Abstract
OBJECTIVE
Muscle weakness and atrophy of the lower limbs may develop in patients with diabetes, increasing their risk of falls. The underlying basis of these abnormalities has not been fully explained. The aim of this study was to objectively quantify muscle strength and size in patients with type 2 diabetes mellitus (T2DM) in relation to the severity of neuropathy, intramuscular noncontractile tissue (IMNCT), and vitamin D deficiency.
RESEARCH DESIGN AND METHODS
Twenty patients with T2DM and 20 healthy control subjects were matched by age, sex, and BMI. Strength and size of knee extensor, flexor, and ankle plantar and dorsiflexor muscles were assessed in relation to the severity of diabetic sensorimotor polyneuropathy (DSPN), amount of IMNCT, and serum 25-hydroxyvitamin D (25OHD) levels.
RESULTS
Compared with control subjects, patients with T2DM had significantly reduced knee extensor strength (P = 0.003) and reduced muscle volume of both knee extensors (P = 0.045) and flexors (P = 0.019). Ankle plantar flexor strength was also significantly reduced (P = 0.001) but without a reduction in ankle plantar flexor (P = 0.23) and dorsiflexor (P = 0.45) muscle volumes. IMNCT was significantly increased in the ankle plantar (P = 0.006) and dorsiflexors (P = 0.005). Patients with DSPN had significantly less knee extensor strength than those without (P = 0.02) but showed no difference in knee extensor volume (P = 0.38) and ankle plantar flexor strength (P = 0.21) or volume (P = 0.96). In patients with <25 nmol/L versus >25 nmol/L 25OHD, no significant differences were found for knee extensor strength and volume (P = 0.32 vs. 0.18) and ankle plantar flexors (P = 0.58 vs. 0.12).
CONCLUSIONS
Patients with T2DM have a significant reduction in proximal and distal leg muscle strength and a proximal but not distal reduction in muscle volume possibly due to greater intramuscular fat accumulation in distal muscles. Proximal but not distal muscle strength is related to the severity of peripheral neuropathy but not IMNCT or 25OHD level.
Introduction
Although diabetic polyneuropathy manifests primarily in the form of sensory and autonomic dysfunction, an increasing body of evidence shows that ankle and knee motor dysfunction may also be a major manifestation (1–3). Motor dysfunction presents as muscle weakness, a reduction in muscle mass, and limitations of joint flexibility and range of motion, ultimately affecting gait and whole-body movements (4–6).
Although weakness and atrophy of the distal muscles and decreased ankle mobility and strength have been demonstrated in several studies and related to the severity of neuropathy (7–9), underlying mechanisms have not been explored. Previous studies did not perform a comprehensive assessment of muscle strength in relation to morphology and internal composition. Patients with diabetes and obesity have an increased amount of intramuscular noncontractile tissue (IMNCT), which is highly correlated with insulin resistance and a reduction of muscle strength in the calf and thigh muscles (1,2,10).
Variations in muscle volume (11) may contribute to alterations in strength, and because many patients with diabetes are obese, they may have larger muscle size but greater muscle atrophy due to diabetic neuropathy (7). Previous studies have shown atrophy of the ankle plantar and dorsiflexor muscles and knee extensors in patients with diabetic neuropathy compared with patients without neuropathy and control subjects (2,4,6,8). However, the effect on more-proximal leg muscles (knee extensors and flexors), which confer a major effect on postural stability and gait performance, has not been established. Indeed, maximal isometric muscle strength has been related directly to muscle cross-sectional area (CSA) (11–13).
