Abstract
Prolonged hyperglycemia during diabetes mellitus can cause severe ophthalmic complications affecting both the anterior and posterior ocular segments leading to impaired vision or blindness. Diabetes-induced corneal pathologies are associated with decreased wound healing capacity, corneal edema, and altered epithelial basement membrane. The mechanism by which diabetes modulates structure and function within the corneal stroma are unknown. In our study, we characterized the effects of diabetes on extracellular matrix, lipid transport, and cellular metabolism by defining the entire metabolome and lipidome of Type 1 and Type 2 human diabetic corneal stroma. Significant increases in Collagen I and III were found in diabetic corneas suggesting that diabetes promotes defects in matrix structure leading to scarring. Furthermore, increased lipid content, including sphingosine-1-phosphate and dihydrosphingosine, in diabetic corneas compared to healthy controls were measured suggesting altered lipid retention. Metabolomics analysis identified elevated tryptophan metabolites, independent of glucose metabolism, which correlated with upregulation of the Kynurenine pathway in diabetic corneas. We also found significant upregulation of novel biomarkers aminoadipic acid, D,L-pipecolic acid, and dihydroorotate. Our study links aberrant tryptophan metabolism to end-stage pathologies associated with diabetes indicating the potential of the Kynurenine pathway as a therapeutic target for inhibiting diabetes-associated defects in the eye.
Keywords: diabetes mellitus, corneal fibrosis, kynurenine, cellular metabolism, lipidomics
1. INTRODUCTION
Roughly 371 million people worldwide currently have diabetes mellitus (Organization). By 2030, this population is predicted to rise to over 500 million people affected by this metabolic disease (Whiting et al., 2011). The incidence of diabetes mellitus has doubled over the last three decades (Danaei et al., 2011) due to elevated caloric intake and prevalence of obesity (Forouhi and Wareham, 2014). This steep growth of the diabetic population has led to a significant increase in the clinical burden and demand for effective treatments for diabetes-induced complications. Diabetes mellitus classification is further divided into Type 1 and Type 2 diabetes based on the pathophysiology of disease onset and progression. Type 1 diabetes also known as “insulin-dependent” diabetes is caused by the autoimmune destruction of the β-cells in the pancreas, whereas Type 2 diabetes is linked to excessive elevation of blood glucose levels leading to insulin resistance (Alberti and Zimmet, 1998). Type 1 and Type 2 diabetes differ from each other based on their mechanism of development and physiological characteristics, including association to obesity, age of onset, and insulin-insufficiency (Taylor, 2013; Van Belle et al., 2011). However, both conditions are associated with systemic hyperglycemia leading to microvascular and macrovascular complications in a tissue-dependent response to elevated blood glucose levels during the onset and progression of the disease (Howard, 1987).
As a systemic disease, diabetes can cause multiple pathologies, such as neuropathy, nephropathy, inflammation, and oxidative stress leading to permanent organ damage (1993; Leppin et al., 2014; Liu et al., 2015). Ophthalmic complications, such as diabetic retinopathy, cataract development, corneal erosions, and corneal scarring affect over 80% of diabetic patients resulting in significant defects in visual acuity and even blindness (Klein and Klein, 1995). Damage to the corneal surface are common in diabetic patients and are often difficult to treat. The diabetic cornea is associated with epithelial defects due to reduced migration and proliferation of epithelial cells and stromal fibrosis linked to activation of keratocytes (Herse, 1988; Ljubimov et al., 1998; Rehany et al., 2000; Schultz et al., 1981). Reports have shown increased corneal thickness in diabetic patients compared to healthy controls (Goldich et al., 2009; Lee et al., 2005; Su et al., 2008). However, the long-term effects of diabetes on stromal collagen deposition are not well understood.
The extracellular matrix (ECM) within the corneal stroma is secreted and assembled by resident keratocytes. Functionality of keratocytes is influenced by the nutritional status and bioenergetics within the stroma. Metabolite and lipid composition plays a significant role in maintaining normal cellular function and any alterations in biomolecule flux can promote apoptosis, proliferation, or differentiation (Drucker, 2003; Vander Heiden et al., 2009). In tissues exposed to systemic blood flow, excess glucose is channeled into the polyol pathway with the generation of sorbitol at the expense of nicotinamide adenine dinucleotide hydride (NADH) (Brownlee, 2005). As an avascular tissue, the cornea relies on glucose provided by the aqueous humor and tear film in order to generate high-energy adenosine triphosphate (ATP) and NADH substrates (Pirie, 1960). Flux of macromolecules into the stroma from the aqueous humor is regulated by the corneal endothelial layer (Kumagai et al., 1994). Sugars, lipids, and amino acids uptake are essential for energy production, plasma membrane structure and lipid-mediated signaling, and protein synthesis, respectively. To-date, studies have concentrated on corneal epithelial defects and nerve deterioration in diabetics (Kabosova et al., 2003; Tavakoli et al., 2015; Xu et al., 2009). Our study, to the authors’ knowledge, is the first to characterize the flux of the major biomolecules in the human corneal stromal layer in order to determine if the defects in ECM deposition are related to dysregulation in cellular and lipid metabolism.
Amino acid flux directly modulates protein assembly during translation and has been reported to play an important role in a number of diseases, including chronic granulomatous disease, Alzheimer’s, and Huntington’s disease (Romani et al., 2008; Stoy et al., 2005; Widner et al., 2000). The metabolism of the aromatic amino acid, tryptophan, is regulated by the Kynurenine pathway and involves conversion to kynurenic acid and xanthurenic acid, which eventually generates nicotinate and nicotinamide adenine dinucleotide (NAD). Elevated tryptophan levels have been associated with streptozotocin-induced diabetes in rats (Mackenzie and Trulson, 1978). In our study, we sought to determine the role of glucose, lipid, and amino acid metabolism in the human diabetic corneal stroma that may contribute to development of fibrosis. We report here a novel role for the Kynurenine pathway and its potential as a marker for diabetes-induced stromal damage.
2. MATERIALS AND METHODS
2.1. Ethics and inclusion criteria
Institutional review board approval was received prior to initiation of experiments described in this study (#4509). All parts of the study met the tenets of the Declaration of Helsinki. Corneal samples were obtained from the National Development and Research Institute (NDRI) and the Oklahoma Lions Eye Bank. Inclusion criteria for the diabetic donors included clinical diagnosis of Type 1 or 2 diabetes and absence of other unrelated diseases or ocular pathology. The control group included corneas isolated from cadavers with no history of ocular trauma or systemic diseases (Table 1). The causes of death for the diabetic groups were considered to be diabetic-related complications (acute cerebral infarction, cerebrovascular accident, complications from end stage renal disease, respiratory failure) with the cause of death for healthy controls varying from accidental to non-diabetic related diseases (Blunt force trauma, head trauma, end stage renal disease, acute segment elevation myocardial infarction, subarachnoid hemorrhage, cardiac arrest). The average age for each group was as follows: (healthy) 64.25±5.53 years, (T1DM) 56.25±3.82 years, and (T2DM) 67.87±1.18 years and the duration of diabetes for all the diabetic donors was 10+ years.
Table 1.
