SUMMARY
Nowadays, the transposition of microvascular free flaps is the most popular method for management of head and neck defects. However, not all patients are suitable candidates for free flap reconstruction. In addition, not every defect requires a free flap transfer to achieve good functional results. The aim of this study was to assess whether pedicled flap reconstruction of head and neck defects is inferior to microvascular free flap reconstruction in terms of complications, functionality and prognosis. The records of consecutive patients who underwent free flap or pedicled flap reconstruction after head and neck cancer ablation from 2006 to 2015, from a single surgeon, in the AOUC Hospital, Florence Italy were analysed. A total of 93 patients, the majority with oral cancer (n = 59), were included, of which 64 were pedicled flap reconstructions (69%). The results showed no significant differences in terms of functional outcome, flap necrosis and complications in each type of reconstruction. Multivariate regression analysis of flap necrosis and functional impairments showed no associated factors. Multivariate regression analysis of complicated flap healing showed that only comorbidities remained an explaining factor (p = 0.019). Survival analysis and proportional hazard regression analysis regarding cancer relapse or distant metastasis, showed no significant differences in prognosis of patients concerning both types of reconstruction. In this retrospective, non-randomised study cohort, pedicled flaps were not significantly inferior to free flaps for reconstruction of head and neck defects, considering functionality, complications and prognosis.
KEY WORDS: Head and neck reconstruction, Oral cavity reconstruction, Pedicled flap, Free flap, Head and neck cancer
RIASSUNTO
La trasposizione di lembi liberi microvascolari rappresenta oggi la procedura maggiormente diffusa nelle ricostruzioni del distretto testacollo. Tuttavia, non tutti i pazienti sono candidati ideali per ricostruzioni microvascolari, né tutti i difetti richiedono necessariamente lembi microvascolari per ottenere buoni risultati funzionali. Lo scopo di questo studio è quello di valutare se la ricostruzione di difetti del distretto testa-collo mediante lembi peduncolati sia inferiore alle ricostruzioni microvascolari in termini di complicanze, outcome funzionale e prognosi. In una coorte di pazienti consecutivi che sono stati sottoposti a resezione maggiore per carcinomi del distretto testa collo, abbiamo confrontato i dati delle ricostruzioni mediante lembi peduncolati con quelli delle ricostruzioni microvascolari. Tutti gli interventi sono stati eseguiti da un unico chirurgo dal 2006 al 2015. Sono stati inclusi un totale di 93 pazienti, la maggior parte dei quali affetti da carcinoma del cavo orale (n = 59), di cui 64 hanno subito ricostruzione tramite lembo peduncolato (69%). Nei due gruppi non si sono registrate differenze significative in termini di necrosi del lembo, complicanze ed outcome funzionale. L'analisi multivariata ha mostrato che le comorbidità preoperatorie rappresentano l'unico fattore significativo per il rischio di complicanze nella guarigione del lembo (p = 0,019). Nei due gruppi l'analisi di sopravvivenza e l'analisi di regressione proporzionale al rischio di recidiva di malattia o metastasi a distanza non hanno mostrato differenze significative. In questo studio retrospettivo di coorte, non randomizzato, i lembi peduncolati non sono risultati significativamente inferiori rispetto ai lembi liberi in termini complicanze, outcome e prognosi.
Introduction
The head and neck area is a particularly complex region providing very important functions: respiration, voice production, articulation and swallowing. Head and neck cancer resection results in loss of functioning tissue, which can lead to a broad range of functional impairments and in some cases to disfigurement. Only small defects in this region are amenable for primary closure and in general medium sized and large or complex defects require reconstruction 1. Currently, tumour resection and reconstruction are conducted as a single stage procedure; optimal reconstructive outcomes aim at enhancing residual functions and allowing good mobility of the preserved structures around the resected area 2. The inevitable substitution of dynamic structures by static ones has obvious limitations and thus a thoughtful analysis of the anticipated defect and impairment is mandatory.
Nowadays, the most popular method for the management of defects in the head and neck area is represented by the transposition of microvascular free flaps. The introduction of free flaps in reconstructive surgery has provided the head and neck surgeon with a broad variety of available tissues, such as skin, muscle and bone, for optimal restoration of form and function 3-5. This reconstructive method represents a major evolution in the management of head and neck cancer, with a success rate, as defined by flap survival, of approximately 94% 4 5, resulting in a reduced utilisation of pedicled flaps. Overall, there are no validated contraindications for microvascular reconstruction in head and neck surgery, and in high volume institutions around the world the indications for free flaps are extended to even fragile patients or patients presenting with disadvantageous anatomical conditions (e.g. previous vessel depleted neck or previous chemo-radiation). However, not every defect requires a microvascular free flap reconstruction in order to achieve good functional results 6. Moreover, surgeons frequently deal with both elderly patients suffering from severe medical comorbidities 7 and pretreated patients presenting recurrent disease or second primary malignancies 8 9, which may preclude or overburden a microvascular procedure 10.
The surgeon must be cautious with the application of advanced reconstructive techniques and should always carefully evaluate the general status and regional anatomy of each patient, in order to select and propose the most appropriate reconstructive solution 11 12; this calls for the evaluation of valid alternatives.
Several reports have indicated the reliability and good functional results of alternative pedicled flaps 6 7 11-15, which may still have an important role even in the free flap era.
The primary goal of this study was to investigate whether pedicled flap reconstruction in head and neck cancer treatment is inferior to microvascular free flap reconstruction in terms of healing results (flap necrosis and complicated healing of the flap) and functional outcome (deglutition and speech). Additionally, survival and follow-up status of patients were documented in an effort to assess whether the type of flap employed for their reconstruction was associated with a different prognosis.
Materials and methods
This study is retrospective in nature and is therefore discharged from the local institutional review board; nonetheless, the study abided the guidelines of the Declaration of Helsinki.
