Abstract
IMPORTANCE
New methods are needed to quantify the change in the outer retinal structures in retinitis pigmentosa (RP).
OBJECTIVE
To implement an alternate method for tracking ellipsoid zone (EZ) changes in RP by quantifying the EZ area on en face spectral domain–optical coherence tomographic (SD-OCT) images.
DESIGN, SETTING, AND PARTICIPANTS
Data for this observational case study were collected at the Department of Ophthalmology, University of California, Los Angeles, from May 1 to July 30, 2015, and included SD-OCT images of a subset of patients from the Trial of Oral Valproic Acid for Retinitis Pigmentosa. To be eligible for the en face OCT subanalysis, the preserved EZ area was required to be limited to the SD-OCT scanning field. Cases in which the EZ band extended to the margins of any B-scan or the most superior or inferior B-scan were excluded. The SD-OCT images of all included cases were imported into the manufacturer’s software to generate en face images at the level of the EZ. Two certified SD-OCT graders independently delineated the boundaries of the preserved EZ on the en face images.
MAIN OUTCOMES AND MEASURES
Comparison of the 2 masked gradings of the generated en face images of patients with RP for agreement between the graders and the validity of the method.
RESULTS
Of the 43 available patients with volume SD-OCT data, 45 eyes of 24 patients met the eligibility criteria and were included in this subanalysis. Every patient had 2 visits that were 1 year apart, which included a total of 90 en face OCT images that were graded. The mean (SD) absolute difference and percentage difference between the 2 independent graders for each visit were 0.08 (0.10) mm2 and 4.5% (5.9%), respectively. The EZ area determined by the 2 graders showed excellent agreement with an intraclass correlation coefficient of 0.996 (95% CI, 0.995–0.997; P < .001).
CONCLUSIONS AND RELEVANCE
Quantification of the preserved EZ area on en face SD-OCT images of patients with RP is a valid and reproducible method. En face SD-OCT quantification may be a useful tool for monitoring the EZ changes of patients with advanced RP and a useful outcome measurement variable in therapeutic trials.
The term retinitis pigmentosa (RP) is defined as a group of hereditary disorders that diffusely involve the outer retina, with initial involvement more severe in the rods than the cones. In these disorders, a progressive but slow loss of photoreceptor cells starts from the retinal periphery and expands centripetally. As a result of this particular pattern of disease progression, patients’ visual fields constrict progressively, and after many years, patients lose their central vision owing to the depletion of the foveal photo receptor cells.1
In recent years, new therapeutic approaches have been considered for RP.2 Because of the relatively slow deterioration of the visual function in this disease,3 detecting visual function changes during a typical clinical trial presents a challenge. Thus, as a surrogate measure for the disease status, investigators have attempted to quantify the change in the outer retinal structures in patients with RP. One of the most useful tools for quantifying these changes is spectral domain–optical coherence tomography (SD-OCT), which allows the various layers of the outer retina to be distinctly visualized.4
Many previous studies using SD-OCT have demonstrated a progressive reduction in the total thickness of various outer retinal structures owing to RP,5,6 which was correlated with the loss of visual field sensitivity in these patients.7,8 In 2013, Birch et al9 evaluated the progression rate of RP by serial measurements of the ellipsoid zone (EZ) band width using SD-OCT. They also showed that the mean rate of decline in the EZ band width (7%) translates into a mean rate of change of 13% for the equivalent area of functioning retina. They noted that this rate of change is consistent with the rates of decline reported for visual fields and full-field electroretinograms. Unlike visual fields and electroretinograms, however, the repeated variability for EZ band width is less than the annual rate of change. Thus, the EZ band width could serve as a reliable remeasurement tool to monitor progression over a time frame practical for a therapeutic clinical trial. However, measurement of the EZ band width only samples a small portion of the region of preserved photoreceptors. Ideally, one would measure the entire area of preserved EZ, because that could further reduce the noise in the measure and improve repeatability. This result is particularly important if rates of EZ changes in different meridians vary. Ramachandran et al10 segmented the EZ band on every SD-OCT B-scan to produce such a comprehensive EZ contour map by interpolating between the EZ edge points throughout the volume scan. Although this method is very precise, it may be time-consuming (particularly if segmentation errors from an automated algorithm require correction) and may not be cost-effective or feasible in the context of large clinical trials. In the present study, we take advantage of an en face SD-OCT approach to quantify more efficiently and reliably the EZ area in patients with RP and demonstrate its use in defining EZ changes over time.
