SUMMARY
Obstructive sleep apnoea syndrome (OSAS) is a sleep disorder that leads to metabolic abnormalities and increased cardiovascular risk. This study aimed to define the expression and clinical significance of biomarkers involved in oxidative stress in patients with OSAS. A prospective study was designed to compare outcomes of oxidative stress laboratory tests in three groups of subjects. The study involved the recruitment of three groups of subjects, 10 patients with obstructive sleep apnoea syndrome with AHI > 30; 10 patients suffering from snoring at night with AHI < 15; 10 patients with nasal respiratory impairment with AHI < 5. Patients were subjected to skin prick tests for common aero-allergens, nasal endoscopy, active anterior rhinomanometry, fibrolaryngoscopy and polysomnography; and extra-routine diagnostic tests and procedures; analysis of oxidative and antioxidant (plasma thiol groups) biomarkers in blood and urine samples. No statistical differences in age, sex distribution or body mass index were present between the three groups (p > 0.05). There were significant differences in AHI among the three groups of patients (p < 0.05). No statistical significance was found in the Analysis of Variance (ANOVA) test (p > 0.05) between the levels of biomarkers of oxidative stress in the three populations studied. The results of our study show that the nose can play a role in the pathogenesis of OSAS through the production of biomarkers of oxidative stress.
KEY WORDS: OSAS, Oxidative damage, Biomarkers of oxidative stress, Polysomnography
RIASSUNTO
La sindrome delle apnee ostruttive del sonno (OSAS) è una malattia che può portare ad alterazioni metaboliche e a un'aumentata incidenza di patologie cardiovascolari. Questo studio ha lo scopo di definire l'espressione e il significato clinico di biomarkers coinvolti nello stress ossidativo nei pazienti con diagnosi di OSAS. I risultati degli esami di laboratorio dello stress ossidativo sono stati confrontai prospetticamente in tre gruppi di soggetti: 10 con sindrome delle apnee ostruttiva del sonno con Apnea Hypopnea Index (AHI) > 30; 10 con roncopatia notturna e AHI < 15 e 10 con insufficienza respiratoria nasale e AHI < 5. I pazienti sono stati sottoposti a test cutanei per aero-allergeni comuni, rinoscopia anteriore, rinomanometria anteriore attiva, fibrolaringoscopia e polisonnografia. Per la ricerca dei biomarkers dello stress ossidativo sono stati effettuati test diagnostici in campioni di sangue e urine. I gruppi sono risultati omogenei per età, sesso e distribuzione del Body Mass Index (BMI) (p > 0.05). Ci sono state differenze significative nell'AHI tra i tre gruppi di pazienti (p < 0.05). Nessuna significatività statistica è stata identificata (p > 0.05) tra i livelli di biomarkers di stress ossidativo nelle tre popolazioni studiate. I risultati del nostro studio hanno mostrato che il naso può svolgere un ruolo nella patogenesi dell' OSAS, attraverso la produzione di biomarkers di stress ossidativo.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is a sleep disorder characterised by repeated episodes of partial or complete obstruction of the upper airways during sleep, resulting in a reduction (hypoapnoea) or abolition (apnoea) of airflow 1. The prevalence of clinically significant apnoea during sleep, in middle age, is about 4% in men and 2% in women 2. OSAS is associated with important clinical symptoms such as obstructive respiratory symptoms, insomnia, awakenings and excessive daytime sleepiness. In addition, important clinical sequelae such as neuropsychiatric complications, resulting in sleep fragmentation and daytime sleepiness, which lead to an impairment of professional, family and social life, cardiovascular and metabolic complications, resulting in intermittent hypoxia, such as pulmonary and systemic hypertension, arrhythmias, heart attack, heart failure, stroke and diabetes may be present. It is also associated with increased morbidity and mortality from cardiovascular disease 1.
The starting point for the diagnosis of OSAS is clinical history and physical examination, which allow for risk stratification in each patient. More specific examinations for diagnosis are: a) multiple sleep latency test (MSLT) to quantify daytime sleepiness and is considered an objective measure of the tendency to sleep during the day; b) sleep endoscopy, which is an endoscopic evaluation of the site of obstruction; Muller's manoeuver; c) polysomnography, which is considered the gold standard for diagnosis, but is expensive and laborious, which has pushed the search for more practical diagnostic systems such as nocturnal oximetry and portable monitors 3-5.
