophilic dermatoses.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Natasha Bollegala, Jenna Tessolini, Ahmed Al-Mazroui, Michael A. Scaffidi, and Samir C. Grover were responsible of study conception and design. Adrienne Showler, Natasha Bollegala, Ahmed Al-Mazroui, Rishad Khan, Jenna Tessolini, and Errol Colak were responsible of acquisition of data. Rishad Khan, Errol Colak, and Samir C. Grover were responsible of analysis and interpretation of data. Natasha Bollegala, Rishad Khan, Ahmed Al-Mazroui, and Samir C. Grover were responsible of drafting of the manuscript. Natasha Bollegala, Ahmed Al-Mazroui, Michael A. Scaffidi, Jenna Tessolini, Rishad Khan, Adrienne Showler, and Samir C. Grover were responsible of critical revision of the manuscript for important intellectual content. Natasha Bollegala, Rishad Khan, Michael A. Scaffidi, Ahmed Al-Mazroui, and Samir C. Grover assisted in writing of the manuscript. Errol Colak and Samir C. Grover were responsible of administrative, technical, or material support. Samir C. Grover was responsible of study supervision. Natasha Bollegala, Jenna Tessolini, Ahmed Al-Mazroui, Michael A. Scaffidi, Rishad Khan, Adrienne Showler, Errol Colak, and Samir C. Grover approved the final manuscript.
References
- 1.Stone M. A., Mayberry J. F., Baker R. Prevalence and management of inflammatory bowel disease: a cross-sectional study from central England. European Journal of Gastroenterology and Hepatology. 2003;15(12):1275–1280. doi: 10.1097/00042737-200312000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kugathasan S., Judd R. H., Hoffmann R. G., et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. Journal of Pediatrics. 2003;143(4):525–531. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 3.Rocchi A., Benchimol E., Bernstein C. N., et al. Inflammatory bowel disease: a Canadian burden of illness review. Canadian Journal of Gastroenterology. 2012;26(11):811–817. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auvin S., Molinié F., Gower-Rousseau C., et al. Incidence, clinical presentation and location at diagnosis of pediatric inflammatory bowel disease: a prospective population-based study in Northern France (1988–1999) Journal of Pediatric Gastroenterology and Nutrition. 2005;41(1):49–55. doi: 10.1097/01.mpg.0000162479.74277.86. [DOI] [PubMed] [Google Scholar]
- 5.André M. F. J., Piette J.-C., Kémény J.-L., et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine. 2007;86(3):145–161. doi: 10.1097/md.0b013e18064f9f3. [DOI] [PubMed] [Google Scholar]
- 6.Lamport R. D., Cheskin L. J., Moscatello S. A., Nikoomanesh P. Sterile epidural and bilateral psoas abscesses in a patient with Crohn's disease. American Journal of Gastroenterology. 1994;89(7):1086–1089. [PubMed] [Google Scholar]
- 7.Actis G. C., Ottobrelli A., Lavezzo B., et al. Recurrent necroinflammatory disease of multiple organs and colon. Digestive Diseases and Sciences. 1996;41(10):2100–2105. doi: 10.1007/bf02093616. [DOI] [PubMed] [Google Scholar]
- 8.Tirpitz C. V., Buchwald H.-J., Lang G. K., Adler G., Reinshagen M. Simultaneous onset of pyoderma gangrenosum and bitemporal abscesses of the upper eyelids during a flare of ulcerative colitis. Inflammatory Bowel Diseases. 1998;4(2):98–100. doi: 10.1002/ibd.3780040206. [DOI] [PubMed] [Google Scholar]
- 9.Murata I., Satoh K., Yoshikawa I., Masumoto A., Sasaki E., Otsuki M. Recurrent subcutaneous abscess of the sternal region in ulcerative colitis. American Journal of Gastroenterology. 1999;94(3):844–845. doi: 10.1016/S0002-9270(99)00012-X. [DOI] [PubMed] [Google Scholar]
- 10.Hara H., Wakui F., Fujitsuka A., Ochiai T., Morishima T. Subcutaneous abscesses in a patient with ulcerative colitis. Journal of the American Academy of Dermatology. 2000;42(2):363–365. doi: 10.1016/S0190-9622(00)90113-0. [DOI] [PubMed] [Google Scholar]
- 11.Coat N., Le Berre-Heresbach N., Poinsignon Y., et al. Maladie de Crohn compliquée d'abcès aseptiques multiples et récidivants de la rate. Gastroentérologie Clinique et Biologique. 2001;25(4):p. 425. [PubMed] [Google Scholar]