Methods
Description of the Cohort
The Trial of Oral Valproic Acid for Retinitis Pigmentosa11 is a phase 2 multiple-site, randomized, placebo-controlled clinical trial of the safety and efficacy of valproic acid for autosomal dominant RP. Ninety patients with RP who were enrolled in this study underwent physical examination, perimetry, electroretinography, color fundus photography, and SD-OCT imaging at multiple time points. From June 1, 2013, to May 31, 2014, the Doheny Image Reading Center received the data from this ongoing study. Forty-three of the 90 patients had completed the study term during this time frame and had SD-OCT images available at baseline and the week 52 visit. This subset of patients underwent evaluation in our study from May 1 to July 30, 2015. The research protocol was approved by the Institutional Review Board of UCLA (the University of California, Los Angeles), and the research adhered to the tenets set forth in the Declaration of Helsinki.12 All patients provided written informed consent for the original trial.11
Key Points.
Question
Can changes in the ellipsoid zone (EZ) on spectral domain–optical coherence tomographic (SD-OCT) images track progression of retinitis pigmentosa (RP)?
Findings
In this case series, the mean (SD) absolute difference and percentage difference between the 2 graders who delineated the boundaries of the preserved EZ on en face SD-OCT images were 0.08 (0.10) mm2 and 4.5% (5.9%), respectively. The EZ area showed excellent agreement between graders with an intraclass correlation coefficient of 0.996.
Meaning
Quantifying the preserved EZ area on en face SD-OCT images of patients with RP appears to be a reproducible method for tracking the EZ changes.
The SD-OCT scans were obtained in both eyes using a single device (Spectralis OCT; Heidelberg Engineering). These scans were captured in the high-speed mode using a 20° × 20° protocol (approximately 6 × 6-mm scan) centered on the fovea. Raster scans consisted of 97 horizontal B-scans, each containing 512 vertical A-scans.
Grading Protocol
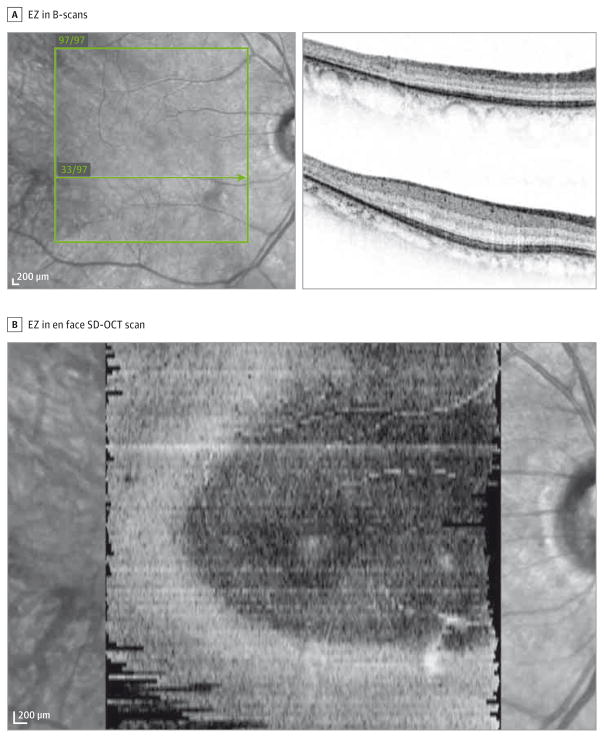
Because the true EZ area can only be quantified if the full extent of the EZ falls within the scanning window, all volume scans were initially inspected to identify the full extent of the EZ. If the EZ was observed to extend to the nasal or temporal border of any B-scan, or if the EZ was visible on the most inferior or superior B-scan at the baseline visit, the eye was excluded (Figure 1).
Figure 1. Ellipsoid Zone (EZ) of an Ineligible Eye.