The therapy is focused on three main points. 1) lifestyle intervention. In obese patients, weight loss is a very effective strategy for the treatment of OSAS. In recent years, bariatric surgery procedures are used more frequently for treatment of severe obesity 6. 2) CPAP (Continuous Positive Airway Pressure). Ventilation with continuous positive airway pressure is today the most effective treatment available for patients with OSAS since the positive pressure acts as a mechanic stent to the upper airway. The main problem associated with CPAP treatment is poor patient compliance due to its side effects 7 8. 3) Surgery. Surgical therapies for the treatment of OSAS aim to improve airway patency, acting at the level of the specific site (or sites) of obstruction. Because the obstruction may localise to different levels along the upper airway, different surgical techniques have been developed: nasal, oropharynx, tongue and finally multi-level surgery 9-12.
OSAS has a multifactorial pathogenesis, and the main pathogenic mechanism is represented by collapse of the upper airway during sleep as a result of anatomical alterations (including congenital) or changes in neuromuscular control 13-15.
Materials and methods
The study was designed to compare outcomes of oxidative stress laboratory tests in three groups of subjects: 10 patients with obstructive sleep apnoea syndrome (OSAS) with AHI > 30; 10 patients suffering from snoring at night with AHI < 15; 10 control subjects with nasal respiratory impairment and AHI < 5. The inclusion criteria for the study groups were: a) age 18-60 years; b) no previous treatment for OSAS; c) snoring at night with AHI < 15; d) Apnoea-hypopnoea index (AHI) > 30 assessed with polysomnography; e) willingness to provide free and informed consent. The inclusion criteria of the control group were: a) age 18-60 years; b) nasal respiratory impairment (RAA with total nasal resistance > 0.25 Pa/cm3/s at 150 Pa; c) non-allergic vasomotor rhinitis. The exclusion criteria were the same both for the study and control groups: a) smoking; b) subjects exposed to environmental irritants; c) no comorbidity that increases oxidative stress; d) diabetes; e) obesity; f) asthma-nasal polyposis; g) allergic rhinitis; h) hypertension.
The study protocol considered that both patients (subjects with OSAS and snoring) and controls were subjected to the following routine diagnostic tests: a) prick test for common aero-allergens b) otolaryngology visit with active anterior rhinomanometry, examination that evaluates nasal respiratory function by measuring nasal flow and resistance to the passage of air through the nasal passages; c) nasal endoscopy: examination of the nasal cavity and nasopharynx via an endoscope with flexible optics (2.4 mm diameter); d) polysomnography, which is reference technique for the study of sleep.
The starting point for the diagnosis of nasal respiratory impairment represented by the clinical history and physical examination. Physiologically, the nasal structure generates airflow resistance that can reach -50% of the total respiratory resistance. Therefore, the starting point for the diagnosis of nasal impairment is the Active Anterior Rhinomanometry (AAR) for physiologic estimation of nasal pressure and airflow during normal inspiration and expiration. It is considered the standard technique for a quantitative measure of nasal airflow resistance when performed according to the rules suggested by the Committee on Standardisation. Normal rates for total nasal resistance are < 0.25 Pa/cm3/s at 150 Pa 16.
Both patients and controls were also subjected to the following extra-routine diagnostic tests: dosage of biomarkers of oxidative stress in the blood and urine; a) Non-Protein- Bound Iron (NPBI); b) Advanced Oxidation Protein Products (AOPP); c) plasmatic and urinary isoprostanes; d) thiol.
Pro-oxidant and antioxidant assays were performed at the Oxidative Stress laboratory of the Neonatal Unit of Siena Hospital.
NPBI it is a low molecular mass iron form with no highaffinity binding to transferrin. Lowering of plasma pH (acidosis), as occurs during ischaemia, involves the activation of a release cascade of the iron and production of free radicals that can cause serious damage to cells 17 18.
Protein carbonyls are formed as a result of a variety of oxidative mechanisms and are sensitive indices of oxidative damage; such mechanisms include the action of Reactive Oxygen Species (ROS) of lipid oxidation products and reducing sugars or their oxidation products.
Isoprostanes are a series of prostaglandin-like compounds formed by direct ROS attack on arachidonic acid (AA) 19. Thiols are a qualitatively significant component of the antioxidant plasma barrier. In fact, thiol groups (-SH) of the compounds present in plasma (for example, plasma proteins, P-SH) oppose the propagation of radical chain reactions and neutralise the tissue-damaging action of hydroxyl radicals (HO*).