Summary of donors’ age and gender, Group 1: Controls, Group 2: Type 1 diabetic mellitus patients (T1DM), and Group 3: Type 2 diabetic mellitus patients (T2DM).
| Patient (Serial No.) |
HEALTHY | Patient (Serial No.) |
T1DM | Patient (Serial No.) |
T2DM | |||
|---|---|---|---|---|---|---|---|---|
| Age (Years) |
Gender | Age (Years) |
Gender | Age (Years) |
Gender | |||
| 1 | 84 | F | 1 | 54 | F | 1 | 66 | F |
| 2 | 84 | F | 2 | 66 | F | 2 | 69 | F |
| 3 | 66 | F | 3 | 66 | F | 3 | 70 | F |
| 4 | 38 | M | 4 | 54 | M | 4 | 62 | M |
| 5 | 49 | M | 5 | 55 | M | 5 | 66 | M |
| 6 | 63 | M | 6 | 67 | M | 6 | 67 | M |
| 7 | 63 | M | 7 | 54 | n.d. | 7 | 70 | M |
| 8 | 67 | M | 8 | 34 | n.d. | 8 | 73 | M |
2.2. Tissue Processing
Corneal tissues were received within 24 hours of death on ice and preserved in optisol corneal storage medium. The corneal epithelium and endothelium were removed by scraping with a surgical scalpel with complete removal of scleral and limbal regions. Corneal stromal tissues were cut and divided for Western blot, metabolomics, and lipidomics analysis.
2.3. Western blot analysis
Tissue lysates were used for isolated western blot analysis. Briefly, preparation of tissue lysates was initiated by using RIPA buffer (50 mM Tris, pH 8, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate) (Abcam; Cambridge, MA) for extracting tissue lysates to which protease and phosphatase inhibitors (Sigma Aldrich; St. Louis, MO) were added. The tissue samples were homogenized with metal beads in the bullet blender homogenizer for 3 minutes and immediately replaced on ice to avoid protein denaturing followed by brief centrifugation and storage at −20°C until further usage. BCA assay (Thermo Scientific, IL) was initiated for total protein concentration and purity assessment. Equal amounts of proteins were loaded onto 4%–20% Tris-Glycine gels (Novex, Life technologies, Carlsbad, CA) for gel electrophoresis followed by protein transfer to a nitrocellulose membrane (Novex, Life Technologies) and further incubation in 5% BSA blocking solution (Thermo Scientific). The membranes were then incubated with primary antibodies: anti-Collagen I (ab34710; Abcam; Cambridge; MA), Collagen III (ab7778; Abcam; Cambridge; MA), Collagen V (ab94673; Abcam; Cambridge; MA), α-Smooth Muscle Actin (ab5694; Abcam; Cambridge; MA), IGF1(Cambridge; MA), 1GF1R(Abcam; Cambridge; MA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, ab9485; Abcam; Cambridge; MA). Antibodies were used at 1:1000 dilution in TBST overnight at 4 °C with rocking followed by washing of the membranes and incubation with a secondary antibody (Alexa Fluor® 568 Donkey anti-Rabbit, IgG [H+L], Abcam) at 1:2000 dilutions for 1 hr. UVP imaging system was used for band detection and quantification by densitometry. Net intensities were normalized to the loading control (GAPDH) and depicted as fold change.
2.4. Metabolite Extraction and Analysis
2.4.1. Sample preparation
Metabolites was isolated from corneal tissue as previously described (Webhofer et al., 2013). Briefly, samples were rinsed in 1xPBS and lysed with ice-cold 80% methanol, incubated on dry ice for 15 minutes, and homogenized to ensure complete cell lysis. Samples were then centrifuged (14,000 g, overnight, 4°C) and metabolites were isolated and stored at −80°C until further processing.
2.4.2. Mass spec processing
Samples were processed for targeted tandem mass spectrometry (LC-MS/MS), as previously described (Webhofer et al., 2013). Briefly, 5 µL were injected and analyzed using a hybrid 5500 QTRAP triple quadrupole mass spectrometer (AB/SCIEX) coupled to a Prominence UFLC system (Shimadzu) using an Amide HILIC column (Waters) and analyzed with selected reaction monitoring (SRM) with positive/negative polarity switching. Peak areas from the total ion current for 291 metabolites were obtained (Webhofer et al., 2013).
2.4.3. Data analysis
Metabolomics data was analyzed using CIMminer available from the genomics and bioinformatics group (http://discover.nci.nih.gov/cimminer/home.do) to generate a color-coded Clustered Image Map (CIM). Raw net intensity data for all metabolites present in at least n=3 were input into the freely available software to generate a one matrix CIM. Metabolites were clustered according to abundance (Weinstein et al., 1997).
2.5. Lipid Isolation
Tissue samples were processed as previously described (Priyadarsini et al., 2014). Briefly, after the initial processing of the cornea they were cut into small pieces of about 3×3mm size and transferred to Eppendorf tubes and stored at −80°C until further use. The extracted lipids were then analyzed using untargeted LC MS/MS methods using Shimadzu Nexera UPLC and a hybrid triple quadrupole linear ion trap (AB SCIEX 6500).
2.6. Statistical Analysis
Data was generated based on results from at least four different donors for each group, and repeated at least three times: Type 1 diabetes, Type 2 diabetes, and healthy controls with no history of ocular disease. Statistical analysis was conducted using Graph Pad Prism 6 and a oneway ANOVA was performed. P≤0.05 was considered statistically significant.
3. RESULTS
3.1. Fibrotic Markers in Diabetic Corneas
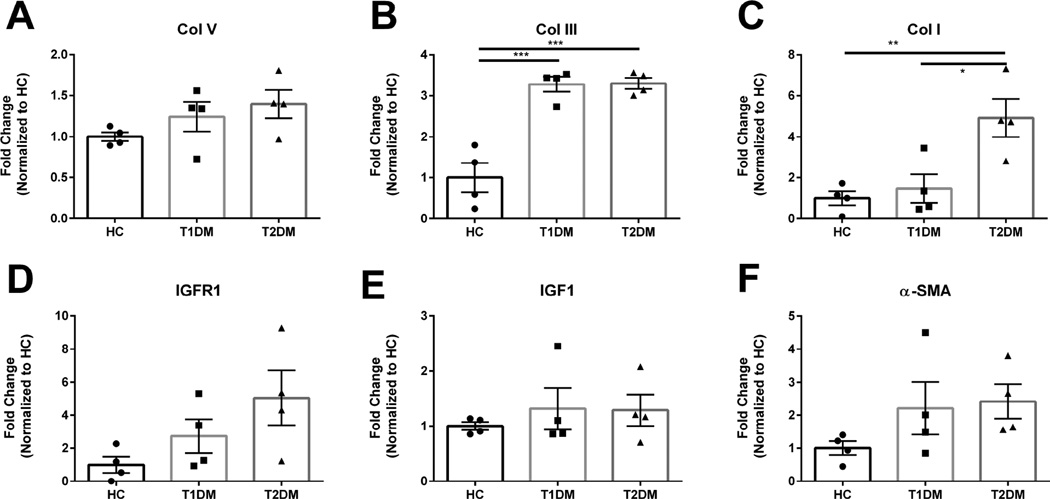
The structural integrity of the human cornea within the stroma is composed primarily of collagens, crystallins, and proteoglycans. Collagen I and V are the dominant collagen isoforms present in the cornea in a ratio of 5:1 with little Collagen III in the normal, uninjured stroma (Birk et al., 1988). Diabetic patients are known to have reduced healing capabilities in multiple tissues, including the cornea, with a predisposition to scarring and epithelial defects (Skarbez et al., 2010). In order to determine the effects of diabetes on ECM deposition in the cornea, we quantified the expression of Collagen I, III, and V in the human corneal stroma isolated from Type 1 and Type 2 diabetic patients (Figure 1). No significant difference in Collagen V expression was measured in normal, Type 1, and Type 2 corneas (Figure 1A). However, we found a significant increase in the collagen isoform associated with fibrosis, Collagen III, by 3.5–fold (p≤0.001) in both Type 1 and Type 2 diabetic corneal stroma compared to healthy controls (Figure 1B). Collagen I expression was also elevated 5-fold in Type 2 diabetic corneas compared to healthy controls (p≤0.01) and Type 1 diabetic corneas (p≤0.05) (Figure 1C). These results suggest that thickening of the cornea caused by diabetes may be due toelevated secretion of predominantly Collagen III in both Type 1 and Type 2 diabetes. Smooth muscle actin (SMA), a well-known marker for corneal myofibroblast presence was slightly upregulated in both Type I and Type II diabetes (Figure 1F), when compared to healthy controls, but did not reach significance. This result was not a surprise given that our corneal tissue samples are from end-stage diabetesand the transient nature of myofibroblast differentiation may have occurred during earlier stages.