A, The EZ is discernible in the uppermost B-scan (B-scan 97/97 on upper right) and the nasal end of B-scan 33/97 (lower right). B, In the resultant en face spectral domain–optical coherence tomographic image, the preserved EZ extends beyond the scanned area.
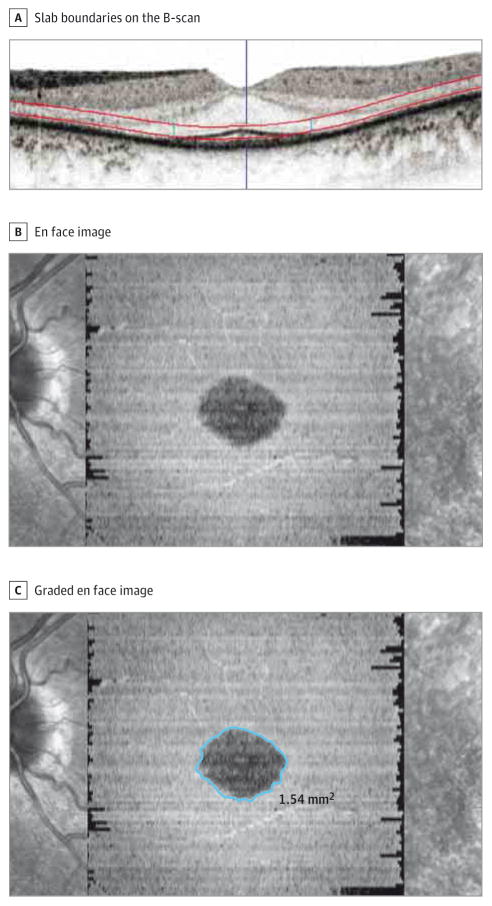
The SD-OCT images of all included patients were imported into the OCT manufacturer’s software (Heidelberg Eye Explorer, version 1.9.10.0; Heidelberg Engineering). For every case, after generating the 3-dimensional view, a transverse display was selected. To generate the en face SD-OCT image of the EZ, the segmentation line following the contour of the boundary of the retinal pigment epithelium (RPE) and Bruch membrane was considered to be the reference structure from which the offsets for the en face slab were selected. First, this line was displaced to 5 μm above the inner RPE boundary to define the outer boundary of the slab. Then, the line was placed on the external limiting membrane to define the inner boundary of the slab (Figure 2A). To achieve the highest contrast between the preserved EZ and the surrounding area, the minimum intensity projection on a black-and-white color table was selected. With this setting, the preserved EZ area is seen as a dark region against a brighter background (Figure 2B).
Figure 2. Generation of Graded en Face Spectral Domain–Optical Coherence Tomographic (SD-OCT) Image.
A, Red lines on the B-scan illustrate the slab boundaries used to generate the en face SD-OCT image. B, En face SD-OCT image. C, Graded en face image using the “draw region” tool in the SD-OCT manufacturer’s software. The exact location of the drawn boundary on all corresponding B-scans is discernible (2 small vertical blue lines within the slab in part A).
Two certified OCT graders (A.H.H. and H.Y.Z.) independently generated en face images and manually delineated the boundaries of the preserved EZ on every en face SD-OCT image using the “draw region” tool in the software. When using that option, the exact location of the drawn boundary on all of the corresponding B-scans is also visible to the grader (Figure 2C). Once the preserved EZ region has been drawn, the software provides a quantitative area result in square millimeters. To compare this alternate method and the current method of quantifying preserved EZ in patients with RP, maximum EZ band width of all the eligible eyes measured at our reading center during the main study was retrieved from the archive, and the correlation of this variable and the EZ area was calculated.
Statistical Analysis
We compared the results of the 2 independent graders. Intergrader reproducibility was assessed with the intraclass correlation coefficient (ICC). A higher ICC indicates better reproducibility of the variable. We also generated Bland-Altman plots13 to show the agreement between the graders. We calculated the correlation between the EZ area and maximum EZ band width using the Pearson correlation coefficient. Statistical analyses of the data were performed using SPSS (version 19.0; SPSS, Inc) and MedCalc (version 12; MedCalc Software bvba) software. Unless otherwise indicated, data are expressed as mean (SD).