The clinical and pathological data of patients who underwent microvascular free flap reconstruction or pedicled flap reconstruction following cancer ablation, treated by the senior author (AD), between July 2006 until December 2015 at the Department of Surgery and Translational Medicine, University of Florence, Italy, were reviewed. In all cases the reconstruction restored a separation between different compartments, created by the surgical approach for tumour resection 1 (upper aerodigestive tract and neck contents, oral cavity and nasal/sinonasal cavities, orbital and cranial contents). This aspect represented the first inclusion criteria.
Patients were excluded from this study in case of simultaneous free flap and pedicled flap reconstruction; in case of overlay pectoralis myofascial flap transposition for pharyngeal suture enforcement during salvage total laryngectomy after chemo-radiation failure; in case of flap transposition during the postoperative course of a non-flap surgery for healing problems (e.g. fistula formation 16); in case of reconstruction by local flaps or skin grafts. The remaining exclusion criterion was inadequate follow-up data.
Out of 143 reconstructive procedures reviewed, 93 patients met the inclusion criteria. Information about age, gender, date of procedure, tumour status (first primary, recurrence or second primary), anatomical site and subsite of cancer, TNM-stage, previous treatments in the head and neck region, vessel depleted neck status, comorbidities, type of tissue defect resulting from resection, type of flap used for reconstruction, surgical margins status, presence of extracapsular tumour spread in positive lymph nodes, adjuvant therapy, functional assessment, length of hospital stay and flap outcome were obtained. Finally, followup time and follow-up status were acquired from the last outpatient consultation; the survival status was recovered from the records or checked through telephonic survey.
Functional assessment of swallowing
In order to assess postoperative swallowing function, dietary status was evaluated at the last follow-up consultation of each patient; with regular diet indicating normal swallowing function; moist or soft diet indicating moderate swallowing impairment; liquid diet indicating severe swallowing impairment and tube-dependent intake indicating inability to swallow. Not being able to assess the swallowing function (e.g. passing away of the patient rapidly after surgery) led to a 'not recordable' status. Any impairment regarding swallowing was noted as a 'swallowing disorder' in the functional impairment assessment.
Functional assessment of speech
In order to assess postoperative speech function, the intelligibility was evaluated at the last follow-up consultation; with always intelligible indicating normal speech function; usually intelligible, but frequent repetition or face-to-face contact required, indicating moderate speech impairment; difficult intelligibility, even with face-to-face contact, indicating severe speech impairment; and never intelligible, written communication required, indicating the inability to speak. Not being able to assess the speech function (e.g. total laryngectomy) led to a 'not recordable' status. Any impairment regarding speech function was noted as a 'speech disorder' in the functional impairment assessment.
Statistical analysis
All data were analysed with professional statistics software (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). Data were expressed as mean ± SD, unless otherwise indicated, for continuous variables. Number of cases and percentages were represented for categorical variables. Univariate analysis by means of the Students t-test was applied for parametric continues values, while the χ2-test was applied for categorical variables, for comparing pedicled flap and free flap outcome measures. Also, for comparing functional outcome in pedicled flap and free flap reconstructions per anatomical site the χ2-test was used, if the number of cases was small (n ≤ 5) Fisher's exact test was used. Patients with a 'not recordable' status were excluded from this analysis.
Subsequently, binary logistic regression analysis was applied to assess independent correlations of existing functional impairments after reconstruction, complicated healing of the flap and flap necrosis. Target variables were converted into binary variables, in order to appropriately fit into a logistic regression model. The co-variates used in this model were age, type of flap used for reconstruction, tumour status (first primary, recurrence or second primary), anatomical site of cancer, previous treatment for cancer, type of tissue defect resulting from resection, and existing comorbidities.
Furthermore, to compare the survival distribution of patients who received either pedicled flap or free flap reconstruction, a Log-Rank test was applied and a Kaplan- Meier survival curve was computed regarding patients with a follow-up time over three years, but inferior to the 95th-percentile of the follow-up time. In addition, due to their important prognostic role, the presence of extracapsular tumour spread in positive lymph nodes and the status of surgical margins were analysed in both types of reconstruction by the χ2-test. Patients who did not receive a neck dissection (previous vessel depleted neck) were excluded from the analysis for extracapsular tumour spread. Finally, with the purpose to assess an estimate of the effect regarding the type of flap used for reconstruction on the development of recurrences, distant metastases or second primaries, after adjustment for explanatory variables, a Cox-regression was applied. Covariates were age, type of flap used for reconstruction, tumour status (first primary, recurrence or second primary), T-stage, N-stage, surgical margin status and the presence of extracapsular tumour spread in positive lymph nodes. A p value of < 0.05 was regarded as statistically significant.
Results
The rate of total flap necrosis in this series was 1 of 93 (1%), while 7 patients (8%) required further surgery for flap healing complications. Analysis of the pathological reports regarding tumour resection showed that there was no significant difference in surgical margin status between both types of reconstruction, nor concerning extracapsular tumour spread in positive lymph nodes. Table I and Table II display the characteristics of the population comparing pedicled flap with free flap reconstructions.
Table I.