Figure 1. Expression of Collagen I, III, V, and α-smooth muscle actin (α-SMA), insulin growth factor-1 (IGF-1) and insulin growth factor-1 Receptor (IGFR-1).
(A) Representative Western blots and (B–G) quantification of bands normalized to loading control. n≥4 for HC, T1DM, and T2DM corneal buttons. Error bars represent standard error of the mean. A one-way ANOVA was performed and P≤0.05 considered statistically significant. Each data point corresponds to individual patients.
Diabetes is known to reduce insulin growth factor-1 (IGF-1) expression in heart and vascular smooth muscle tissue (Bornfeldt et al., 1992). IGF-1 is also known to strongly associate with bound proteins resulting in a molecular weight of 115-155kDa (Ranke et al., 2007). Effects of Type 1 and Type 2 diabetes on IGF-1 and IGF-1 receptor (IGFR-1) expression within the cornea are not well understood. We found slight increases, though not significant, of IGFR-1 expression in both Type 1 and 2 diabetic corneas (Figure 1D). Moreover, we found no significant difference in expression of IGF1 (Figure 1E) which may be representative of the temporal regulation of IGF-1 based on nutritional or therapeutic status.
3.2. Glucose Metabolism in the Diabetic Cornea
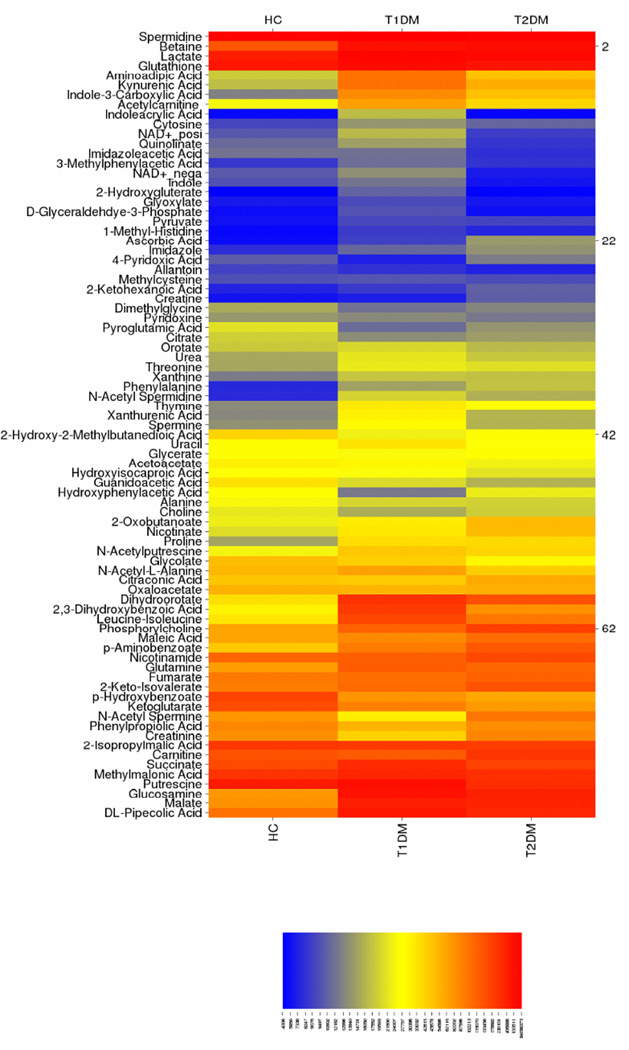
Having seen alterations in collagen expression in diabetic corneal stroma, we investigated whether these are mediated by alterations in cellular metabolism. Since the cornea is dependent on systemic flux of metabolites present in the aqueous humor and tear film, we hypothesized that diabetes promotes changes in bioenergetics within the cornea that may contribute to increased collagen expression. We used a shot-gun metabolomics approach to measure 291 metabolites with more than 82 metabolites detected in all samples. We generated a heat map from the net intensities in order to determine metabolites with substantial flux in Type 1 and Type 2 diabetic corneal stroma (Figure 2). Significantly regulated metabolites were associated with kynurenic and amino acid metabolism. Metabolites with the highest detected concentrations included glucosamine, pipecolic acid, spermidine, and betaine. Interestingly, metabolites associated with glycolysis, such as pyruvate and glyceraldehyde-3-phosphate, were present in low concentrations compared to lactate, malate, and succinate.
Figure 2. Heat map of metabolites isolated from corneal stromal tissue from healthy controls (HC), Type 1 (T1DM) and Type 2 (T2DM) diabetic mellitus patients.
Color scheme depicts metabolite present in high amounts (orange), intermediate amounts (yellow), and low amounts (blue). Metabolites at similar concentrations are grouped together along the y-axis. n≥3 for HC, T1DM, and T2DM corneal buttons.