The statistical analysis is based on the comparison between the frequencies of events in a case-control study with independent data, in which the events considered are represented by the positivity to each of the biomarkers examined in the study. Therefore, once the positivity/ negativity of each biomarker is defined according to a predetermined reference value (cut-off), cases and controls were compared for the frequencies of subjects positive and negative to the different biomarkers.
For cases we considered both patients with OSAS (AHI > 30) and patients with nocturnal snoring (AHI < 15). Next, statistical analysis was conducted separately for the two diseases, but using the same control subjects, consisting of patients suffering from non-allergic vasomotor rhinitis.
The goal of the study was to determine if the proportions of subjects negative and positive for each biomarker are significantly different between cases and controls.
Because of the difficulty of enrolment, the sample size was rather low, at 10 cases for each of the 2 pathologies and 10 control subjects, for a total of 30 patients.
Statistical analysis used Fisher's exact test or analysis of variance (ANOVA). The objective of the analysis was to determine if the difference between the means (variability between groups) is greater than the internal variability in each group (variability within groups) to verify if the variability provides information on the causes of the phenomena and their relationship. The ANOVA is used to assess the relative importance, in terms of statistical significance, of the different sources of variation (systematic or accidental); the principle underpinning the ANOVA is the relationship between two variances (the systematic and that due to error). This type of analysis is indicated when the populations are more than two (in our case, three). In addition, given the low sample size in our study, this is the only possible analysis that ensures an acceptable level of accuracy.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Results
The three groups were homogeneous for age and BMI (Table I). There were significant differences between the AHI values of the three groups of patients. No significant difference was found in the analysis with ANOVA test (p > 0.05) between the various levels of biomarkers of oxidative stress in the three populations.
Table I.
Demographic and clinical data of the study and control groups.
| Nasal Stenosis |
OSAS | Snoring | P value | |
|---|---|---|---|---|
| Number of subjects | 20 | 20 | 20 | |
| Age | 39 | 47.3 | 39,2 | > 0.05 |
| Sex (male/female) | 13/7 | 12/8 | 12/8 | > 0.05 |
| BMI | 23.54 | 25.62 | 25.46 | > 0.05 |
| AHI | 1.3 | 36.04 | 10.18 | < 0.0001 |
Plasma isoprostanes were 60.64 ± 58.79 vs 48.44 ± 32.2 vs 66.19 ± 54.99 in patients with OSAS, patients with snoring and patients with nasal stenosis, respectively, p > 0.005 (Fig. 1). AOPP (Advanced Oxidation Protein Products) were 109.1 ± 33.0 vs 103 ± 31.7 vs 116.7 ± 29.24 in patients with OSAS, patients with snoring and patients with nasal stenosis, respectively, p > 0.005 (Fig. 2).
Fig. 1.
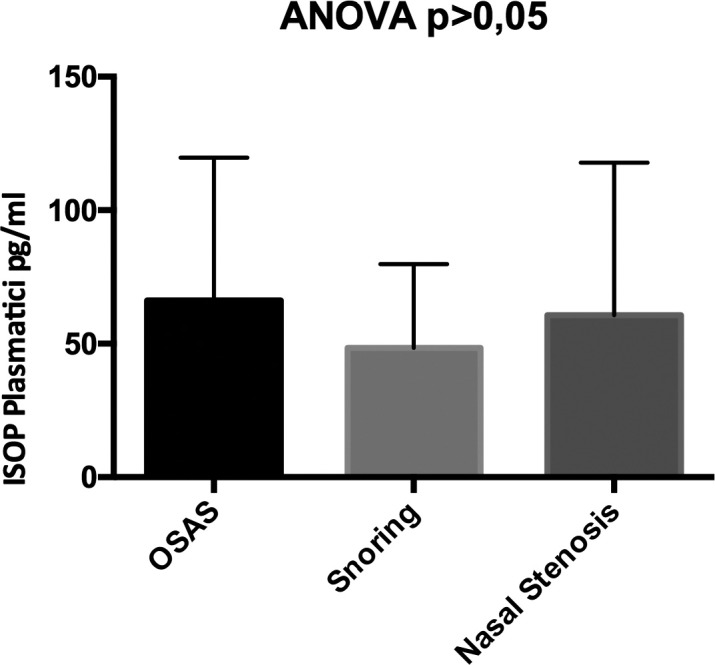
Differences in the levels of plasma isoprostanes in patients with OSAS, patients with snoring and patients with nasal stenosis.