- 12.Kinjo F., Miyazato S., Hokama A., Kugai Y., Saito A. Aseptic subcutaneous abscess associated with ulcerative colitis. Journal of Gastroenterology and Hepatology. 2003;18(10):1214–1215. doi: 10.1046/j.1440-1746.2003.03148.x. [DOI] [PubMed] [Google Scholar]
- 13.Holstein A., Egberts E., Von Herbay A. Rheumatoid‐like nodules in the spleen: new extraintestinal manifestation of Crohn's disease? Journal of Gastroenterology and Hepatology. 2006;21(1):295–298. doi: 10.1111/j.1440-1746.2006.04001.x. [DOI] [PubMed] [Google Scholar]
- 14.Li K. J., Yu C. L., Lin W. C., Lu M. C., Wu C. H., Hsieh S. C. Concomitant aseptic subcutaneous abscess and immunoglobulin M nephropathy—rare extraintestinal manifestations in ulcerative colitis. Digestive Diseases and Sciences. 2006;51(2):401–405. doi: 10.1007/s10620-006-3144-9. [DOI] [PubMed] [Google Scholar]
- 15.Coyne J. D. Crohn's disease with inflammatory splenic granuloma. Journal of Clinical Pathology. 2006;59(8, article 889) doi: 10.1136/jcp.2005.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renna S., Mocciaro F., Perricone G., et al. Is splenectomy a treatment option for aseptic abscesses in patients with Crohn's disease? European Journal of Gastroenterology and Hepatology. 2009;21(11):1314–1316. doi: 10.1097/MEG.0b013e32832bab85. [DOI] [PubMed] [Google Scholar]
- 17.Zakout R., Fonseca M., Santos J. M., et al. Multiple aseptic liver abscesses as the initial manifestation of crohn's disease: report of a case. Diseases of the Colon and Rectum. 2009;52(2):343–345. doi: 10.1007/dcr.0b013e318199db60. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz B., Yüksel O., Çoban Ş., Çakmak I., Başar Ö., Ekiz F. Rare complication of ulcerative colitis: aseptic nasal septal abscess. Inflammatory Bowel Diseases. 2011;17(7, article no. E71) doi: 10.1002/ibd.21732. [DOI] [PubMed] [Google Scholar]
- 19.Brooks J., Ghaffari G. Aseptic splenic abscess as precursory extraintestinal manifestation of inflammatory bowel disease. Case Reports in Medicine. 2014;2014:4. doi: 10.1155/2014/684231.684231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakharpe A. K., Sakharpe A. K., Mirmanesh M., et al. A case and review of aseptic liver abscess in Crohn’s disease. International Journal of Colorectal Disease. 2016;31(3):787–788. doi: 10.1007/s00384-015-2288-5. [DOI] [PubMed] [Google Scholar]
- 21.Boschetti G., Assaad S., Balme B., Boyer S., Flourié B., Nancey S. A challenging case of multiple splenic and pancreatic lesions in a patient with Crohn's disease. Gut. 2016;65(2):p. 295. doi: 10.1136/gutjnl-2015-310130. [DOI] [PubMed] [Google Scholar]
- 22.André M. F. J., Kémény J.-L., Aumaître O. Aseptic abscesses: entity already described in Crohn's disease: Comment on the case report by Holstein et al. Journal of Gastroenterology and Hepatology. 2007;22(5):p. 765. doi: 10.1111/j.1440-1746.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- 23.Callen J. P. Pyoderma gangrenosum. Lancet. 1998;351(9102):581–585. doi: 10.1016/s0140-6736(97)10187-8. [DOI] [PubMed] [Google Scholar]
- 24.Lindor N. M., Arsenault T. M., Solomon H., Seidman C. E., McEvov M. T. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clinic Proceedings. 1997;72(7):611–615. doi: 10.1016/s0025-6196(11)63565-9. [DOI] [PubMed] [Google Scholar]
- 25.Fett D. L., Gibson L. E., Su W. P. D. Sweet's syndrome: systemic signs and symptoms and associated disorders. Mayo Clinic Proceedings. 1995;70(3):234–240. doi: 10.4065/70.3.234. [DOI] [PubMed] [Google Scholar]
- 26.Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr. Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology. 1976;70(3):439–444. [PubMed] [Google Scholar]
- 27.Mary J. Y., Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: A Prospective Multicentre Study. Groupe d'Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30(7):983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]