A decline in muscle strength and muscle size with increased intramuscular fat infiltration and a reduction in physical performance in healthy elderly subjects may be related to vitamin D deficiency (14,15). Motor dysfunction can occur in those with mild and particularly severe vitamin D deficiency (14,16). Furthermore, 93% of patients complain of nonspecific musculoskeletal pain, which may be attributed to vitamin D deficiency (17). The degree of vitamin D deficiency is currently categorized according to circulating levels of 25-hydroxyvitamin D (25OHD) such that adequate is defined as >75 nmol/L (>30 ng/mL), insufficient as 50–75 nmol/L (20–30 ng/mL), deficient as 25–50 nmol/L (10–20 ng/mL), and severely deficient as <25 nmol/L (<10 ng/mL) (18). The underlying basis of vitamin D deficiency–related muscle symptoms and dysfunction is likely to be complex, but proximal myopathy is a major manifestation in severe vitamin D deficiency (17). Vitamin D receptor levels decline in elderly subjects (17,19,20), and vitamin D deficiency is associated with atrophy of skeletal muscle fibers (type II) and a decline in muscle strength, leading to an increased risk of falls (17,21). We have previously shown a high prevalence of vitamin D deficiency in patients with diabetes (22), and vitamin D levels have been inversely correlated with obesity, diabetes, and high triglyceride levels (23).
Although previous studies have investigated specific aspects of motor function in patients with type 2 diabetes, there has not been a comprehensive assessment of skeletal muscle strength, morphology, and internal composition in relation to neuropathy, IMNCT, and 25OHD. The purpose of the present study was to investigate muscle strength deficits in distal and proximal extensors and flexors in the lower limb of patients with type 2 diabetes and to relate these to muscle size, severity of peripheral neuropathy, IMNCT, and vitamin D deficiency.
Research Design and Methods
Twenty patients with type 2 diabetes and 20 control subjects without diabetes were assessed at the muscle function laboratory of Manchester Metropolitan University (Manchester, U.K.). Individuals with severe musculoskeletal problems; neurological, orthopedic, or surgical problems; severe foot deformities; foot ulcers; and amputations or who were pregnant were excluded. The study was approved by the U.K. National Health Service ethics committee and local research ethics committees at the University of Manchester and the Manchester Metropolitan University, and written informed consent was obtained from all subjects before participation. This research adhered to the tenets of the Declaration of Helsinki.
Assessment of Neuropathy
All patients with diabetes underwent assessment of BMI, blood pressure, HbA1c, lipid profile (total cholesterol, LDL, HDL, triglycerides), albumin creatinine excretion ratio, estimated glomerular filtration rate, and 25OHD. Symptoms of diabetic polyneuropathy were assessed with the Neuropathy Symptom Profile. Neurological deficits were evaluated with the simplified Neuropathy Disability Score, which comprises vibration perception, pin prick and temperature sensations, and presence or absence of ankle reflexes. Vibration perception threshold was tested with a Horwell Neurothesiometer (Scientific Laboratory Supplies, Wilford, Nottingham, U.K.). Cold and warm thresholds and cold-induced and warm-induced pain were established on the dorsolateral aspect of the foot by using a TSA-II NeuroSensory Analyzer (Medoc Ltd., Ramat-Yishai, Israel). Electrodiagnostic studies were performed with a Dantec Keypoint system (Dantec Dynamics Ltd., Bristol, U.K.) equipped with a Defense Information Systems Agency temperature regulator to keep a constant limb temperature of 32–35°C. Sural sensory nerve amplitude, sural sensory nerve conduction velocity, and peroneal motor nerve conduction velocity and amplitude were assessed by a consultant neurophysiologist. Diabetic sensorimotor polyneuropathy (DSPN) was defined according to the Toronto criteria (24). Control subjects were assessed only for vibration perception threshold and Neuropathy Disability Score.