Fig. 2.

Differences in levels of Advanced Oxidation Protein Products (AOPP) in patients with OSAS, patients with snoring and patients with nasal stenosis.
NPBI were 4.38 ± 5.65 vs 3.49 ± 3.38 vs 2.66 ± 2.56 in patients with OSAS, patients with snoring and patients with nasal stenosis, respectively, p > 0.005 (Fig. 3).
Fig. 3.
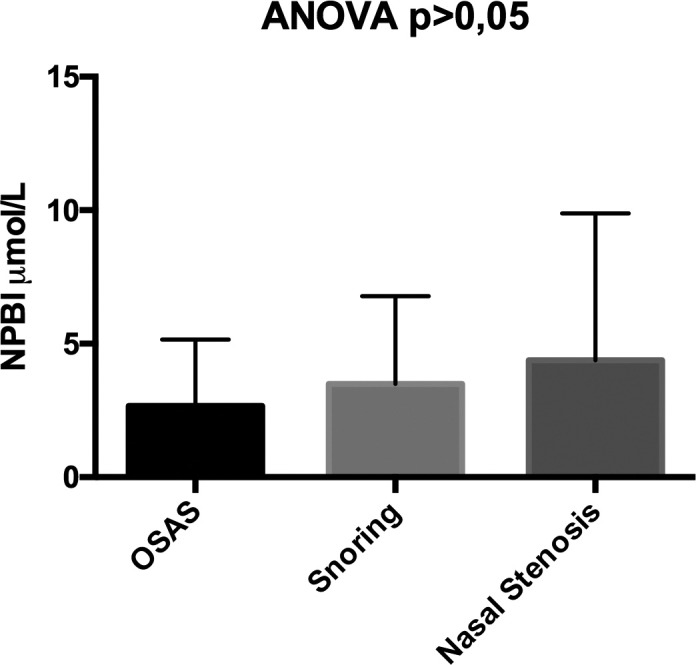
Differences in the levels of Non-Protein-Bound Iron (NPBI) in patients with OSAS, patients with snoring and patients with nasal stenosis.
Plasma thiol groups were 451 ± 57 vs 443 ± 92 vs 470 ± 46.96 in patients with OSAS, patients with snoring and patients with nasal stenosis, respectively, p > 0.005 (Fig. 4).
Fig. 4.
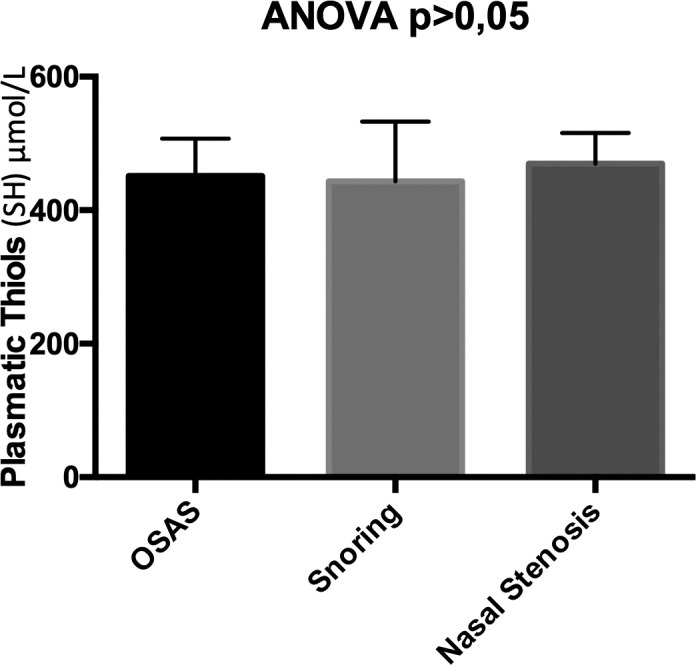
Differences in the levels of plasma thiol groups in patients with OSAS, patients with snoring and patients with nasal stenosis.
Urinary isoprostanes were 412 ± 494 vs 137 ± 64 vs 311 ± 328.03 in patients with OSAS, patients with snoring and patients with nasal stenosis, respectively, p > 0.005 (Fig. 5).
Fig. 5.