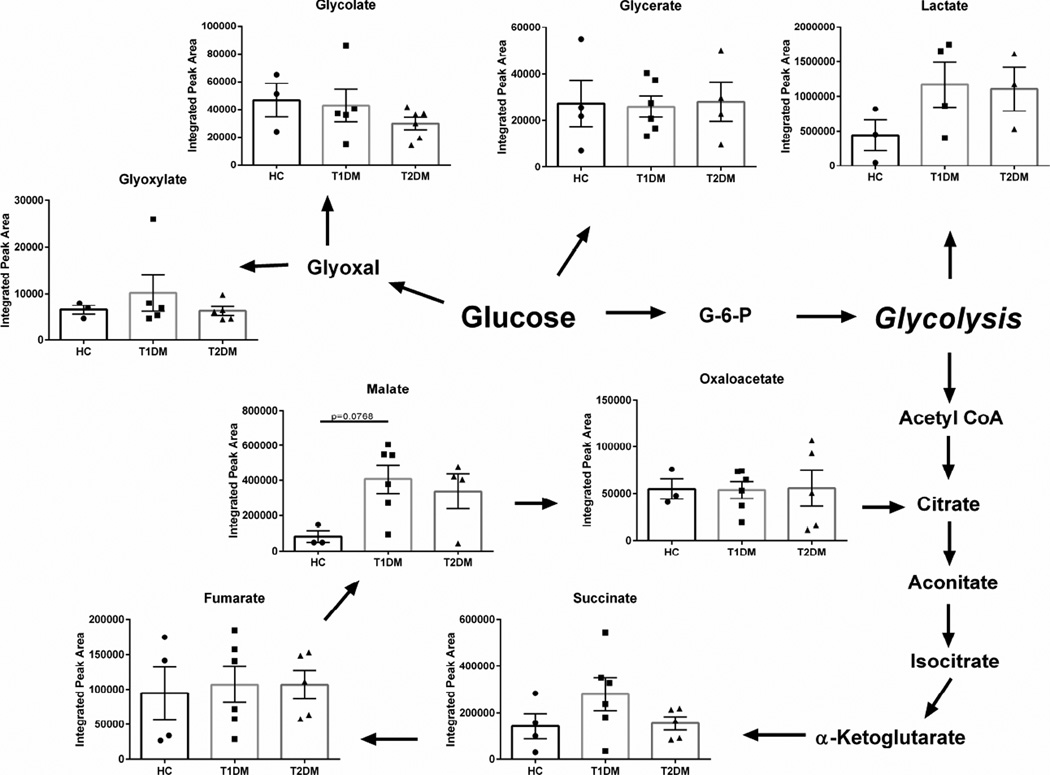
Given that diabetes is a metabolic disease associated with defects in insulin production or response, we sought to determine if diabetes-induced corneal dystrophies are linked to altered glucose metabolism. Studies have identified glyoxylate as a biomarker for early detection of Type 2 diabetes (Nikiforova et al., 2014). In our study, we measured little variation between glyoxylate levels in the diabetic cornea compared to healthy controls (Figure 3). We also found no changes in the relative concentrations of glucose-derived metabolites glycolate and glycerate (Figure 3). Similar findings were seen in the flux of citric acid cycle intermediates, including succinate, fumarate, malate, and oxaloacetate. The effects of diabetes on the citric acid cycle was minimal in the cornea with a slight increase in malate (p=0.0768) in Type 1 diabetic corneal stroma compared to healthy controls (Figure 3). These results suggest that the readily-metabolized glucose derivatives may not serve as detectable biomarkers of diabetes-induced pathologies compared to long-lasting, stable metabolites.
Figure 3. Glucose metabolism in corneal tissue isolated from healthy controls (HC), Type 1 (T1DM) and Type 2 (T2DM) patient samples.
Schematic depicts flux of glucose-derived metabolites (glycolate, glycerate, glyoxylate, and lactate), as well as citric acid cycle intermediates (succinate, fumurate, malate, oxaloacetate). n≥3 forHC, T1DM, and T2DM corneal buttons. Error bars represent standard error of the mean. A one-way ANOVA was performed and P≤0.05 considered statistically significant. Each data point corresponds to an individual patient.
3.3. Lipid Trafficking in the Diabetic Cornea
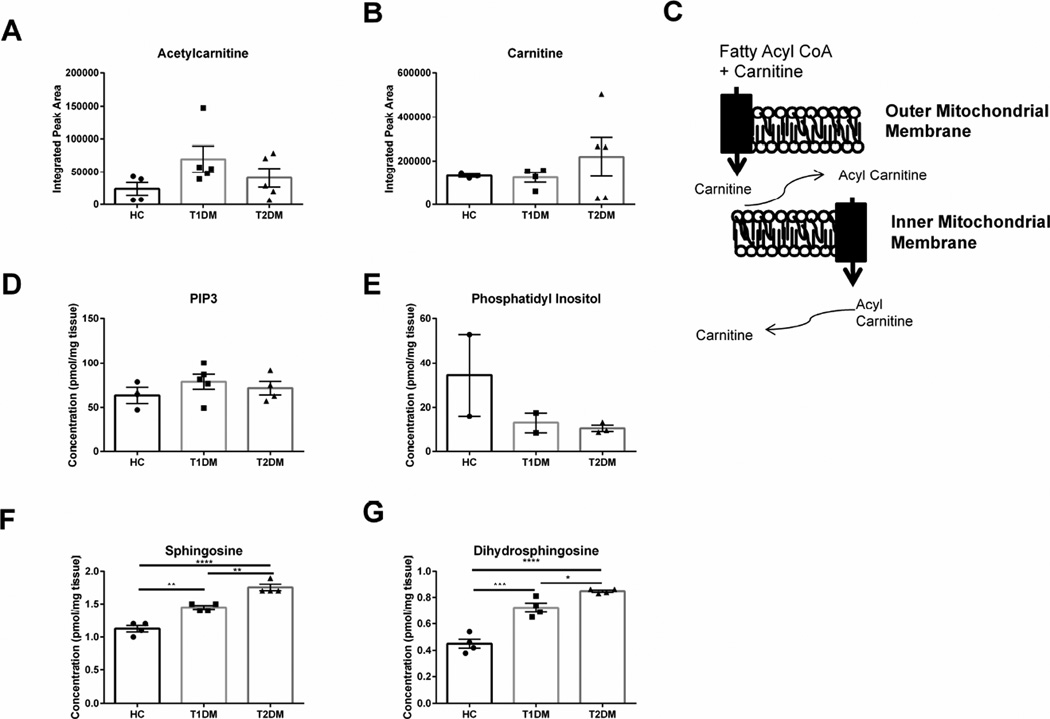
We have previously reported increased monohexosylceramides and sphingomyelin levels in both Type 1 and 2 diabetic patients (Priyadarsini et al., 2014). In order to determine if changes in metabolic flux associated with lipid trafficking contribute to lipid retention in the diabetic cornea, we quantified the expression of carnitine and acetyl carnitine, the primary carrier proteins responsible for transport of lipids to the mitochondria (Figure 4A–C). Though we measured substantial increases in carnitine levels in some Type 2 diabetic corneal samples, total acetylcarnitine and carnitine levels were not significantly different compared to the healthy control (Figure 4A–B). We also quantified the expression of phosphatidyl 1,3,5-triphosphate with no variability between corneas and a slight reduction in phosphatidyl inositol expression in both Type 1 and 2 diabetic corneas (Figure 4D–E). Interestingly, we measured a significant increase in sphingosine (1.5-fold, p≤0.01) and dihydrosphingosine (2-fold, p≤0.001) in Type 1 diabetic corneal stroma (Figure 4F–G). Type 2 corneal stroma also showed significantly increased sphingosine and dihdryosphingosine amounts 1.8-fold (p≤0.0001) and 2.2-fold (p≤0.0001), respectively, with significantly higher concentrations compared even to Type 1 diabetic corneas (Figure 4F–G). These results suggest that lipid metabolism is altered in diabetic corneal stroma independent of carnitine-mediated transport.
Figure 4. Lipids and transport mediators found in corneal stromal tissue isolated from healthy controls (HC), Type 1 (T1DM) and Type 2 (T2DM) diabetes mellitus patients.