Cohort characteristics.
| N = 93 | Pedicled Flap reconstruction (n = 64) |
Free Flap reconstruction (n = 29) |
P-value |
|---|---|---|---|
| Age: | 64.5 (SD ± 9.7) | 58.2 (SD ± 10.4) | 0.005* |
| Gender: | 0.874† | ||
| Male (n = 62) | 43 (67%) | 19 (65%) | |
| Female (n = 31) | 21 (33%) | 10 (35%) | |
| Anatomical site: | 0.073† | ||
| Oral Cavity (n = 59) | 39 (61%) | 20 (69%) | |
| Oropharynx (n = 17) | 9 (14%) | 8 (28%) | |
| Larynx (n = 6) | 6 (9%) | 0 (0%) | |
| Hypopharynx (n = 4) | 4 (6%) | 0 (0%) | |
| Oesophagus (n = 3) | 3 (5%) | 0 (0%) | |
| Other (n = 4) | 4 (6%) | 1 (3%) | |
| Previous treatment: | 0.006† | ||
| None (n = 52) | 29 (45%) | 23 (79%) | |
| Previous RT (n = 3) | 1 (2%) | 2 (7%) | |
| Previous surgery (n = 15) |
11 (17%) | 4 (14%) | |
| Previous surgery + RT (n = 15) |
15 (23%) | 0 (0%) | |
| Previous CT+RT (n = 3) | 3 (5%) | 0 (0%) | |
| Previous surgery and CT+RT (n = 5) |
5 (8%) | 0 (0%) | |
| Vessel Depleted Neck: | 0.004† | ||
| None (n = 69) | 41 (64%) | 28 (97%) | |
| Unilateral (n = 12) | 11 (17%) | 1 (3%) | |
| Bilateral (n = 12) | 12 (19%) | 0 (0%) | |
| Comorbidity: | < 0.001† | ||
| None (n = 55) | 28 (44%) | 27 (94%) | |
| Diabetes (n = 2) | 2 (3%) | 0 (0%) | |
| Neurological disease (n = 1) |
0 (0%) | 1 (3%) | |
| Severe cardiovascular disease (n = 19) |
18 (28%) | 1 (3%) | |
| Multiple (n = 16) | 16 (25%) | 0 (0%) |
Student's t-test
χ2-test. Bold script indicates significant values.
Table II.
Tumour-defect characteristics.
| N = 93 | Pedicled Flap reconstruction (n = 64) |
Free Flap reconstruction (n = 29) |
P-value |
|---|---|---|---|
| Tumour Status: | 0.005† | ||
| First primary (n = 54) |
30 (47%) | 24 (83%) | |
| Recurrence (n = 27) | 23 (36%) | 4 (14%) | |
| Second primary (n = 13) |
11 (17%) | 1 (3%) | |
| Tissue Defect: | 0.045† | ||
| Soft Tissue (n = 56) | 39 (61%) | 17 (59%) | |
| Bony + Soft Tissue (n = 17) |
8 (12%) | 9 (31%) | |
| Soft Tissue + marginal (n = 20) |
17 (27%) | 3 (10%) | |
| pT-stage (grouping): | 0.262† | ||
| T1-T2 (n = 21) | 15 (23%) | 6 (21%) | |
| T3 (n = 19) | 10 (16%) | 9 (31%) | |
| T4 (n = 26) | 16 (25%) | 10 (35%) | |
| rT1-rT2 (n = 8) | 7 (11%) | 1 (3%) | |
| rT3 (n = 5) | 4 (6%) | 1 (3%) | |
| rT4 (n = 14) | 12 (19%) | 2 (7%) | |
| pN-stage (grouping): | 0.075† | ||
| N0 (n = 28) | 20 (31%) | 8 (28%) | |
| N+ (n = 38) | 21 (33%) | 17 (58%) | |
| rN0 (n = 17) | 15 (23%) | 2 (7%) | |
| rN+ (n = 10) | 8 (13%) | 2 (7%) | |
| Adjuvant therapy: | < 0.070 † | ||
| None (n = 35) | 29 (45%) | 6 (21%) | |
| RT (n = 32) | 20 (31%) | 12 (41%) | |
| CT+RT (n = 26) | 15 (24%) | 11 (38%) | |
| Present (n = 31) | 19 (37%) | 12 (41%) |
Student's t-test
χ2-test. Bold script indicates significant values.
Patients who underwent a pedicled flap reconstruction were older than patients who underwent a free flap re-construction (mean 64.5 vs 58.2, p = 0.005), and more often presented a recurrence or a second primary (36% and 17% vs 14% and 3%, p = 0.005). Furthermore, patients who underwent a pedicled flap reconstruction were more often identified with a unilateral or bilateral vessel depleted neck (17% and 19% vs 3% and 0%, p = 0.004) and suffered more often from comorbidities (56% vs 6%, p < 0.001) than patients who underwent free flap reconstruction. Finally, the resulting defect more frequently involved soft tissue or soft tissue and marginal mandibular resection in patients who underwent pedicled flap reconstruction (88% vs 69%); while reconstruction for segmental bony tissue defects (which also include soft tissue defects) was more frequently achieved by means of a free flap reconstruction (12% vs 31%, p = 0.045). Table III shows that, when corrected for the anatomical site of cancer resection, the degrees of impairment for swallowing function and speech between pedicled flap and free flap reconstructions were not statistically significant.
Table III.
Functional assessment outcome in different types of reconstruction per anatomical site.