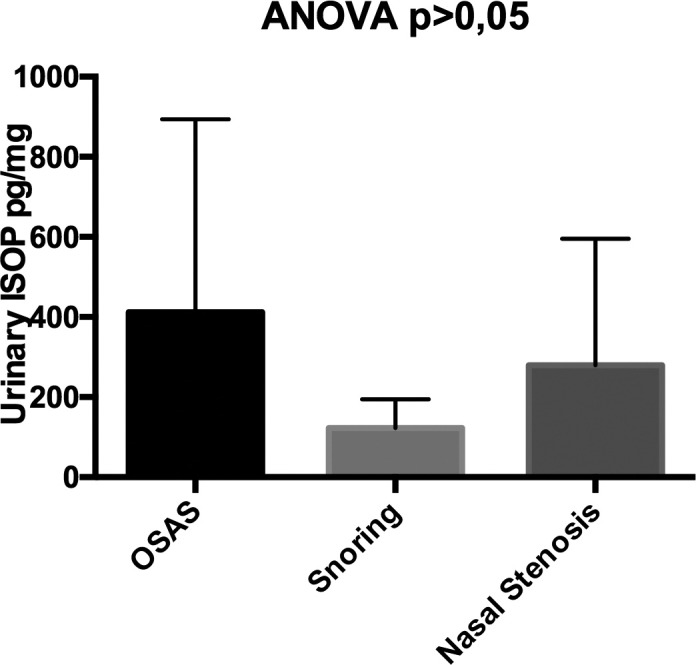
Differences in levels of urinary isoprostanes in patients OSAS, patients with snoring and patients with nasal stenosis.
Discussion
Although the primary site of obstruction in patients with OSAS has been identified in the oropharynx-hypopharynx, several studies, based on the induction of nasal obstruction in patients with OSAS, have revealed a positive and significant association between nasal obstruction and OSAS, although the exact nature of this relationship has not been fully clarified 20.
Several epidemiological studies have demonstrated a relationship between the measurement of the air flow at the level of the nose and snoring, but the attempt to find a linear correlation between obstruction, and therefore increase in the resistance, nasal and sleep apnoea has been less successful 21.
The nose is responsible for more than 50% of the total resistance of the upper airway and plays an important role in various physiological functions such as humidification, heating and air filtration. Among the areas of the nose of greater resistance to air flow, there are the vestibule and nasal valve, circumscribed by the alar cartilages, septum and inferior turbinates 22.
The nasal mucosa is a dynamic organ regulated by the autonomic nervous system. The condition of periodic congestion and nasal decongestion was called the "nasal cycle" of Heetderks. This cycle occurs in about 80% of the adult population. In patients with permanent unilateral nasal obstruction, the nasal cycle can contribute to a significant increase in the total resistance of the airways. In healthy individuals, the lateral decubitus increases congestion in the homolateral nasal cavity and reduces the resistance of the air flow in the contralateral nasal cavity. This does not occur due to a hydrostatic effect, but rather as a reflex caused by the asymmetrical pressure on the body. This reflex stops the nasal cycle. However, in the evaluation of both nasal cavities, significant changes were observed in the cross section when comparing supine and lateral decubitus 23.
To fully explain the relationship between the air flow, nasal obstruction and sleep apnoea, some dynamic theories of physics should be explained. Several mechanisms have been proposed to explain the role of the nose in the pathophysiology of OSAS and to clarify the relationship between air flow and nasal breathing during sleep. Such mechanisms include the so-called "Starling resistor model", the instability of the mouth breathing, nasal breathing reflex and the role of nitric oxide (NO) 24.
According to the model of Starling, the function of the upper airway can be represented as a hollow tube with a constriction in the vicinity of the entrance hole, which corresponds to the nostrils, and a posterior segment of folding, which corresponds to the oropharynx. This model predicts that the presence of an additional obstructive factor upstream (nose) generates a suction force and a negative intraluminal pressure downstream (oropharynx), with consequent collapse of the pharynx in predisposed individuals.
The closure of the mouth and correct dental occlusion stabilise the flow in the upper airway. When nasal resistance exceeds a certain level, there is a sort of air by-pass which leads to an oral breathing, responsible for a narrowing of the retrolingual space because of the retraction of the tongue, narrowing of the pharyngeal lumen, and an increase of the oscillations and vibration of the soft palate and the tissues surrounding the pharynx. This passage from nasal to oral breathing is physiologically disadvantageous for the individual, leading to an unstable breathing pattern 25.