(A–B) Lipid transport mediators, acetylcarnitine and carnitine levels have little variation between groups. (C) Schematic showing function of carnitine in transporting lipids into the mitochondria. (D–E) Concentrations of phosphatidyl inositiol triphosphate-3 (PIP3) and phosphatidyl inositol are unchanged in diabetic samples. (F–G) Sphingosine and dihydrosphingosine are significantly increased in both T1DM and T2DM samples. n≥3 for HC, T1DM, and T2DM corneal buttons. Error bars represent standard error of the mean. A one-way ANOVA was performed and P≤0.05 considered statistically significant. (*represents p≤0.05, **represents p≤0.01, ***represents p≤0.001.) Each data point corresponds to an individual patient.
3.4. Tryptophan Metabolism and Kynurenine Pathway Induced by Diabetes Mellitus
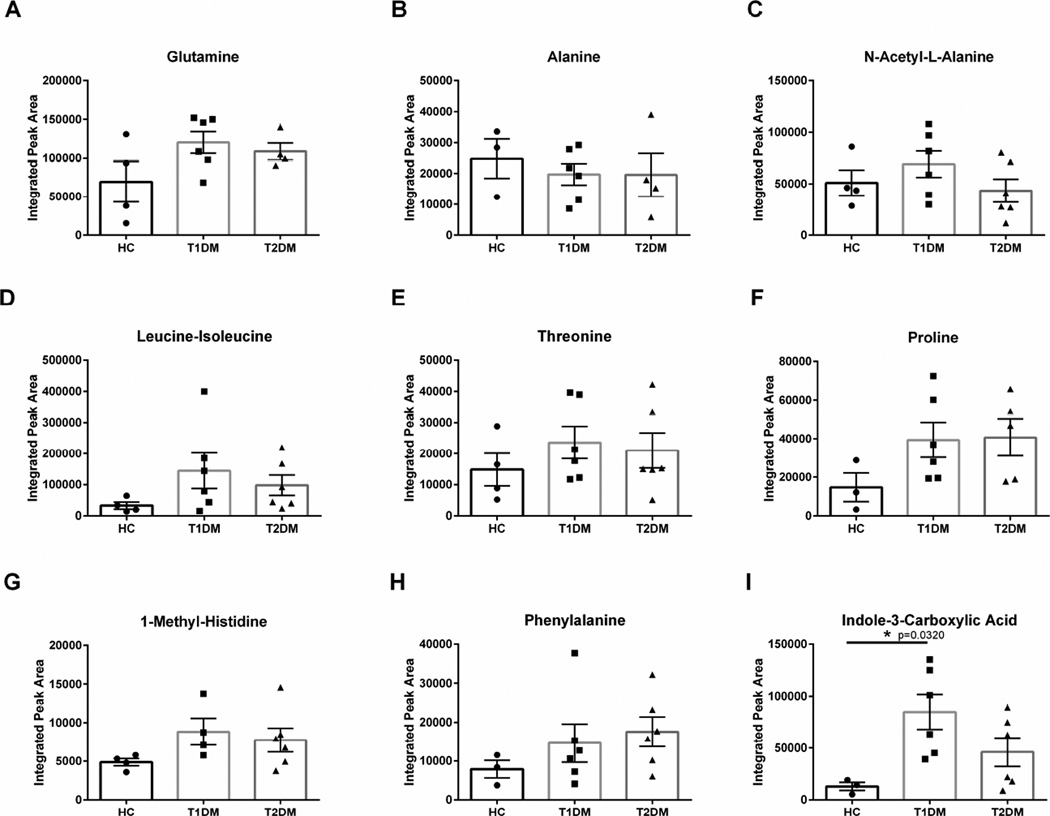
In order to determine if protein metabolism was modulated by variations in amino acid flux, we measured the relative amounts of amino acids and related derivatives in control and Type 1 and Type 2 diabetic corneal stroma. Glutamine, alanine, N-acetyl-L-alanine, leucine-isoleucine, and threonine levels were not significantly altered in the diabetic cornea (Figure 5A–E). In order to determine if the upregulation in Collagen I and III expression was related to increased monomers, we measured expression of proline levels in healthy and Type 1 and 2 diabetic corneas. Proline is an abundant and essential amino acid present in collagen fibrils (Shoulders and Raines, 2009). We measured substantially elevated proline, although not significantly different, in both (2-fold) Type 1 and 2 diabetic corneas compared to healthy controls (Figure 5F). Aromatic amino acid levels, including phenylalanine and 1-methyl-histindine, were not significantly altered with diabetes (Figure 5G–H). Interestingly, the tryptophan derivative, indole-3-carboxylic acid, was significantly increased (4-fold, p=0.0320) in Type 1 diabetic corneas compared to healthy controls (Figure 5I). Tryptophan is known to be an important metabolite that is regulated by the kynurenine pathway, which is used to generate NAD, an essential substrate for production of NADH in glycolysis and the citric acid cycle.
Figure 5. Flux of amino acid metabolites in corneal stromal tissue isolated from healthy controls (HC), Type 1 (T1DM) and Type 2 (T2DM) diabetes mellitus patients.
(A–C) glutamine, alanine, and N-acetylamine-alanine, (D–F) leucine-isoleucine, threonine, and proline, (G–I) 1-methyl-histidine, phenylalanine, and indole-3-carboxylic acid levels. n≥3 forHC, T1DM, and T2DM corneal buttons. Error bars represent standard error of the mean. A one-way ANOVA was performed and P≤0.05 considered statistically significant. Each data point corresponds to an individual patient.
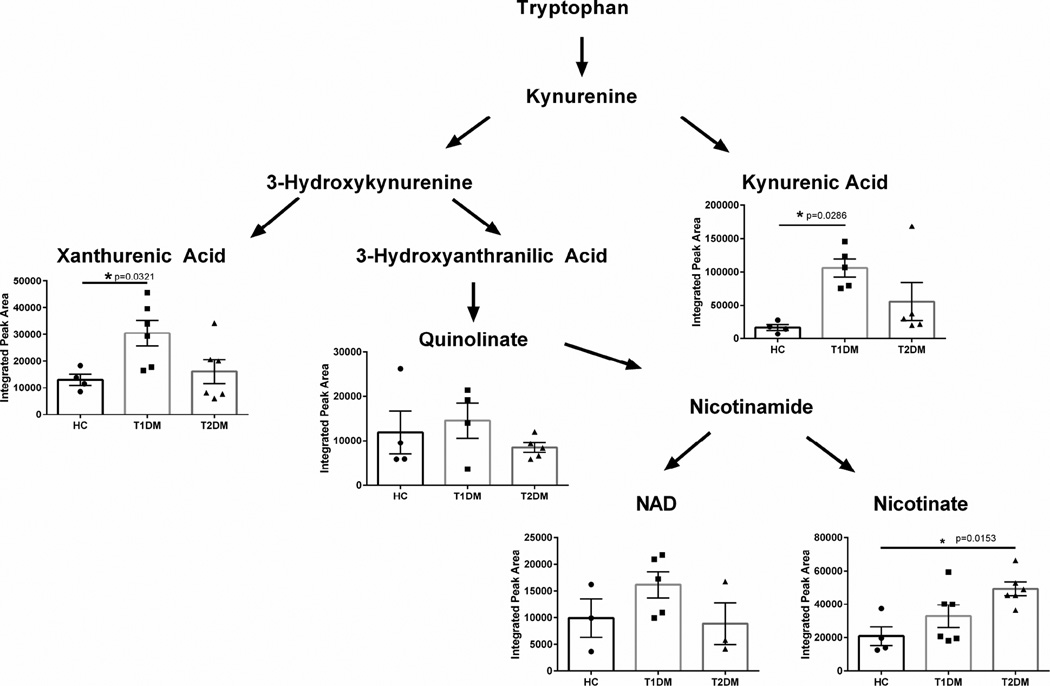
In order to determine if corneal pathologies caused by diabetes affects the Kynurenine pathway, we quantified the concentrations of xanthurenic acid, quinolinate, and kynurenic acid. We found significant upregulation of both xanthurenic acid (2-fold, p=0.0321) and kynurenic acid (3-fold, p=0.0286) in Type 1 diabetic corneal stroma (Figure 6). Interestingly, Type 2 diabetic corneas did not show a significant upregulation in these metabolites, but instead showed a significant increase in nicotinate (p=0.0153) levels compared to healthy controls suggesting divergent regulation depending on form of diabetes. These results suggest that dysregulation of kynurenine metabolism occurs in the human cornea in Type 1 diabetic patients with upregulation of kynurenic acid and xanthurenic acid, with Type 2 diabetes modulating the downstream product of tryptophan metabolism with increased nicotinate levels. This effect may be related to the end-stage disease state depending on the type of diabetes.