| Functional Impairment (n = 76) |
None (%) (n = 39) |
Swallowing Disorder (%) (n = 20) |
Speech Disorder (%) (n = 3) |
Both (%) (n = 14) | P value |
|---|---|---|---|---|---|
| Oral Cavity (n = 57) | 0.614* | ||||
| Pedicled Flap (n = 38) | 18 (48%) | 11 (29%) | 2 (5%) | 7 (18%) | |
| Free Flap (n = 19) | 11 (58%) | 6 (31%) | 0 (0%) | 2 (11%) | |
| Oropharynx (n = 14) | 0.626* | ||||
| Pedicled Flap (n = 6) | 2 (33%) | 1 (17%) | 1 (17%) | 2 (33%) | |
| Free Flap (n = 8) | 4 (50%) | 2 (25%) | 0 (0%) | 2 (25%) | |
| Other (n = 5) | 0.800† | ||||
| Pedicled Flap (n = 4) | 3 (75%) | 0 (0%) | 0 (0%) | 1 (25%) | |
| Free Flap (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Diet (n = 91) | Regular diet without restriction (%) (n = 48) |
Moist or soft diet (%) (n = 37) |
Liquid diet (%) (n = 6) |
P value |
|---|---|---|---|---|
| Oral Cavity (n = 57) | 0.747* | |||
| Pedicled Flap (n = 38) | 20 (52%) | 17 (45%) | 1 (3%) | |
| Free Flap (n = 19) | 11 (58%) | 8 (42%) | 0 (0%) | |
| Oropharynx (n = 17) | 0.549* | |||
| Pedicled Flap (n = 9) | 3 (33%) | 5 (56%) | 1 (11%) | |
| Free Flap (n = 8) | 4 (50%) | 4 (50%) | 0 (0%) | |
| Other (n = 17) | 0.689* | |||
| Pedicled Flap (n = 16) | 9 (56%) | 3 (19%) | 4 (25%) | |
| Free Flap (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) |
| Speech (n = 76) | Always intelligible (%) (n = 59) |
Usually intelligible (%) (n = 12) |
Difficult intelligibility or never intelligible (%) (n = 5) |
P value |
|---|---|---|---|---|
| Oral Cavity (n = 57) | 0.371* | |||
| Pedicled Flap (n = 38) | 30 (79%) | 7 (18%) | 1 (3%) | |
| Free Flap (n = 19) | 17 (90%) | 1 (5%) | 1 (5%) | |
| Oropharynx (n = 14) | 0.150* | |||
| Pedicled Flap (n = 6) | 2 (33%) | 2 (33%) | 2 (33%) | |
| Free Flap (n = 8) | 6 (75%) | 2 (25%) | 0 (0%) | |
| Other (n = 5) | 0.800† | |||
| Pedicled Flap (n = 4) | 3 (75%) | 0 (0%) | 1 (25%) | |
| Free Flap (n = 1) | 1 (100%) | 0 (0%) | 0 (0%) |
χ2-test
Fisher's exact test.
Table IV shows that the differences in healing results and flap related complications were not statistically significant between the two groups, although patients who underwent a free flap reconstruction were admitted for a longer period of time than those who underwent a pedicled flap reconstruction (mean 21.1 days vs 17.6 days, p = 0.028).
Table IV.
Healing outcomes.
| Flap Healing (n = 93) | Healing uneventful (%) (n = 81) |
Minor complications (%) (n = 5) |
Further surgery required (%) (n = 7) |
P value |
|---|---|---|---|---|
| Pedicled Flap | 56 (87%) | 3 (5%) | 5 (8%) | 0.902* |
| Free Flap | 25 (86%) | 2 (7%) | 2 (7%) | |
| Flap Necrosis (n = 93) | None (%) (n = 85) | Partial Necrosis (%) (n = 7) | Total Necrosis (%) (n = 1) | |
| Pedicled Flap | 59 (92%) | 4 (6%) | 1 (2%) | 0.634* |
| Free Flap | 26 (90%) | 3 (10%) | 0 (0%) | |
| Admission length (n=93) | Mean | Standard deviation | Standard error mean | |
| Pedicled Flap (n = 64) | 17.6 | ± 6.8 | 0.9 | 0.028† |
| Free Flap (n = 29) | 21.1 | ± 7.8 | 1.5 |
χ2-test
Students t-test. Bold script indicates significant values
In multivariate regression analysis regarding the development of functional impairments, no associated factors were found; also, no associated factors were found in multivariate regression analysis regarding necrosis of the flap after reconstruction. However, a considerable trend towards significance concerning comorbidities was found regarding the probability of facing a flap necrosis (p = 0.057); and the multivariate regression analysis showed that only the presence of comorbidities remained an explaining factor for complicated flap healing (p = 0.019, OR = 2.018), Table V. Of note, a pedicled flap reconstruction provided uneventful healing process in 81% of fragile patients who suffered from severe comorbidities.
Table V.
Estimation of probability of complicated flap healing.
| Variable | Coefficient | Standard error | P value | OR* | 95% CI† | |
|---|---|---|---|---|---|---|
| Age | 0.00 | 0.37 | 0.998 | 1.000 | 0.930 | 1.076 |
| Type Flap | -1.440 | 1.132 | 0.203 | 0.237 | 0.026 | 2.179 |
| Tumour status | -2.018 | 1.765 | 0.253 | 0.133 | 0.004 | 4.230 |
| Anatomical Site | 0.602 | 0.482 | 0.211 | 1.826 | 0.710 | 4.692 |
| Previous treatment | 0.275 | 0.522 | 0.598 | 1.317 | 0.473 | 3.663 |
| Tissue Defect | -0.130 | 0.497 | 0.793 | 0.878 | 0.331 | 2.326 |
| Comorbidity | 0.702 | 0.300 | 0.019 | 2.018 | 1.121 | 3.634 |
OR odds ratio.
CI confidence interval. Bold script indicates significant values.