All subjects were scanned with laser in vivo corneal confocal microscopy (Heidelberg Retina Tomograph III Rostock Cornea Module; Heidelberg Engineering GmbH, Heidelberg, Germany); all images were captured through the section mode of Heidelberg Eye Explorer software, and approximately six high-clarity images per subject were analyzed from the central sub-basal nerve plexus. Four parameters were established to assess corneal nerve fiber damage: corneal nerve fiber density (the total number of nerve fibers per square millimeter), corneal nerve branch density (the total number of nerve branches per square millimeter), and corneal nerve fiber length (the total length [mm] of all nerve fibers per square millimeter) within the area of cornea and corneal nerve fiber tortuosity (the degree of nonlinearity of the nerve fibers). These parameters were quantified with a semiautomated, purpose-written, proprietary software (CCMetrics; M. A. Dabbah, Imaging Science Biomedical Engineering, University of Manchester, Manchester, U.K.). Intraepidermal nerve fiber density was quantified in skin biopsy samples from the dorsum of the foot, using established techniques (25).
Isokinetic Dynamometer
Maximal isometric muscle strength for knee extensors and ankle plantar flexors was assessed with an isokinetic dynamometer (Cybex NORM; Cybex International, Ronkonkoma, NY). The dynamometer measured joint torque (in newton meters [Nm]) at the knee and ankle, which reflects the net forces acting around the respective joints and the internal tendon moment arm lengths. Because joint torque primarily reflects the force produced by the major muscle groups acting around the joints (knee and ankle extensors) and for the purpose of optimizing clinical understanding, we use the term muscle strength to refer to the measurement of joint torque.
Tests were performed at three different angles for both the knee and the ankle joints of the dominant leg. To test knee extensor joint torque, subjects were seated and secured on the chair of the dynamometer with their knees flexed at 90° (0° = full knee extension) and their hip angle at 85° (0° = supine position). Three maximal voluntary isometric contractions of the knee extensors were performed at three knee joint angles in random order: 85°, 70°, and 55° of knee flexion with a 2-min rest interval between contractions and the highest value recorded.
To test ankle plantar flexor joint torque, subjects were positioned prone on the dynamometer with the knee in full extension and the ankle secured to the footplate. Maximal voluntary isometric plantar flexor joint torque was assessed at three joint angles in random order: 0° (neutral position [i.e., right angle between footplate and tibia]), −5° dorsiflexion, and −10° dorsiflexion. Three maximal isometric contractions also were performed, and the highest value was recorded (Nm). Each maximum isometric contraction for both knee and ankle was held for ∼3–4 s with a 60-s rest interval between contractions within each angle and a 2-min rest between contractions at different angles. A range of joint angles were tested to ensure that we encompassed the joint angle where torque peaked (i.e., the optimum angle) for each subject, thereby taking into account slight variations in the muscle force-length relationship between groups.
Magnetic Resonance Imaging
A 0.25-T MRI peripheral scanner (G-scan; Esaote, Milan, Italy) was used to scan the upper and lower regions of the leg with a T1 gradient echo scanning sequence using the following parameters: field of view = 200 × 200 mm; matrix = 256 × 192 pixels; slice thickness = 10 mm; interslice gap = 1 mm; time to echo = 16 ms; time to repetition = 685 ms; and flip angle = 90°. Serial axial plane images were obtained of the upper and lower leg from which the CSA of specific muscles were analyzed. Major exclusion criteria for MRI were women who were or could be pregnant, ferromagnetic foreign bodies, cardiac pacemakers/cardioverter defibrillators, cochlear implants, intrauterine devices, and implanted drug infusion pumps.