Results
Because the present study is an SD-OCT substudy of the Trial of Oral Valproic Acid for Retinitis Pigmentosa, the Doheny Image Reading Center received SD-OCT images obtained at baseline and week 52 from 43 of the 90 enrolled patients. Forty-five eyes from 24 patients in this subset met the eligibility criteria for grading the EZ area from en face SD-OCT images and were included in our analysis.
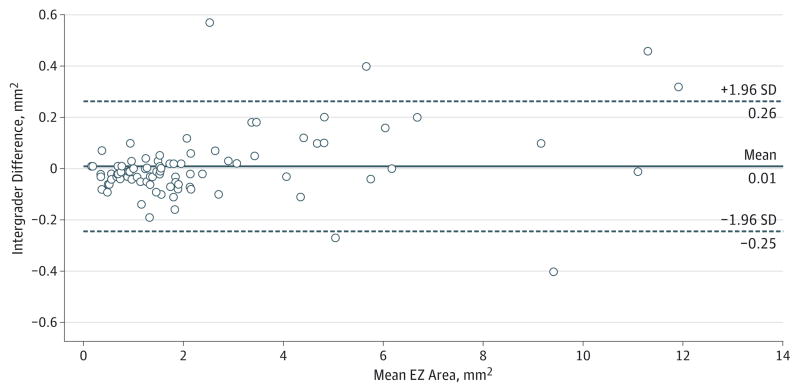
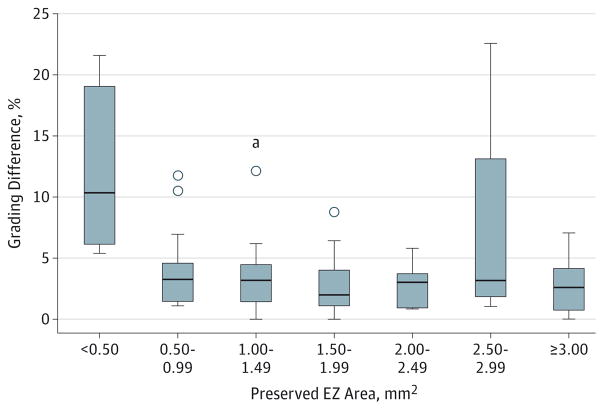
The mean EZ areas measured by the 2 independent graders were 2.54 (2.67) and 2.53 (2.63) mm2. The mean absolute difference and percentage difference between these 2 gradings were 0.08 (0.10) mm2 and 4.5% (5.9%), respectively. The EZ area measurements showed excellent agreement with an ICC of 0.996 (95% CI, 0.995–0.997; P < .001). The coefficient of variation between these 2 grading sessions was 0.04, and the mean repeatability statistic was 0.01 (0.13) mm2. The level of agreement is also illustrated in the Bland-Altman plot in Figure 3. We found a slight suggestion of larger absolute differences between the graders in larger preserved EZ areas, but relatively few cases had areas greater than 4 mm2 of preserved EZ. To evaluate the effect of the size of the preserved EZ area on the percentage difference between the graders, all included eyes were segregated into groups based on the mean of the 2 different grades (Figure 4). When the EZ area was smaller than 0.5 mm2, the percentage difference between gradings was much higher. Furthermore, after applying square root transformation,14 the mean absolute difference between the graders decreased to 2.3% (3.0% [range, 0%–11.3%]).
Figure 3. Bland-Altman13 Plot of Intergrader Reproducibility.
Comparison of the measurement of the ellipsoid zone (EZ) area on en face spectral domain–optical coherence tomographic images between the 2 graders is shown. The mean preserved EZ area is calculated from measurements by both graders. Data points indicate individual images (45 eyes).
Figure 4. Effect of the Size of the Preserved Ellipsoid Zone (EZ) Area on the Percentage Difference Between the Graders.
Box and whisker plot illustrates the effect of the size of the preserved EZ area on the percentage difference between the 2 graders using the drawn boundary method. Horizontal lines indicate 25th, 50th, and 75th percentiles; boxes, amounts that are between 25th and 75th percentiles; whiskers connect 25th percentile and 75th percentile to minimum and maximum amount of preserved EZ within every group; and points beyond the whiskers, outliers.
aIndicates an extreme outlier that is 3 interquartile ranges above the 75th percentile of the box.