Figure 6. Kynurenine pathway in corneal stromal tissue isolated from healthy controls (HC), Type 1 (T1DM) and Type 2 (T2DM) diabetes mellitus patients.
Significant upregulation of tryptophan metabolism is measured in T1DM corneal tissue with increased xanthurenic acid and kynurenic acid. Nicotinate levels were significantly increased in T2DM corneal samples. n≥4 for HC, T1DM, and T2DM corneal buttons. Error bars represent standard error of the mean. A one-way ANOVA was performed and P≤0.05 considered statistically significant. Each data point corresponds to an individual patient.
3.5. Novel mediators in the Human Diabetic Cornea
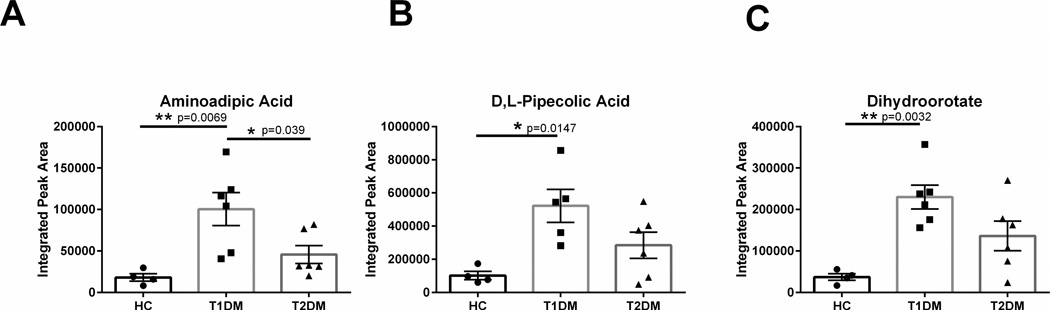
Aminoadipic acid is a derivative of lysine metabolism, though its functional role as a biomolecule remains unknown. Elevated aminoadipic acid levels in plasma and skin have been reported as a predictor of development of Type 2 diabetes (Sell et al., 2007; Wang et al., 2013). To the authors’ knowledge, no reports have established the role of aminoadipic acid in the diabetic cornea, which is avascular and therefore somewhat excluded from systemic distributions within circulation. We found significantly elevated aminoadipic acid (3-fold, p=0.0069) in Type 1 diabetic corneas compared to healthy controls and Type 2 (p=0.039) (Figure 7A). These results suggest that diabetes promotes aminoadipic acid production in multiple tissue systems, including the cornea. Pipecolic acid was also significantly elevated (4-fold, p=0.0147) in Type 1 diabetic corneas compared to healthy controls (Figure 7B). In addition, dihydroorotate levels were also increased in Type 1 diabetic corneas by 4-fold (p=0.0032) compared to healthy controls (Figure 7C).
Figure 7. Novel biomarkers identified in corneal stromal tissue isolated from healthy controls (HC), Type 1 (T1DM) and Type 2 (T2DM) diabetes mellitus patients.
Aminoadipic acid, D,L-pipecolic acid, and dihydroorotate was significantly increased in T1DM corneal tissue. n≥4 for HC, T1DM, and T2DM corneal buttons. Error bars represent standard error of the mean. A one-way ANOVA was performed and P≤0.05 considered statistically significant. Each data point corresponds to an individual patient.
4. DISCUSSION
Diabetes is a systemic metabolic disease that affects nearly every tissue in the body, including the cornea (Henriques et al., 2015; Liu et al., 2015). Current therapeutic options for diabetic wound care do not solve the problem and thus only delay or arrest further complications, such as infection. In the case of the cornea, pathologies are even more pronounced with studies focusing on the epithelial and nerve defects resulted due to chronic diabetes in patients (Hyndiuk et al., 1977; Midena et al., 2006). Surprisingly, the corneal stroma is severely under investigated despite all the defects seen, such as fibrosis, erosions, and thinning (Karamichos et al., 2010). It is well-known that corneal stroma collagen fibrils provide corneal structure and mechanical integrity (Karamichos et al., 2007, 2009), while healthy resident keratocytes are required for maintenance and assembly of the ECM. Collagen I and V are the dominant types present within a healthy cornea, where Collagen III is associated with complications and defects, particularly fibrosis (Karamichos et al., 2010). Collagen III is mainly a result of the transformation of keratocytes to myofibroblasts in response to some kind of assault such as injury. In the case of diabetes, the prolonged exposure to hyperglycemic environment, as well as abundance of reactive oxygen species, leads to keratocyte activation and eventually scars and ulcer development (Hyndiuk et al., 1977). Diabetes has been linked to increased water retention within various tissues, including the cornea (Weston et al., 1995). Increased edema due to an extracellular environment high in glucose can lead to the appearance of stromal thickening. The increase in corneal thickness found in a number of diabetic patients likely results from increased edema as well as increased collagen deposition. In this study we showed that the human diabetic corneal stroma has elevated levels of Collagens I and III but not V suggesting that collagen modulation occurs in an isoform-dependent manner. Our results support clinical data showing increased scarring in the diabetic cornea (Brightbill et al., 1978) and further define the mechanism by determining that diabetes primarily promotes Collagen III rather than Collagen V. Though we measured a significant increase in Collagen III within the T1DM and T2DM corneas and increased Collagen I only in T2DM, we cannot distinguish whether the collagen has been crosslinked into the matrix or is simply secreted into the extracellular environment. Collagen III deposition within the cornea proper is incorporated by myofibroblasts following wounding and is associated with scarring. The presence of Collagen III within the cornea has been reported to be localized to isolated fibrils in a random distribution compared to that found in other tissues, including the skin and tendons where it is present in copoloymers with Collagen I (Keene et al., 1987).