Muscle Volume Calculation
Serial CSAs of the knee extensors (vastus medialis, vastus intermedius, vastus lateralis, rectus femoris), knee flexors (semimembranosus, biceps femoris, semitendinosus), ankle plantar flexors (soleus, medial and lateral heads of gastrocnemius muscles), and ankle dorsiflexors (tibialis anterior, extensor digitorum longus) were analyzed by digitizing software (OsiriX; Pixmeo, Geneva, Switzerland). The CSA of each muscle was manually analyzed from the serial axial plane scans. To establish how many CSAs were required to be analyzed for each specific muscle to provide a representative and accurate muscle volume calculation, three subjects were randomly selected and all the available CSAs analyzed consecutively. The muscle volume calculated from all available slices was then compared against calculations from measurements from every second and third slice. As a result of the analysis, for the soleus, ankle dorsiflexors, vastus medialis, vastus intermedius, vastus lateralis, semimembranosus, biceps femoris, and semitendinosus, every third slice was used and for the medial and lateral heads of gastrocnemius and rectus femoris, every slice was used in the calculation of muscle volume. The sum of all CSAs for each muscle was calculated (∑CSA cm2) and multiplied by the distance between each muscle section d (m) to derive the muscle volume (cm3) as shown in Eq. 1:
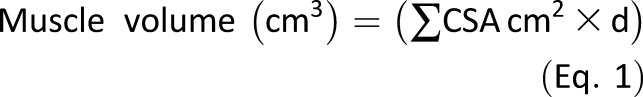 |
Intramuscular Noncontractile Tissue
The density of various tissues is reflected by a different MRI signal intensity. Connective tissue yields low signal intensity values, whereas fat tissue produces very high signal intensity values, with the signal intensity of skeletal muscle falling between these two tissues. By measuring the frequency distribution of the signal intensity from a given area of the MRI scan, it is possible to determine shifts in signal intensity, indicating changes in tissue composition. OsiriX software was used to measure the signal intensity for all muscles from the region outlined as their CSA. The signal intensity was quantified in each muscle studied, and a frequency distribution of the signal intensity in that CSA was obtained. The signal intensity value with the highest frequency from the selected muscle CSA was recorded when this signal intensity value comprised >10% of the pixel number from the total number of pixels within that specific muscle CSA. Three different levels along the subject’s leg were chosen (proximal, mid, distal) according to the anatomical structure of the muscle. The sum of the three signal intensity values from each muscle was selected and used for further analysis.
Statistical Analysis
An independent samples Student t test was used to test between-group differences in the measured variables. Pearson correlation coefficients were used to test the relationship between muscle strength and other parameters. Data are presented as mean ± SD unless otherwise stated.
With Eq. 2, we performed a power analysis before the study (a priori power calculation) by using the ankle joint strength (torque) results of previous studies (7,26):
The power analysis indicated that we needed 14 subjects in each group to detect a difference of 22 Nm between groups (∼20% difference between groups), with an α-level of 0.05 and a β-level of 0.9 (i.e., power of 90%). To account for dropout and potential data problems, we recruited 20 subjects into each group.
Results
Subjects
Twenty healthy control subjects (13 male, 7 female) and 20 patients with type 2 diabetes (15 male, 5 female [8 with DSPN, 12 with no DSPN]) were assessed. Age, height, and BMI were matched in patients with diabetes compared with control subjects (Table 1). Patients had reasonable control of their glycemia and lipid levels and evidence of mild neuropathy based on neurological examination, quantitative sensory testing, neurophysiology, corneal confocal microscopy, and skin biopsy (Table 1).
Table 1.