The correlation coefficient between maximum EZ width and EZ area was 0.956 (95% CI, 0.935–0.970; P < .001).
We evaluated longitudinal changes in the EZ area by using the mean results of the 2 graders. The mean EZ area at the baseline visit was 2.67 (2.67) mm2, which decreased to 2.40 (2.62) mm2 at week 52. This translates to a reduction of 13% in EZ area during the 1-year span of our study.
Discussion
In this study, we evaluated a method to measure the EZ area from en face SD-OCT images obtained from the eyes of patients with autosomal dominant RP. Using our approach, we observed good agreement between graders for measurement of the EZ area with a mean absolute difference of 0.08 mm2 (4.5%).
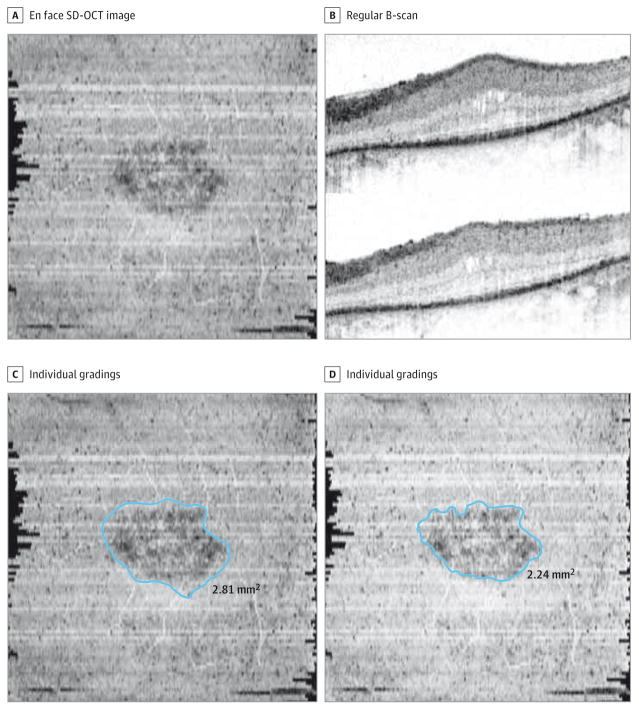
During post hoc review of cases with a mean percentage difference between the 2 gradings of more than 4.5%, we observed that the main reason for the discrepancy between the 2 graders was the clarity of the demarcation of the boundary of the preserved EZ area. Figure 5 illustrates anoutlier case with a percentage difference of 23% (this case is the result of the mistake of one of the graders instead of being a legitimate outlier). In inspecting the corresponding B-scans at these poorly demarcated boundaries, the principal cause appeared to be the presence of attenuated or dying photoreceptor cells, which manifest as a thinner and less bright band close to the level of the EZ. Although we placed the lower boundary of the en face slab 5 μm above the RPE inner boundary, we presumed that attenuated or dying photoreceptor cells would still be included within the slab and would be evident as a slightly less dark (but still darker than background) region in the resultant en face SD-OCT image, thus making the assessment of the border of the preserved EZ more challenging in these areas. Inspection of the corresponding B-scans in these poorly demarcated regions may be of help in refining the final boundary position (Figure 2). However, these areas of attenuated EZ at the margin may be difficult to segment, even on the individual B-scans. Other possibilities that can interfere with generating a high-quality en face image and cause difficulty in the graders’ assessment include (but are not limited to) any unusual breakup in the EZ that can generate a saw tooth pattern, local dimness of the EZ band, and regional irregularities where the EZ meets the RPE band.
Figure 5. An Eye With a High Percentage Difference Between the Graders.
The difference between the graders was 23%. A and B, The precise boundaries of the preserved ellipsoid zone (EZ) band are difficult to discern on the en face spectral domain–optical coherence tomographic (SD-OCT) image and regular B-scans. C and D, The individual gradings are illustrated side by side. One of the graders who measured the preserved EZ area as 2.81 mm2 was not very precise in the grading; the other grader who measured the area as 2.24 mm2 was more meticulous.