A third factor is the nasal respiratory reflex. The experimental application of local anaesthetics in the nasal mucosa of healthy patients leads to a significant increase in obstructive and central apnoea, of the same magnitude as those reported in presence of complete nasal obstruction. Similar results from other experiments have confirmed that activation of the nasal receptor during nasal breathing has a direct positive effect on spontaneous ventilation, leading to greater respiratory rate at rest and minute ventilation. Oral breathing reduces the activation of these nasal receptors, with deactivation of the nasal respiratory reflex and reduction of spontaneous ventilation, which can trigger respiratory events in susceptible individuals with subclinical OSAS or aggravate episodes of apnoea.
Finally, nitric oxide (NO) appears to play a role in maintaining the patency of the upper airways, as a transmitter between the nose, pharyngeal muscles and lungs. NO is produced in significant amounts in the nose and paranasal sinuses and it has been shown (including in clinical practice) that is a potent pulmonary vasodilator, improving oxygenation, ventilation and perfusion. Since the total amount of inspired NO varies according to the nasal flow, it seems logical that a decrease in nasal breathing would result in the reduced transport of NO to the lungs and a reduction in blood oxygenation. NO also plays a role in maintaining muscle tone, in the regulation of neuromuscular pathways of the pharyngeal muscles, spontaneous breathing and in the regulation of sleep. In general, the role of NO in the regulation of nasal function in OSAS, although probably not significant, has not been completely understood 24.
Conclusions
Several studies in the literature have underlined the importance of oxidative stress in the pathogenesis of OSAS; our study, in particular, examined specific biomarkers of oxidative stress in order to define their role and clinical significance in patients with nasal respiratory failure. The levels of these biomarkers were higher in patients compared to the control group, although these differences, as mentioned, were not statistically significant. In light of these results, and considering that the study protocol excluded persons suffering from conditions that can increase oxidative stress or otherwise exposed to factors, such as smoking or particular environmental irritants, which may cause oxidative stress, the increase of those biomarkers in these individuals is probably associated with the underlying condition of nasal respiratory impairment; this shows how the nose, in addition to a mechanical commitment, can be functionally involved in the pathogenesis of these conditions, through the production of biomarkers of oxidative stress that can support an inflammatory state, at first regional and subsequently systemic. The very term nasal respiratory impairment aims to emphasise the wider and systemic importance of the nose in the genesis of oxidative stress damage, a condition that is known to be involved in long-term systemic damage related to OSAS. The common underlying pathological changes between OSAS and subjects with only nasal respiratory impairment may indicate the nose as a key factor in OSAS development or even identify a new category in OSAS syndrome (nasal-OSAS).
Our study may be useful to differentiate patients with mild OSAS from those with moderate or severe disease. It would thus be of value to increase the cohort size with the aim to clarify the role of these biomarkers even in diagnosis with the intent to limit, at least in some patients, the use of polysomnography, which is an expensive and laborious examination, for assessment of disease severity and evaluation of treatment response.