Subject clinical characteristics
| Parameter | Control | Type 2 diabetes | P value |
|---|---|---|---|
| Subjects (n) | 20 | 20 | |
| Male | 13 | 15 | |
| Female | 7 | 5 | |
| DSPN (n) | NA | ||
| With | NA | 8 | |
| Without | NA | 12 | |
| Age (years) | 61.5 ± 6.0 | 63.1 ± 10.8 | 0.56 |
| Height (m) | 1.69 ± 0.09 | 1.67 ± 0.09 | 0.53 |
| Body mass (kg) | 78.1 ± 11.5 | 82.6 ± 18.2 | 0.34 |
| BMI (kg/m2) | 27.2 ± 3.9 | 29.4 ± 4.1 | 0.09 |
| Ethnicity (n) | |||
| Asian | 9 | 2 | |
| European | 11 | 18 | |
| Duration of diabetes (years) | NA | 14.9 ± 9.9 | |
| 25OHD (nmol/L) | 78.9 ± 48.8 | 72.6 ± 43.5 | 0.66 |
| 25OHD/BMI | 2.9 ± 1.9 | 2.5 ± 1.6 | 0.48 |
| 25OHD/BSA | 1.91 ± 0.17 | 1.95 ± 0.25 | 0.54 |
| HbA1c (%) | NA | 7.34 ± 1.52 | NA |
| Cholesterol (mmol/L) | NA | 4.01 ± 0.73 | NA |
| HDL (mmol/L) | NA | 1.37 ± 0.887 | NA |
| LDL (mmol/L) | NA | 1.76 ± 0.62 | NA |
| Triglycerides (mmol/L) | NA | 1.80 ± 1.82 | NA |
| NDS (0–10) | 0.4 ± 1.0 | 3.1 ± 2.6 | 0.000 |
| VPT (Hz) | 6.4 ± 3.0 | 14.7 ± 11.0 | 0.003 |
| CT (°C) | NA | 26.1 ± 3.3 | NA |
| WT (°C) | NA | 41.6 ± 4.9 | NA |
| SNCV (m/s) | NA | 10.4 ± 11.5 | NA |
| SNAP (μV) | NA | 5.2 ± 10.7 | NA |
| PMNCV (m/s) | NA | 40.0 ± 11.2 | NA |
| PMNAP (mV) | NA | 4.7 ± 4.1 | NA |
| CNFD (n/mm2) | NA | 28.0 ± 9.2 | NA |
| CNBD (n/mm2) | NA | 95.9 ± 41.4 | NA |
| CNFL (mm/mm2) | NA | 23.6 ± 8.57 | NA |
| CNFT (TC) | NA | 20.50 ± 4.2 | NA |
| IENFD (n/mm) | NA | 7.8 ± 5.4 | NA |
Data are mean ± SD unless otherwise indicated. BSA, body surface area; CNBD, corneal nerve branch density; CNFD, corneal nerve fiber density; CNFL, corneal nerve fiber length; CNFT, corneal nerve fiber tortuosity; CT, cold threshold; IENFD, intraepidermal nerve fiber density; NA, not applicable; PMNAP, peroneal motor nerve amplitude; PMNCV, peroneal motor nerve conduction velocity; SNAP, sural sensory nerve amplitude; SNVC, sural sensory nerve conduction velocity; TC, tortuosity coefficient; WT, warm threshold.
Muscle Strength
Knee and ankle muscle strength (Nm/kg) was significantly lower in patients with type 2 diabetes compared with control subjects at three different angles. Knee strength at 55° (1.3 ± 0.4 vs. 2.1 ± 0.7; P = 0.002), 70° (1.3 ± 0.5 vs. 2.0 ± 0.8; P = 0.002), and 85° (1.3 ± 0.4 vs. 1.8 ± 0.6; P = 0.009) was significantly reduced in patients compared with control subjects. Ankle strength at 0° (0.6 ± 0.2 vs. 0.9 ± 0.3; P = 0.000), −5° (0.7 ± 0.2 vs. 1.04 ± 0.3; P = 0.001), and −10° (0.7 ± 0.2 vs. 1.1 ± 0.36; P = 0.001) was significantly reduced in patients compared with control subjects. Accordingly, the average knee extensor (1.3 ± 0.5 vs. 1.9 ± 0.7; P = 0.003) and ankle plantar flexor (0.6 ± 0.2 vs. 1.05 ± 0.3; P = 0.001) strength was significantly lower in patients compared with control subjects (Table 2).
Table 2.