In addition, we found a slight tendency for larger absolute differences between graders for larger preserved EZ areas. This tendency among EZ areas is very similar to the dependence of the geographic atrophy growth rate on baseline lesion size, which may be addressed in part by applying a square root transformation.14
Sujirakul et al15 observed a significantly lower mean rate of structural progression in patients with RP who had a smaller preserved EZ band, which suggests that the progression rate decreases as the disease approaches the fovea. We observed a tendency toward higher variability in grading of the EZ area in eyes with a smaller preserved EZ area in our study. These findings suggest that, very similar to the geographic atrophy trials,16,17 a size limit for preserved EZ should be considered when enrolling patients for RP trials.
The en face SD-OCT approach described in this study depends on having a reliable segmentation of the boundary between the RPE and the Bruch’s membrane, because this is the reference structure from which the offsets for the en face slab are selected. We inspected the segmentation in all of our cases before proceeding to EZ area grading. Although we did not observe automated segmentation errors of the RPE–Bruch’s membrane boundary in our study, the errors could occur, particularly in other retinal degenerations, which may also feature more severe RPE disruption. Such disruption would require manual correction of the boundary, which would increase the time needed for EZ area measurement. In such cases or in diseases requiring extensive manual correction, one could consider measurement of EZ width on a single central B-scan as a potential surrogate.
The use of the EZ bandwidth from a single B-scan has been reported by Birch and colleagues.9 In their study of eyes with X-linked RP, they also observed good repeatability of EZ band width grading with mean (SD) test-retest differences in EZ width of 0.08° (0.22° [range, –0.30° to 0.60°]). The EZ area and maximum EZ band width obtained from the 2 different grading sessions of our cases were highly correlated (0.956). The EZ area, however, has some advantages over the EZ band width because it provides a more complete assessment of the total EZ in all meridians and uses more of the information available in the SD-OCT images. This advantage may be of particular importance for longitudinal evaluation of cases, which may show different rates of contraction of the EZ in different meridians. Another advantage of sampling the entire EZ rather than a single B-scan is that the metric may be more resilient to grading errors or difficulty in visualizing the EZ boundary in a particular meridian. Finally, measuring an EZ area rather than a width may better facilitate structure-function correlations because the preserved EZ area may be compared more easily with the preserved island of vision from perimetric assessments. As such, the EZ area offers important clinically relevant benefits compared with the maximum EZ band width. Moreover, with the inclusion of en face analysis tools in many commercial SD-OCT systems, the EZ area has the potential to be obtained rapidly by the health care professional treating these patients, even in the context of a busy clinical practice.
The most important value of EZ assessments is the longitudinal assessment of progression in eyes with RP. In their study of patients with X-linked RP, Birch and colleagues9 demonstrated a mean annual decrease in EZ band width of 7%, which they extrapolated to yield a 13% mean annual rate of change for the equivalent area of the EZ. In our study, longitudinal evaluation of en face SD-OCT images showed a similar rate of reduction in the EZ area during the 1-year span of the study. However, our substudy is part of a larger ongoing therapeutic trial, the reading center is masked to the treatment assignment of the patients, and thus the rates of EZ changes observed in our analysis should not be considered as the natural history of the disease. Nonetheless, the rate of change over time is important because it sets the benchmark for the level of repeatability in EZ measurement that is required for the use of this metric in the longitudinal assessment of eyes with RP. A major challenge for the use of perimetry and electroretinography to measure RP progression is the considerable retest variability, which requires a long period of evaluation to be confident that a true change has occurred,18 something that may not be feasible in the context of a therapeutic trial. In our study, we observed a repeated mean variability in EZ measurement of 0.01 (0.13) mm2 that was considerably less than the 0.27-mm2 change in the EZ area during the 1-year span of our study.