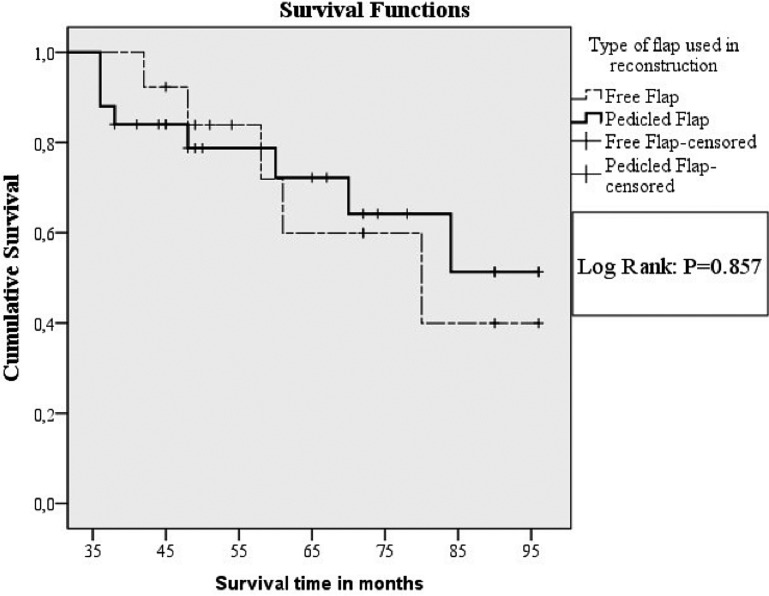
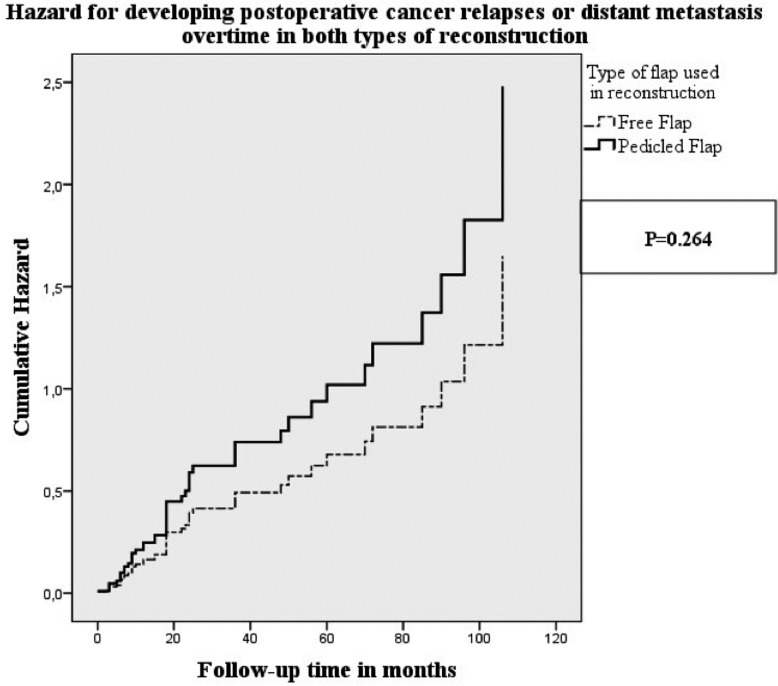
Figure 1 displays the overall survival of patients with a follow-up time over 36 months, receiving pedicled flap and free flap reconstructions; the differences were not statistically significant (Log Rank: p = 0.857). Figure 2 displays the development of recurrences, distant metastasis or second primary cancers, overtime, after pedicled flap and free flap reconstructions. Although the odds (hazard ratio = 0.665) for developing a recurrence, distant metastasis or second primary cancer were higher for patients who underwent pedicled flap reconstruction, as compared to patients who underwent a free flap reconstruction, this difference was not statistically significant corrected for age, tumour status (first primary, recurrence or second primary), T-stage, N-stage, surgical margin status and the presence of extracapsular tumour spread in positive lymph nodes.
Fig. 1.
Kaplan Meier curves of overall survival in patients with a follow-up time over 36 months, but less than 97 months, who underwent either a pedicled flap (n = 25, bold line) or a free flap (n = 13, dotted line).
Fig. 2.
Cumulative hazard curves regarding the development of recurrences, distant metastasis or second primary cancers in patients who underwent either pedicled flap (n= 64, bold line) or free flap (n = 29, dotted line) reconstruction.
Discussion
In our study, comparison of outcomes in patients who underwent a pedicled flap or a free flap reconstruction showed no statistically significant differences in terms of functional outcomes, flap necrosis, or complications. Furthermore, multivariate regression analysis showed that only the existence of comorbidities remained an explaining factor for complicated flap healing. In addition, there was no significant differences in terms of overall survival between patients who underwent a pedicled flap reconstruction and those who underwent a free flap reconstruction. Finally, proportional hazard regression analysis, regarding the development of recurrences, distant metastases or second primary cancers, showed no significant differences between patients who underwent a pedicled flap reconstruction and patients who underwent a free flap reconstruction. In our cohort, pedicled flap reconstruction did not seem be inferior to free flap reconstruction in terms of complications, functional outcome, survival, development of cancer relapse, or distant metastases.
The type of flap used for reconstruction depends on the needs of the recipient site; in some situations free flaps are required (e.g. in segmental bony reconstructions), whereas pedicled flaps cannot always offer the amount or type of desired tissue 17. Furthermore, the anatomical site of the defect can sometimes be out of reach for a pedicled flap, since the length of the vascular pedicle limits the required distance of transfer. However, premorbid patient factors and regional anatomy (e.g. comorbidity or previous head and neck cancer treatment) are also important in deciding which flap is employed for reconstruction 18.
Randomised controlled trials are not feasible; consequently, the nature of studies comparing the outcome of reconstruction in head and neck surgery is restricted to descriptive reports, stratifying, wherever possible, for patient and tumour factors. Thus, the outcome of free flap and pedicled flap reconstructions cannot easily be compared and bias is inevitable.
The small cohort and heterogeneity of the reconstructed defects represent the major limitation of our study. Nevertheless, this series replicates a comparable scenario of many low volume centres where a careful selection of patients undergoing microvascular reconstructive surgery is performed. All reconstructive surgical procedures and follow-up consultations were conducted by a single surgeon (AD), diminishing inter-patient variability to minimise bias concerning treatment and evaluation. Furthermore, all reconstructions with local flaps or grafts were excluded a priori, focusing only on major resections with flap transposition.
Several authors have reported that free flaps have advantages over pedicled flaps in head and neck reconstruction. Firstly, tissue dimensions and thickness can be tailored to the size of the defect and vascularised bone can be used to reconstruct complex defects, which leads to superior aesthetic results 19. Secondly, some reports state that free flaps provide superior speech outcome over pedicled flaps 18 20. Thirdly, it is reported that swallowing function, following free flap reconstruction in comparison to pedicled flap reconstruction, is improved 20, while other authors were unable to substantiate this finding 18 21. Considering the results of our study, we cannot support these findings, either for superior swallowing function or for superior speech function.