Muscle strength, volume, and IMNCT (MRI signal intensity values) in patients with type 2 diabetes and control subjects, with a priori statistical power analysis, statistical difference, and the percentage difference between groups
| Variable | Control | Type 2 diabetes | Statistical power | P value | % difference |
|---|---|---|---|---|---|
| Muscle strength (Nm/kg) | |||||
| Knee extensors | 1.9 ± 0.7 | 1.3 ± 0.5 | 0.99 | 0.003 | −32 |
| Ankle PFs | 1.0 ± 0.3 | 0.6 ± 0.2 | 1 | 0.001 | −34 |
| MV (cm3) | |||||
| MV SOL | 418.7 ± 114.8 | 420.2 ± 132.4 | 0.06 | 0.97 | 0 |
| MV MG | 184.8 ± 52.8 | 170.3 ± 68.5 | 0.28 | 0.46 | −7 |
| MV LG | 106.3 ± 35.8 | 92.4 ± 36.7 | 0.53 | 0.24 | −13 |
| Sum ankle PF | 709.9 ± 186.5 | 617.9 ± 291.2 | 0.53 | 0.23 | −12 |
| Ankle DF | 218.2 ± 49.9 | 205.3 ± 55.3 | 0.29 | 0.45 | −5 |
| MV VM | 342.3 ± 98.8 | 328.9 ± 80.2 | 0.16 | 0.64 | −3 |
| MV VI | 342.3 ± 106.4 | 293.1 ± 62.8 | 0.83 | 0.08 | −14 |
| MV VL | 368.5 ± 106.7 | 330.6 ± 94.9 | 0.51 | 0.24 | −10 |
| MV RF | 148.5 ± 47.8 | 117.5 ± 48.7 | 0.89 | 0.05 | −20 |
| Sum knee extensors | 1,201.6 ± 323.2 | 968.4 ± 395.6 | 0.83 | 0.04 | −19 |
| MV SM | 227.7 ± 58.4 | 194.1 ± 56.7 | 0.83 | 0.07 | −14 |
| MV BF | 288.4 ± 77.3 | 246.4 ± 68.3 | 0.83 | 0.08 | −14 |
| MV ST | 152.2 ± 51.4 | 131.5 ± 40.5 | 0.64 | 0.16 | −13 |
| Sum knee flexors | 668.5 ± 172.9 | 517.5 ± 219.6 | 0.96 | 0.01 | −22 |
| IMNCT (pixel intensity) | |||||
| SOL | 3,453.3 ± 356.0 | 3,736.0 ± 240.0 | 1 | 0.006 | 8 |
| MG | 2,751.8 ± 325.3 | 2,901.7 ± 464.9 | 0.52 | 0.25 | 5 |
| LG | 2,039.6 ± 282.5 | 2,231.4 ± 314.9 | 0.89 | 0.05 | 9 |
| DF | 3,748.7 ± 321.8 | 4,103.6 ± 414.2 | 1 | 0.005 | 9 |
| VM | 3,628.4 ± 138.3 | 3,636.2 ± 251.5 | 0.07 | 0.90 | 0 |
| VI | 3,553.8 ± 155.2 | 3,594.0 ± 205.9 | 0.26 | 0.49 | 1 |
| VL | 3,670.4 ± 239.7 | 3,668.1 ± 349.6 | 0.05 | 0.98 | 0 |
| RF | 3,303.1 ± 206.1 | 3,437.6 ± 306.1 | 0.76 | 0.11 | 4 |
| SM | 3,571.3 ± 342.5 | 3,543.6 ± 353.9 | 0.10 | 0.80 | 0 |
| BF | 3,411.3 ± 214.5 | 3,457.9 ± 276.9 | 0.21 | 0.56 | 1 |
| ST | 3,513.2 ± 360.7 | 3,715.4 ± 427.9 | 0.74 | 0.12 | 5 |
An independent sample t test was used to represent the statistical differences in muscle strength, volume, and IMNCT in control subjects vs. patients with diabetes. Boldface data denotes statistical significance. BF, biceps femoris; DF, dorsiflexor; LG, lateral gastrocnemius; MG, medial gastrocnemius; MV, muscle volume; PF, plantar flexor; RF, rectus femoris; SM, semimembranosus; SOL, soleus; ST, semitendinosus; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
Muscle Volume
Muscle volume for the knee extensors (P = 0.04) and flexors (P = 0.01) was significantly lower in patients with type 2 diabetes compared with control subjects (Table 2). No significant reduction was found in ankle plantar (P = 0.23) and dorsiflexor (P = 0.45) muscle volume between groups (Table 2).