References
- 1.Staevska MT, Mandajieva MA, Dimitrov VD. Rhinitis and sleep apena. Currt Allerg Asthma Rep. 2004;4:193–199. doi: 10.1007/s11882-004-0026-0. [DOI] [PubMed] [Google Scholar]
- 2.Jennum P, Riha RL. Epidemiology of spleep apnoea/hypopnea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33:904–914. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 3.McNicholas WT. Diagnosis of obstructive sleep apnea in adults. Proc Am Thorac Soc. 2008;5:154–160. doi: 10.1513/pats.200708-118MG. [DOI] [PubMed] [Google Scholar]
- 4.Corso E, Bastanza G, Della Marca G, et al. Drug-induced sleep endoscopy as a selection tool for mandibular advancement therapy by oral device in patients with mild to moderate obstructive sleep apnoea. Acta Otorhinolaryngol Ital. 2015;35:426–432. doi: 10.14639/0392-100X-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corso E, Fiorita A, Rizzotto G, et al. The role of druginduced sleep endoscopy in the diagnosis and management of obstructive sleep apnoea syndrome: our personal experience. Acta Otorhinolaryngol Ital. 2013;33:405–413. [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz AR, Patil SP, Laffan AM, et al. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;15:185–192. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushida CA, Chediak A, Berry RB, et al. Guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4158 [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon P, Sanders MH. Positive airway pressure therapy for obstructive spleep apnea/hypopnea syndrome. Thorax. 2000;60:68–75. doi: 10.1136/thx.2003.007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics. 2012;9:710–716. doi: 10.1007/s13311-012-0141-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bican A, Kahraman A, Bora I, et al. What is the efficacy of nasal surgery in patients with obstructive sleep apnea syndrome. J Craniofac Surg. 2010;21:1801–1806. doi: 10.1097/SCS.0b013e3181f40551. [DOI] [PubMed] [Google Scholar]
- 11.Salamanca F, Costantini F, Mantovani M, et al. Barbed anterior pharyngoplasty: an evolution of anterior palatoplasty. Acta Otorhinolaryngol Ital. 2014;34:434–438. [PMC free article] [PubMed] [Google Scholar]
- 12.Scarano E, Della Marca G, Corso E, et al. Hyoid myotomy without suspension: a surgical approach to obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2014;34:362–367. [PMC free article] [PubMed] [Google Scholar]
- 13.Patil SP, Schneider H, Schwartz AR, et al. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132:325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remmers JE, Groot WJ, Sauerland EK, et al. Pathogenesys of upper airway occlusion during sleep. J Appl Physiol. 1979;46:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 15.King ED, O'Donnell CP, Smith PL, et al. A model of obstructive sleep apnea in normal humans. Role of the upper airway. Am J Respir Crit Care Med. 2000;161:1979–1984. doi: 10.1164/ajrccm.161.6.9904096. [DOI] [PubMed] [Google Scholar]
- 16.Shelton DM, Eiser NM. Evaluation of active anterior and posterior rhinomanometry in normal subjects. Clin Otolaryngol Allied Sci. 1992;17:178–182. doi: 10.1111/j.1365-2273.1992.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 17.Shouman BO, Mesbah A, Aly H. Iron metabolism and lipid peroxidation products in infants with hypoxic ischemic encephalopathy. J Perinatol. 2008;28:487–491. doi: 10.1038/jp.2008.22. [DOI] [PubMed] [Google Scholar]
- 18.Shadid M, Buonocore G, Groenendaal F, et al. Effect of deferoxamine and allopurinol on non-protein-bound iron concentrations in plasma and cortical brain tissue of newborn lambs following hypoxia-ischemia. Neurosci Lett. 1998;248:5–8. doi: 10.1016/s0304-3940(98)00303-6. [DOI] [PubMed] [Google Scholar]
- 19.Yin H, Gao L, Tai HH, et al. Urinary prostaglandin F2alpha is generated from the isoprostane pathway and not the cyclooxygenase in humans. J Biol Chem. 2007;282:329–336. doi: 10.1074/jbc.M608975200. [DOI] [PubMed] [Google Scholar]
- 20.Passali FM, Bellussi L, Mazzone S, et al. Predictive role of nasal functionality tests in the evaluation of patients before nocturnal polysomnographic recording. Acta Otorhinolaryngol Ital. 2011;31:103–108. [PMC free article] [PubMed] [Google Scholar]
- 21.Toraldo DM, Nuccio F, Benedetto M, et al. Obstructive sleep apnoea syndrome: a new paradigm by chronic nocturnal intermittent hypoxia and sleep disruption. Acta Otorhinolaryngol Ital. 2015;35:69–74. [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa Michels D, Mota Silveira Rodrigues A, Nakanishi M, et al. Nasal involvement in obstructive sleep apnea syndrome. Int J Otolaryngol. 2014;717419 doi: 10.1155/2014/717419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passàli D, Tatti P, Toraldo M, et al. OSAS and metabolic diseases: Round Table, 99(th) SIO National Congress, Bari 2012. Acta Otorhinolaryngol Ital. 2014;34:158–166. [PMC free article] [PubMed] [Google Scholar]
- 24.Passali D, Corallo G, Yaremchuk S, et al. Oxidative stress in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2015;35:420–425. doi: 10.14639/0392-100X-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brevi B, Blasio A, Blasio C, et al. Which cephalometric analysis for maxillo-mandibular surgery in patients with obstructive sleep apnoea syndrome? Acta Otorhinolaryngol Ital. 2015;35:332–337. doi: 10.14639/0392-100X-415. [DOI] [PMC free article] [PubMed] [Google Scholar]