A videofluorographic swallowing study is certainly the golden standard for the assessment of swallowing disorders. However, at our institution, this is not routinely prescribed to all patients, but only in case of aspiration problems. Therefore, we used a rough assessment concerning the quality of swallowing and speech, which was already used in previous reports from both our group 7 15 and from others 22-24, for this assessment was relatively simple to apply during follow-up consultation visits.
Frequently pedicled flaps even seem to be preferable over free flaps 25-27. Many reports regarding the elderly in relation to microvascular free flap reconstruction agree that age is a risk factor for poor surgical outcome 10 28-30. McCrory et al. described that operative time, resection-reconstruction, was statistically much longer for free flap than for pedicled flap procedures (9 hours 35 min vs 4 hours 58 min) 28. Long surgical times was a significant factor for the development of postoperative complications in a series of 104 free flaps in patients aged 65 and older 10. Furthermore, older patients are less capable of coping with large fluid shifts and significant blood loss 10, whereas free flap reconstructions are more often associated with the need for blood transfusion 29. In addition, cardiovascular disease proves to be an important factor in free flap reconstructive failure 10, a condition which proves to affect the majority of adults past the age of 60 years 31, and furthermore with increasing age there is a greater likelihood of postoperative complications after free flap reconstruction 27, even with successful microvascular reconstructions 32.
Besides age, diabetes also appears to interfere with free flap survival 33. However, the impact of diabetes on free flap survival is still much debated. While some authors support that diabetes interferes with free flap survival 7 34, Cooley et al. reported that patients with diabetes are not at increased risk either for flap failure or for abnormal healing of the anastomoses as long as normal glycaemia is maintained 35.
Our study shows that more than 80% of patients suffering from comorbidities who underwent a pedicled flap reconstruction had uneventful healing of the flap. Only 2 patients suffering from comorbidities underwent a free flap reconstruction, 1 of these patients had complicated flap healing. This suggests that a reconstruction by means of a pedicled flap is a safe procedure in patients who are suffering from comorbidities. Further research with a larger population should be conducted in order to assess whether pedicled flap reconstructions in patients suffering from comorbidities are less prone to complicated postoperative healing than free flap reconstructions.
The use of free flaps for reconstruction in previously irradiated patients or patients who underwent prior chemoradiation is also much debated in literature. In a review, Wong et. al. points out that prior chemotherapy and/or radiotherapy can cause significant scarring and vessel damage to the utilised vessels for microvascular free flap reconstruction with obvious negative consequences 36. Furthermore, Schultze-Mosgau et al. reported a reduced clinical success rate (84%) of free vascular grafts in head and neck patients with previous radiotherapy of 60- 70 Gy 37. Moreover, in a study of 429 patients who underwent free flap reconstruction in the head and neck, preoperative radiotherapy (irrespective of irradiation doses) was significantly associated with fistulae formation and wound infection, while previous neck irradiation at doses of more than 60 Gy proved to be a significant risk factor for free flap failure, overall local complications, haematoma, longer duration of enteral nutrition and hospital stay 38. In a multicentre survival analysis after free flap reconstructive surgery of head and neck squamous cell carcinoma by Salvatori et al. 39, pre-treated patients had significantly worse survival than those with first primary tumours (43.1% and 54.1% respectively). Accordingly, also in our series, patients presenting with recurrence or second primaries showed worse survival than those with first primary tumours. However, this was irrespective to the type of employed flap (pedicled flap or free flap). Based on our policy, the greatest majority of pre-treated patients received a pedicled flap reconstruction; among them 21 of 34 patients (62%) who underwent pedicled flap reconstruction were confirmed alive at the end of our study.
Since intake of alcohol ≥ 30 g/day is related to the development of head and neck cancer 40, many head and neck cancer patients suffer from alcohol-related problems. Both acute alcohol withdrawal as well as other alcoholinduced disorders negatively influence the outcome of microvascular free flap tissue transfers 41-43.
Consequently, those patients presenting the above mentioned factors, which are associated with a higher rate of free flap failure or postoperative complications, are less eligible for microvascular free flap reconstructive surgery, whereas locoregional pedicled flaps may offer a reliable alternative for reconstruction 44-49.
Furthermore, a pedicled flap reconstruction brings some additional benefits for both patient and surgeon. Most sites of pedicled flaps have a low donor-site morbidity with donor sites that can be closed primarily. Also, many pedicled flaps can be harvested and transferred rapidly, which leads to decreased operating time and a corresponding decrease in the morbidities of prolonged general anaesthesia.
Our results showed that the admission length of patients who underwent a pedicled flap reconstruction were shorter than in those who underwent a free flap reconstruction. Other papers pointed out that pedicled flap reconstructions were associated with shorter intensive care stay than free flap reconstructions 7 28. Consequently, free flap reconstructions are usually more expensive procedures than pedicled flap reconstructions 15 25 28.
Conclusions
In our patient cohort, pedicled flaps were performed in two-thirds of cases and were not significantly inferior to free flaps in terms of functionality, complications, or prognosis. This study highlights how, in a low volume setting, careful selection of patients receiving free flap reconstruction is advisable in order to maintain high success rates; in this scenario, pedicled flaps are a viable option in patients considered suboptimal for a microvascular reconstruction. A well thought and careful analysis of every patient is needed to offer the best solution in the light of individualised treatment 1.