Our study has several limitations that should also be considered when assessing our findings. First, this study used SD-OCT data from a therapeutic trial, and dense-volume SD-OCT data were not available for all cases in the trial. Thus, our findings may be subject to a selection bias. Moreover, even among the eyes with SD-OCT scans that were submitted to the reading center, only 45 of 86 OCT scans (52%) met the criteria for inclusion in our study; namely, the border of preserved EZ was required to fall within the SD-OCT scan area to yield a quantifiable result. Including cases with preserved EZ extending to the margin of the scan area would lead to an underestimate of the preserved EZ, which would confound longitudinal assessments. Thus, our method may not be feasible for trials that enroll patients with less advanced RP and more extensive preservation of photoreceptors. On the other hand, newer OCT instruments (especially swept source devices), which rapidly scan larger regions, may address this problem. In particular, 55° field lenses are now available to increase the size of the scanned area by the OCT instruments. Another interval solution is to acquire multiple steered OCT volumes, which can then be montaged19 to yield a larger en face OCT image for grading. Our method would not be applicable for patients with severe RP and no remaining clearly visible EZ. Another limitation of our approach is that it requires dense-volume OCT for the instrument software to produce en face SD-OCT images. For patients with poor fixation, acquisition of dense scans may be difficult, although generally not an issue for patients with RP. This issue may also be addressed by faster scanning devices in the future, and motion tracking might help. A final limitation of our study is the lack of correlation with functional data. In the future, perimetric data from the trial should become available and allow this correlation.
Our study and approach also have several strengths, including the use of trained, certified reading center graders and demonstration of a high level of reproducibility. The method described in this report, however, can be learned very easily, which further facilitates its ultimate translation in toother studies and clinical practice. Unlike tedious manual segmentation of the EZ in multiple individual B-scans, this en face SD-OCT approach allows rapid delineation of the entire area of preserved EZ.
Conclusions
The measurement of the EZ area by en face SD-OCT is a reproducible metric that can be studied and tracked longitudinally in RP. This metric may be a useful tool for monitoring EZ changes and assessing the efficacy of new therapeutic agents for patients with degenerations of the outer retinal structures and photoreceptors.
Acknowledgments
Funding/Support: The Trial for Oral Valproic Acid for Retinitis Pigmentosa was supported by the Foundation Fighting Blindness Clinical Research Institute, which also sponsored the ellipsoid zone area analysis described in this report.
Role of the Funder/Sponsor: The funding source actively participated in the design and conduct of this analysis and interpretation of the data and had a limited role in preparation of the manuscript. The funding source was not involved in the review and approval of the manuscript and decision to submit the manuscript for publication.
Footnotes
Group Information: The principal investigators for the Trial of Oral Valproic Acid for Retinitis Pigmentosa Group include Paul S. Bernstein, MD, PhD, Moran Eye Center University of Utah, Salt Lake City; David Birch, PhD, Retina Foundation of the Southwest, Dallas, Texas; John Heckenlively, MD, W. K. Kellogg Eye Center, University of Michigan, Ann Arbor; Alessandro Iannaccone, MD, University of Tennessee Hamilton Eye Institute, Memphis; Byron Lam, MD, Bascom Palmer Eye Institute, University of Miami, Miami, Florida; Mark E. Pennesi, MD, PhD, Casey Eye Institute, Oregon Health & Science University, Portland; Judith Chiostri, MS, Foundation Fighting Blindness Clinical Research Institute, Columbia, Maryland; Laura R. Erker, PhD, and David J. Wilson, MD, Oregon Health & Science University, Casey Eye Institute Reading Center; and Jennifer B. McCormack, MS, The EMMES Corporation, Clinical Coordinating Center, Rockville, Maryland.
Author Contributions: Drs Hariri and Sadda had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hariri, Ho, Francis, Weleber, Sadda.
Acquisition, analysis, or interpretation of data: Hariri, Zhang, Ho, Weleber, Birch, Ferris, Sadda.
Drafting of the manuscript: Hariri, Sadda.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Hariri, Sadda.
Obtained funding: Birch, Sadda.
Administrative, technical, or material support: Hariri, Zhang, Ho, Francis, Birch, Ferris, Sadda.