Intramuscular Noncontractile Tissue
IMNCT was significantly increased in the soleus (P = 0.006), dorsiflexor (P = 0.005), and lateral gastrocnemius (P = 0.05) muscles in patients with type 2 diabetes compared with control subjects (Table 2 and Fig. 1). No significant differences were found in IMNCT in the knee extensors or knee flexors between groups (Table 2).
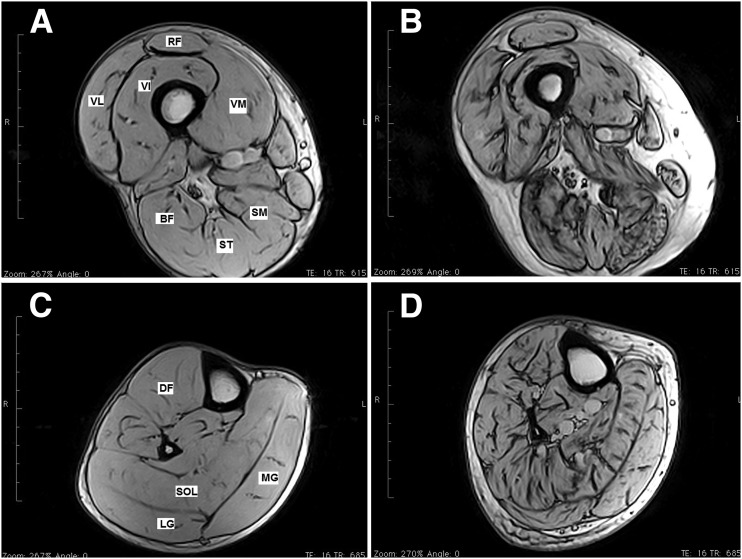
Figure 1.
MRI images of the lower limbs of control subjects vs. patients with type 2 diabetes. Representative lower-limb MRI images from a healthy 69-year-old control group volunteer (A and C) and a 67-year-old patient with diabetic polyneuropathy (B and D). Images are from the midthigh level (A and B) and midtibia level (C and D). Note the substantial increase in IMNCT (dark areas inside the muscle cross-sections are connective tissue) in images from the patient with diabetes. Scale bar = 10 cm. BF, biceps femoris; DF, dorsiflexor; LG, lateral gastrocnemius; MG, medial gastrocnemius; RF, rectus femoris; SM, semimembranosus; SOL, soleus; ST, semitendinosus; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
DSPN Versus No DSPN
Patients with DSPN (n = 8) had significantly lower knee extensor strength (Nm/kg) compared with patients without DSPN (n = 12) (1.0 ± 0.4 vs. 1.5 ± 0.4; P = 0.028). No significant difference was found in ankle plantar flexor strength between patients with and without DSPN (0.59 ± 0.31 vs. 0.76 ± 0.19; P = 0.21).
Low Versus Normal Vitamin D Levels
No significant difference was found in muscle strength (1.2 ± 0.1 Nm/kg vs. 1.3 ± 0.5 Nm/kg; P = 0.32) and volume (932.1 ± 427.3 cm3 vs. 1,122.7 ± 175.1 cm3; P = 0.18) of the knee extensors and ankle plantar flexor strength (0.6 ± 0.2 Nm/kg vs. 0.7 ± 0.2 Nm/kg; P = 0.58) and volume (582.6 ± 306.9 cm3 vs. 768.1 ± 160.5 cm3; P = 0.12) between patients with 25OHD levels <25 nmol/L and those with 25OHD levels >25 nmol/L.