References
- 1.Deganello A. Modern oral cavity reconstruction with free flaps and pedicled flaps. J Aesth Reconstr Surg. 2015;1:4–4. [Google Scholar]
- 2.List MA, Bilir SP. Functional outcomes in head and neck cancer. Semin Radiat Oncol. 2004;14:178–189. doi: 10.1053/j.semradonc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Pellini R, Pichi B, Marchesi P, et al. External monitor for buried free flaps in head and neck reconstructions. Acta Otorhinolaryngol Ital. 2006;26:1–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Varvares MA, Lin D, Hadlock T, et al. Success of multiple, sequential, free tissue transfers to the head and neck. Laryngoscope. 2005;115:101–104. doi: 10.1097/01.mlg.0000150697.54000.eb. [DOI] [PubMed] [Google Scholar]
- 5.Beausang ES, Ang EE, Lipa JE, et al. Microvascular free tissue transfer in elderly patients: the Toronto experience. Head Neck. 2003;25:549–553. doi: 10.1002/hed.10240. [DOI] [PubMed] [Google Scholar]
- 6.Deganello A, Manciocco V, Dolivet G, et al. Infrahyoid fascio-myocutaneous flap as an alternative to free radial forearm flap in head and neck reconstruction. Head Neck. 2007;29:285–291. doi: 10.1002/hed.20512. [DOI] [PubMed] [Google Scholar]
- 7.Deganello A, Gitti G, Parrinello G, et al. Infrahyoid flap reconstruction of oral cavity and oropharyngeal defects in elderly patients with severe general comorbidities. Head Neck. 2012;34:1299–1305. doi: 10.1002/hed.21913. [DOI] [PubMed] [Google Scholar]
- 8.Deganello A, Gitti G, Mannelli G, et al. Risk factors for multiple malignancies in the head and neck. Otolaryngol Head Neck Surg. 2013;149:105–111. doi: 10.1177/0194599813484273. [DOI] [PubMed] [Google Scholar]
- 9.Deganello A, Franchi A, Sardi I, et al. Genetic alterations between primary head and neck squamous cell carcinoma and recurrence after radiotherapy: recurrence, genetically related cancer, or second primary? Cancer. 2010;116:1291–1297. doi: 10.1002/cncr.24854. [DOI] [PubMed] [Google Scholar]
- 10.Serletti JM, Higgins JP, Moran S, et al. Factors affecting outcome in free-tissue transfer in the elderly. Plast Reconstr Surg. 2000;106:66–70. doi: 10.1097/00006534-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Mahieu R, Russo S, Gualtieri T, et al. Oral cavity reconstruction with the masseter flap. Acta Atorhinolaryngol Ital. 2016;36:1–5. doi: 10.14639/0392-100X-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deganello A, Leemans CR. The infrahyoid flap: a comprehensive review of an often overlooked reconstructive method. Oral Oncol. 2014;50:704–710. doi: 10.1016/j.oraloncology.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Gangloff P, Deganello A, Lacave ML, et al. Use of the infra hyoid musculo-cutaneous flap in soft palate reconstruction. Eur J Surg Oncol. 2006;32:1165–1169. doi: 10.1016/j.ejso.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Deganello A, Bree R, Dolivet G, et al. Infrahyoid myocutaneous flap reconstruction after wide local excision of a merkel cell carcinoma. Acta Otorhinolaryngol Ital. 2005;25:50–54. [PMC free article] [PubMed] [Google Scholar]
- 15.Deganello A, Gitti G, Parrinello G, et al. Cost analysis in oral cavity and oropharyngeal reconstructions with microvascular and pedicled flaps. Acta Otorhinolaryngol Ital. 2013;33:380–387. [PMC free article] [PubMed] [Google Scholar]
- 16.Busoni M, Deganello A, Gallo O. Pharyngocutaneous fistula following total laryngectomy: analysis of risk factors, prognosis and treatment modalities. Acta Otorhinolaryngol Ital. 2015;35:400–405. doi: 10.14639/0392-100X-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarsitano A, Ciocca L, Cipriani R, et al. Mandibular reconstruction using fibula free flap harvested using a customised cutting guide: how we do it. Acta Otorhinolaryngol Ital. 2015;35:198–201. [PMC free article] [PubMed] [Google Scholar]
- 18.O'Neill JP, Shine N, Eadie PA, et al. Free tissue transfer versus pedicled flap reconstruction of head and neck malignancy defects. Ir J Med Sci. 2010;179:337–343. doi: 10.1007/s11845-010-0468-4. [DOI] [PubMed] [Google Scholar]
- 19.Rosenthal ELM, Dixon SFM. Free flap complications: when is enough, enough. Curr Opin Otolaryngol Head Neck Surg. 2003;11:236–239. doi: 10.1097/00020840-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Abemayor EBK. Reconstruction of soft tissue defects in the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2000;126:909–912. doi: 10.1001/archotol.126.7.909. [DOI] [PubMed] [Google Scholar]
- 21.Freedlander E, Espie CA, Campsie LM, et al. Functional implications of major surgery for intraoral cancer. Br J Plast Surg. 1989;42:266–269. doi: 10.1016/0007-1226(89)90144-6. [DOI] [PubMed] [Google Scholar]
- 22.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66:564–569. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill JP, Shine N, Eadie PA, et al. Free tissue transfer versus pedicled flap reconstruction of head and neck malignancy defects. Ir J Med Sci. 2010;179:337–343. doi: 10.1007/s11845-010-0468-4. [DOI] [PubMed] [Google Scholar]
- 24.Andrades P, Pehler SF, Baranano CF, et al. Fistula analysis after radial forearm free flap reconstruction of hypopharyngeal defects. Laryngoscope. 2008;118:1157–1163. doi: 10.1097/MLG.0b013e31816f695a. [DOI] [PubMed] [Google Scholar]
- 25.Colletti G, Tewfik K, Bardazzi A, et al. Regional flaops in head and neck reconstruction: a reappraisal. J Oral Maxillofac Surg. 2015;73:571.e1–571.e10. doi: 10.1016/j.joms.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Deganello A, Gitti G, Struijs B, et al. Palliative combined treatment for unresectable cutaneous basosquamous cell carcinoma of the head and neck. Acta Otorhinolaryngol Ital. 2013;33:353–356. [PMC free article] [PubMed] [Google Scholar]