Study supervision: Hariri, Sadda.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Francis reports receiving financial support from Pacific Ophthalmology Consulting, ReNeuron, Retro-Sense Therapeutics, and Sanofi and serving as a paid consultant for Sanofi. DrWeleber reports serving as a paid consultant for Novartis, Pfizer, and Wellstat, being a member of the scientific advisory boards of Applied Genetic Technologies Corp and the Foundation Fighting Blindness, receiving clinical trial support from Sanofi-Fovea (all outside the submitted work), and having US patent 8 657 446 (Method and Apparatus for Visual Field Monitoring, also known as Visual Field Monitoring and Analysis, or VFMA). Dr Birch reports serving as a consultant for and receiving financial support from AGTC, Acucela Inc, Shire Pharmaceuticals, ISIS/GSK, and QLT and serving as a contractor for and receiving financial support from Thrombogenics. Dr Sadda reports serving as a consultant for and receiving financial support from Carl Zeiss Meditec, Optos, Allergan, and Genentech and serving as a paid consultant for Alcon, Novartis, and Iconic. No other disclosures were reported.
References
- 1.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.Sahni JN, Angi M, Irigoyen C, Semeraro F, Romano MR, Parmeggiani F. Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Curr Genomics. 2011;12(4):276–284. doi: 10.2174/138920211795860062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holopigian K, Greenstein V, Seiple W, Carr RE. Rates of change differ among measures of visual function in patients with retinitis pigmentosa. Ophthalmology. 1996;103(3):398–405. doi: 10.1016/s0161-6420(96)30679-9. [DOI] [PubMed] [Google Scholar]
- 4.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31(8):1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(5):2328–2336. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witkin AJ, Ko TH, Fujimoto JG, et al. Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am J Ophthalmol. 2006;142(6):945–952. doi: 10.1016/j.ajo.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rangaswamy NV, Patel HM, Locke KG, Hood DC, Birch DG. A comparison of visual field sensitivity to photoreceptor thickness in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010;51(8):4213–4219. doi: 10.1167/iovs.09-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen Y, Klein M, Hood DC, Birch DG. Relationships among multifocal electroretinogram amplitude, visual field sensitivity, and SD-OCT receptor layer thicknesses in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2012;53(2):833–840. doi: 10.1167/iovs.11-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birch DG, Locke KG, Wen Y, Locke KI, Hoffman DR, Hood DC. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013;131(9):1143–1150. doi: 10.1001/jamaophthalmol.2013.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramachandran R, Zhou L, Locke KG, Birch DG, Hood DC. A comparison of methods for tracking progression in X-linked retinitis pigmentosa using frequency domain OCT. Transl Vis Sci Technol. 2013;2(7):5. doi: 10.1167/tvst.2.7.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ClinicalTrials.gov. [Accessed November 1, 2010];Trial of Oral Valproic Acid for Retinitis Pigmentosa. NCT01233609. https://clinicaltrials.gov/ct2/show/NCT01233609.
- 12.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 14.Feuer WJ, Yehoshua Z, Gregori G, et al. Square root transformation of geographic atrophy area measurements to eliminate dependence of growth rates on baseline lesion measurements: a reanalysis of Age-Related Eye Disease Study report No. 26. JAMA Ophthalmol. 2013;131(1):110–111. doi: 10.1001/jamaophthalmol.2013.572. [DOI] [PubMed] [Google Scholar]
- 15.Sujirakul T, Lin MK, Duong J, Wei Y, Lopez-Pintado S, Tsang SH. Multimodal imaging of central retinal disease progression in a 2-year mean follow-up of retinitis pigmentosa. Am J Ophthalmol. 2015;160(4):786–798. e4. doi: 10.1016/j.ajo.2015.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114(2):271–277. doi: 10.1016/j.ophtha.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunness JS, Applegate CA, Bressler NM, Hawkins BS. Designing clinical trials for age-related geographic atrophy of the macula: enrollment data from the Geographic Atrophy Natural History Study. Retina. 2007;27(2):204–210. doi: 10.1097/01.iae.0000248148.56560.b1. [DOI] [PubMed] [Google Scholar]
- 18.Berson EL, Sandberg MA, Rosner B, Birch DG, Hanson AH. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol. 1985;99(3):240–251. doi: 10.1016/0002-9394(85)90351-4. [DOI] [PubMed] [Google Scholar]
- 19.Mori K, Kanno J, Gehlbach PL. Retinochoroidal morphology described by wide-field montage imaging of spectral domain optical coherence tomography. Retina. 2016;36(2):375–384. doi: 10.1097/IAE.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]