We have previously reported the lipid content of the human diabetic cornea with significant upregulation of monohexosylceramides when compared to healthy controls (Priyadarsini et al., 2014). In an attempt to fully characterize differential lipid species important to the diabetic cornea, we used untargeted mass spectrometry analysis and found significant upregulation of sphingosine and dihydrosphingosine in both Type 1 and Type 2 diabetic corneas compared to healthy controls suggesting altered lipid metabolism in diabetes. Both of these, and especially sphingosine have been show to play a role in diabetes and corneal wound healing (Kawanabe et al., 2007; Lee et al., 2000). Our current study identified differences in lipid composition and transport in the human diabetic corneal stroma. Overall, our data supports the hypothesis that lipid retention may play an important role in the pathological changes that occur during diabetes.
Given the importance of sphingolipid metabolism and lipid regulation, we proceeded with metabolic analysis in order to dissect the pathways that are more involved and possibly responsible for the corneal diabetic defects. We found little to no effect on glucose-derived metabolite flux or the citric acid cycle. While this was rather surprising, we suspect was a result of controlled diabetes and insulin-based medication that these patients receive in their life time.
We identified significant upregulation of the Kynurenine pathway in the diabetic corneal stroma. The Kynurenine pathway is a critical metabolic pathway that leads to the production of NAD+ (Grant et al., 2010). Disruptions in this pathway have been associated with several genetic and inflammatory disorders (Lim et al., 2010). In the cornea, the Kynurenine pathway has been linked to corneal endothelium health as well as oxidative stress (Serbecic et al., 2009; Wang et al., 2014). Our findings suggest that upregulation of the Kynurenine pathway, independent of glucose metabolism, may play an important role in the progression of diabetes-induced fibrosis in the corneal stroma and may contribute to corneal scarring and delayed wound healing. Differences in glucose metabolism may not have been detected due to the possibility of clinical treatment or stabilization of the disease prior to death.
Given the clinical significance of corneal diabetic wound care, our data opens up new avenues towards understanding the main cause of these defects and the development of novel therapeutic solutions. Here, we have identified potential novel biomarkers, aminoadipic acid, pipecolic acid, and dihydroorotate, as significantly upregulated in Type 1 diabetic corneas compared to healthy controls. Elevated aminoadipic acid in plasma has been reported to be a predictor for development of Type 2 diabetes in a human population (Wang et al., 2013). Our results suggest that increased aminoadipic acid levels may also play an important role in the diabetic corneal stroma and may be related to the onset of diabetes-induced pathologies. Further studies are needed to identify if these novel biomarkers can be used to predict development of defects within the diabetic cornea and lead to innovative treatments of diabetic corneal pathologies.
Highlights.
Human corneal diabetes leads to increased expression of collagen type III.
Sphingosine and dihydrosphingosine are elevated in the human diabetic cornea.
Tryptophan-derivatives in the Kynurenine pathway are significantly upregulated.
Aminoadipic acid and pipecolic acid are novel biomarkers in the human diabetic cornea.
Acknowledgments
This work was supported by the National Institutes of Health Grants 5R01EY023568 (DK), 5R01EY020886 (DK) and T32EY023202. We acknowledge the support of the NEI/DMEI Cellular Imaging Core Facility at OUHSC (P30EY021725), an unrestricted grant (DMEI) from Research to Prevent Blindness (New York, NY USA). The authors thank Dr John M Asara Min Yuan and Susanne Breitkopf of Harvard Medical School for technical help with metabolomics experiments. We would also like to acknowledge the research grants from the Veteran’s Administration (VA Merit Review I BX001792 (CEC) and a Research Career Scientist Award 13F-RCS-002 (CEC): from the National Institutes of Health via HL125353 (CEC), CA154314 (CEC). In support of the research project, all the services and products were generated by the VCU Massey Cancer Center Shared supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059. The manuscript contents do not represents the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions
S.P. conducted experiments, analyzed data, and wrote the manuscript. T.B.M. analyzed data and wrote the manuscript. D.K. conceived the project, provided reagents and supplies, and reviewed/edited the manuscript. J.XM reviewed/edited the manuscript. A.S. conducted experiments. C.C. and J.A. executed the lipidomics experiments.
Conflict of Interest: The authors declare that no conflict of interest exists.
REFERENCES
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. The New England journal of medicine. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Alberti KGMM, Zimmet Pf. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Babiarz JP, Linsenmayer TF. Collagen type I and type V are present in the same fibril in the avian corneal stroma. The Journal of cell biology. 1988;106:999–1008. doi: 10.1083/jcb.106.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfeldt KE, Skottner A, Arnqvist HJ. In-vivo regulation of messenger RNA encoding insulin-like growth factor-I (IGF-I) and its receptor by diabetes, insulin and IGF-I in rat muscle. The Journal of endocrinology. 1992;135:203–211. doi: 10.1677/joe.0.1350203. [DOI] [PubMed] [Google Scholar]
- Brightbill FS, Myers FL, Bresnick GH. Postvitrectomy Keratopathys. American Journal of Ophthalmology. 1978;85:651–655. doi: 10.1016/s0002-9394(14)77099-0. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating, G. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Molecular endocrinology. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- Forouhi NG, Wareham NJ. Epidemiology of diabetes. Medicine (Abingdon) 2014;42:698–702. doi: 10.1016/j.mpmed.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldich Y, Barkana Y, Gerber Y, Rasko A, Morad Y, Harstein M, Avni I, Zadok D. Effect of diabetes mellitus on biomechanical parameters of the cornea. Journal of Cataract & Refractive Surgery. 2009;35:715–719. doi: 10.1016/j.jcrs.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Grant R, Nguyen S, Guillemin G. Kynurenine Pathway Metabolism is Involved in the Maintenance of the Intracellular NAD Concentration in Human Primary Astrocytes. International journal of tryptophan research : IJTR. 2010;3:151–156. doi: 10.4137/ijtr.s4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques J, Vaz-Pereira S, Nascimento J, Rosa PC. [Diabetic eye disease] Acta medica portuguesa. 2015;28:107–113. [PubMed] [Google Scholar]
- Herse P. A review of manifestations of diabetes mellitus in the anterior eye and cornea. American journal of optometry and physiological optics. 1988;65:224–230. doi: 10.1097/00006324-198803000-00013. [DOI] [PubMed] [Google Scholar]
- Howard BV. Lipoprotein metabolism in diabetes mellitus. Journal of lipid research. 1987;28:613–628. [PubMed] [Google Scholar]
- Hyndiuk RA, Kazarian EL, Schultz R, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Archives of Ophthalmology. 1977;95:2193–2196. doi: 10.1001/archopht.1977.04450120099012. [DOI] [PubMed] [Google Scholar]