- 27.Deganello A, Gallo O, Cesare JM, et al. Surgical management of surgery and radiation induced peristomal neck ulcerations. B-ENT. 2008;4:169–174. [PubMed] [Google Scholar]
- 28.McCrory AL, Magnuson JS. Free tissue transfer versus pedicled flap in head and neck reconstruction. Laryngoscope. 2002;112:161–165. doi: 10.1097/00005537-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Perisanidis C, Herberger B, Papadogeorgakis N, et al. Complications after free flap surgery: do we need a standardized classification of surgical complications? Br J Oral Maxillofacial Surg. 2012;50:113–118. doi: 10.1016/j.bjoms.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Chick LR, Walton RL, Reus W, et al. Free flaps in the elderly. Plast Reconstr Surg. 1992;90:87–94. doi: 10.1097/00006534-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Laslett LJ, Alagona P, Jr, Clark BA, III, et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the american college of cardiology. J Am Coll Cardiol. 2012;60:S1–S49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Beausang ES, Ang EE, Lipa JE, et al. Microvascular free tissue transfer in elderly patients: the toronto experience. Head Neck. 2003;25:549–553. doi: 10.1002/hed.10240. [DOI] [PubMed] [Google Scholar]
- 33.Valentini V, Cassoni A, Marianetti TM, et al. Diabetes as main risk factor in head and neck reconstructive surgery with free flaps. J Craniofac Surg. 2008;19:1080–1084. doi: 10.1097/SCS.0b013e3181763531. [DOI] [PubMed] [Google Scholar]
- 34.Joo YH, Sun DI, Park JO, et al. Risk factors of free flap compromise in 247 cases of microvascular head and neck reconstruction: a single surgeon's experience. Eur Arch Otorhinolaryngol. 2010;267:1629–1633. doi: 10.1007/s00405-010-1268-1. [DOI] [PubMed] [Google Scholar]
- 35.Cooley BC, Hanel DP, Anderson RB, et al. The influence of diabetes on free flap transfer: I. flap survival and microvascular healing. Ann Plast Surg. 1992;29:58–64. doi: 10.1097/00000637-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Wong KK, Higgins KM, Enepekides DJ. Microvascular reconstruction in the vessel-depleted neck. Curr Opin Otolaryngol Head Neck Surg. 2010;18:223–226. doi: 10.1097/MOO.0b013e32833a2e50. [DOI] [PubMed] [Google Scholar]
- 37.Schultze-Mosgau S, Grabenbauer GG, Radespiel-Troger M, et al. Vascularization in the transition area between free grafted soft tissues and pre-irradiated graft bed tissues following preoperative radiotherapy in the head and neck region. Head Neck. 2002;24:42–51. doi: 10.1002/hed.10012. [DOI] [PubMed] [Google Scholar]
- 38.Benatar MJ, Dassonville O, Chamorey E, et al. Impact of preoperative radiotherapy on head and neck free flap reconstruction: A report on 429 cases. J Plastic Reconstr Aest Surg. 2013;66:478–482. doi: 10.1016/j.bjps.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Salvatori P, Paradisi S, Calabrese L, et al. Patients' survival after free flap reconstructive surgery of head and neck squamous cell carcinoma: a retrospective multicentre study. Acta Otorhinolaryngol Ital. 2014;34:99–104. [PMC free article] [PubMed] [Google Scholar]
- 40.Maasland DH, Brandt PA, Kremer B, et al. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: results from the Netherlands cohort study. BMC Canc. 2014;14:187–187. doi: 10.1186/1471-2407-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallivan KH, Reiter D. Acute alcohol withdrawal and free flap mandibular reconstruction outcomes. Arch Facial Plast Surg. 2001;3:264–266. doi: 10.1001/archfaci.3.4.264. [DOI] [PubMed] [Google Scholar]
- 42.Kuo YR, Jeng SF, Lin KM, et al. Microsurgical tissue transfers for head and neck reconstruction in patients with alcoholinduced mental disorder. Ann Surg Oncol. 2008;15:371–377. doi: 10.1245/s10434-007-9506-5. [DOI] [PubMed] [Google Scholar]
- 43.Cheng NC, Ko JY, Tai HC, et al. Microvascular head and neck reconstruction in patients with liver cirrhosis. Head Neck. 2008;30:829–835. doi: 10.1002/hed.20784. [DOI] [PubMed] [Google Scholar]
- 44.Giordano L, Bondi S, Toma S, et al. Versatility of the supraclavicular pedicle flap in head and neck reconstruction. Acta Otorhinolaryngol Ital. 2014;34:394–398. [PMC free article] [PubMed] [Google Scholar]
- 45.Rigby MH HR. Regional flaps: a move to simpler reconstructive options in the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2014;22:401–406. doi: 10.1097/MOO.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 46.Squaquara R, Kim Evans KF, Spanio di Spilimbergo S, et al. Intraoral reconstruction using local and regional flaps. Semin Plast Surg. 2010;24:198–211. doi: 10.1055/s-0030-1255337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bussu F, Gallus R, Navach V, et al. Contemporary role of pectoralis major regional flaps in head and neck surgery. Acta Otorhinolaryngol Ital. 2014;34:327–341. [PMC free article] [PubMed] [Google Scholar]
- 48.Kucur C, Durmus K, Ozer E. Supraclavicular artery island flap reconstruction of a contralateral partial laryngopharyngeal defect. Acta Otorhinolaryngol Ital. 2015;35:121–124. [PMC free article] [PubMed] [Google Scholar]
- 49.Dell'Aversana Orabona G, Salzano G, Abbate V, et al. Use of the SMAS flap for reconstruction of the parotid lodge. Acta Otorhinolaryngol Ital. 2015;35:406–411. doi: 10.14639/0392-100X-395. [DOI] [PMC free article] [PubMed] [Google Scholar]