- Kabosova A, Kramerov AA, Aoki AM, Murphy G, Zieske JD, Ljubimov AV. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Experimental eye research. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010;51:1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Lakshman N, Petroll WM. Regulation of corneal fibroblast morphology and collagen reorganization by extracellular matrix mechanical properties. Invest Ophthalmol Vis Sci. 2007;48:5030–5037. doi: 10.1167/iovs.07-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Lakshman N, Petroll WM. An experimental model for assessing fibroblast migration in 3-D collagen matrices. Cell motility and the cytoskeleton. 2009;66:1–9. doi: 10.1002/cm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. Journal of dermatological science. 2007;48:53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Keene DR, Sakai LY, Bächinger HP, Burgeson RE. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. The Journal of cell biology. 1987;105:2393–2402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE. Vision disorders in diabetes. Diabetes in America. 1995;1:293. [Google Scholar]
- Kumagai AK, Glasgow BJ, Pardridge WM. GLUT1 glucose transporter expression in the diabetic and nondiabetic human eye. Investigative Ophthalmology and Visual Science. 1994;35:2887–2894. [PubMed] [Google Scholar]
- Lee H, Goetzl EJ, An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. American Journal of Physiology-Cell Physiology. 2000;278:C612–C618. doi: 10.1152/ajpcell.2000.278.3.C612. [DOI] [PubMed] [Google Scholar]
- Lee JS, Oum BS, Choi HY, Lee JE, Cho BM. Differences in corneal thickness and corneal endothelium related to duration in Diabetes. Eye. 2005;20:315–318. doi: 10.1038/sj.eye.6701868. [DOI] [PubMed] [Google Scholar]
- Leppin K, Behrendt AK, Reichard M, Stachs O, Guthoff RF, Baltrusch S, Eule JC, Vollmar B. Diabetes mellitus leads to accumulation of dendritic cells and nerve fiber damage of the subbasal nerve plexus in the cornea. Investigative ophthalmology & visual science. 2014;55:3603–3615. doi: 10.1167/iovs.14-14307. [DOI] [PubMed] [Google Scholar]
- Lim CK, Brew BJ, Sundaram G, Guillemin GJ. Understanding the roles of the kynurenine pathway in multiple sclerosis progression. International journal of tryptophan research : IJTR. 2010;3:157–167. doi: 10.4137/ijtr.s4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sheng M, Liu Y, Wang P, Chen Y, Chen L, Wang W, Li B. Expression of SIRT1 and oxidative stress in diabetic dry eye. International journal of clinical and experimental pathology. 2015;8:7644–7653. [PMC free article] [PubMed] [Google Scholar]
- Ljubimov AV, Huang ZS, Huang GH, Burgeson RE, Gullberg D, Miner JH, Ninomiya Y, Sado Y, Kenney MC. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1998;46:1033–1041. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Trulson M. Effects of insulin and streptozotocin-induced diabetes on brain tryptophan and serotonin metabolism in rats. Journal of neurochemistry. 1978;30:205–211. doi: 10.1111/j.1471-4159.1978.tb07053.x. [DOI] [PubMed] [Google Scholar]
- Midena E, Brugin E, Ghirlando A, Sommavilla M, Avogaro A. Corneal diabetic neuropathy: a confocal microscopy study. Journal of refractive surgery (Thorofare, NJ: 1995) 2006;22:S1047–S1052. doi: 10.3928/1081-597X-20061102-08. [DOI] [PubMed] [Google Scholar]
- Nikiforova VJ, Giesbertz P, Wiemer J, Bethan B, Looser R, Liebenberg V, Ruiz Noppinger P, Daniel H, Rein D. Glyoxylate, a new marker metabolite of type 2 diabetes. Journal of diabetes research. 2014;2014:685204. doi: 10.1155/2014/685204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W.H. Geneva: WHO; 2014. Global Status Report on non-communicable diseases 2014. [Google Scholar]
- Pirie A. The biochemistry of the eye. Proceedings of the Nutrition Society. 1960;19:73–78. doi: 10.1079/pns19600018. [DOI] [PubMed] [Google Scholar]
- Priyadarsini S, Sarker-Nag A, Allegood J, Chalfant C, Karamichos D. Description of the Sphingolipid Content and Subspecies in the Diabetic Cornea. Current eye research. 2014:1–7. doi: 10.3109/02713683.2014.990984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranke MB, Price DA, Reiter EO. Growth hormone therapy in pediatrics: 20 years of KIGS. Karger Medical and Scientific Publishers; 2007. [Google Scholar]
- Rehany U, Ishii Y, Lahav M, Rumelt S. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea. 2000;19:534–538. doi: 10.1097/00003226-200007000-00026. [DOI] [PubMed] [Google Scholar]
- Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- Schultz R, Van Horn D, Peters M, Klewin KM, Schutten W. Diabetic keratopathy. Transactions of the American Ophthalmological Society. 1981;79:180. [PMC free article] [PubMed] [Google Scholar]
- Sell D, Strauch C, Shen W, Monnier V. 2-Aminoadipic acid is a marker of protein carbonyl oxidation in the aging human skin: effects of diabetes, renal failure and sepsis. Biochem J. 2007;404:269–277. doi: 10.1042/BJ20061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbecic N, Lahdou I, Scheuerle A, Hoftberger R, Aboul-Enein F. Function of the tryptophan metabolite, L-kynurenine, in human corneal endothelial cells. Molecular vision. 2009;15:1312–1324. [PMC free article] [PubMed] [Google Scholar]
- Shoulders MD, Raines RT. Collagen structure and stability. Annual review of biochemistry. 2009;78:929. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarbez K, Priestley Y, Hoepf M, Koevary SB. Comprehensive Review of the Effects of Diabetes on Ocular Health. Expert review of ophthalmology. 2010;5:557–577. doi: 10.1586/eop.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy N, Mackay G, Forrest C, Christofides J, Egerton M, Stone T, Darlington L. Tryptophan metabolism and oxidative stress in patients with Huntington's disease. Journal of neurochemistry. 2005;93:611–623. doi: 10.1111/j.1471-4159.2005.03070.x. [DOI] [PubMed] [Google Scholar]
- Su DH, Wong TY, Wong W-L, Saw S-M, Tan DT, Shen SY, Loon S-C, Foster PJ, Aung T, Group SMES. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology. 2008;115:964–968. e961. doi: 10.1016/j.ophtha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle & nerve. 2015;52:363–370. doi: 10.1002/mus.24553. [DOI] [PubMed] [Google Scholar]
- Taylor R. Type 2 diabetes etiology and reversibility. Diabetes care. 2013;36:1047–1055. doi: 10.2337/dc12-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle TL, Coppieters KT, Von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiological reviews. 2011;91:79–118. doi: 10.1152/physrev.00003.2010. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.) 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P, Zou MH. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circulation research. 2014;114:480–492. doi: 10.1161/CIRCRESAHA.114.302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O’Sullivan J, Cheng S, Rhee EP. 2-Aminoadipic acid is a biomarker for diabetes risk. The Journal of clinical investigation. 2013;123:4309. doi: 10.1172/JCI64801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webhofer C, Gormanns P, Reckow S, Lebar M, Maccarrone G, Ludwig T, Putz B, Asara JM, Holsboer F, Sillaber I, Zieglgansberger W, Turck CW. Proteomic and metabolomic profiling reveals time-dependent changes in hippocampal metabolism upon paroxetine treatment and biomarker candidates. J Psychiatr Res. 2013;47:289–298. doi: 10.1016/j.jpsychires.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD. An information-intensive approach to the molecular pharmacology of cancer. Science (New York, N.Y.) 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- Weston BC, Bourne WM, Polse KA, Hodge DO. Corneal hydration control in diabetes mellitus. Investigative ophthalmology & visual science. 1995;36:586–595. [PubMed] [Google Scholar]
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Widner B, Leblhuber F, Walli J, Tilz G, Demel U, Fuchs D. Tryptophan degradation and immune activation in Alzheimer's disease. Journal of neural transmission. 2000;107:343–353. doi: 10.1007/s007020050029. [DOI] [PubMed] [Google Scholar]
- Xu KP, Li Y, Ljubimov AV, Yu FS. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–1085. doi: 10.2337/db08-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]