Correlations
A significant correlation was found between knee extensor strength and knee extensor muscle volume (r = 0.57, P = 0.007) in patients with type 2 diabetes. No significant correlation was found between ankle plantar flexor strength and ankle plantar flexor volume (r = 0.23, P = 0.297) or between knee and ankle muscle strength with IMNCT (r ranged from −0.34 to −0.02 at the ankle and from −0.33 to 0.37 at the knee), severity of DSPN (r = −0.36 at ankle and −0.48 at knee), or 25OHD level (r = −0.12 at ankle and 0.13 at knee) among patients.
Conclusions
The findings show that patients with type 2 diabetes have reduced proximal and distal lower-limb muscle strength compared with age-matched control subjects, which agrees with other studies (2,6,7,27). For the knee extensors, this was associated with muscle atrophy as reflected by the significantly reduced knee extensor muscle volume in the patients with diabetes. In contrast, although the plantar flexor muscle strength was reduced in the patients, no measurable muscle atrophy existed. This finding may be attributed to the increase in intramuscular fat, which may mask muscle atrophy. Essentially, because the muscle is infiltrated by increased levels of intramuscular fat, its CSA and volume appear artificially larger than the actual active contractile area. The knee extensors demonstrated a reduction in both muscle strength and muscle volume in the patients. The increase in intramuscular fat in the lower leg as opposed to the proximal knee extensors and flexors of these patients may well be related to peripheral neuropathy affecting the distal muscles. The exact mechanism that explains the association between an increase in intramuscular fat and peripheral neuropathy in patients with type 2 diabetes is not fully understood. An accumulation of IMNCT, particularly in the thigh, may further contribute to a reduction in muscle blood flow and insulin diffusion capacity, increasing the local concentration of fatty acids and resulting in insulin resistance of the skeletal muscle in patients with type 2 diabetes (28). Aging is also associated with increased IMNCT within muscles of the lower limb in patients with type 2 diabetes (29).
We found marked strength deficits not only in the ankle plantar flexors, which has been shown previously and related to DSPN, but also in proximal knee extensors, which may also be partly attributed to DSPN. Of clinical relevance, the knee extensors are a major antigravity muscle group responsible for propelling and controlling the body during gait; therefore, this abnormality may partially explain the recent observation that balance is impaired in patients with diabetic neuropathy (30). Reduced ankle and knee muscle strength in patients with diabetes, and particularly those with neuropathy, may contribute substantially to gait impairment, increased incidence of falls, and severe injuries with hospitalization. Indeed, resistance training exercises can improve muscle strength and walking speed and reduce the risk of falls (6,31–33).
Accumulation of IMNCT within skeletal muscle may result in insulin resistance but has also been shown to correlate with reduced calf and thigh muscle strength in patients with DSPN (1). MRI allows for accurate quantification of muscle CSA and volume and allows one to differentiate muscle from fat, connective tissue, and bone (34). In the current study, knee extensor and flexor muscle volume was significantly smaller, and there was a trend for a smaller distal plantar flexor muscle volume in the patients with type 2 diabetes. Muscle volume is of course associated with muscle strength and power production (14), as others have found (2,6,35). In addition to the impairment in lower-extremity muscle function, alterations in the cartilage, ligaments, and tendons may also contribute to instability (36). Thus, diabetes also increases the thickness of the Achilles tendon and plantar fascia, resulting in decreased flexibility of the ankle joint and limited dorsiflexion during walking (36). We also found knee extensor muscle strength to be reduced significantly in patients with DSPN compared with those without DSPN. Previous studies have found that the severity of neuropathy contributes to an impairment of physical mobility (1). Thus, the reduction in physical activity may result in a reduction in the use of the major antigravity muscles (37–39), particularly knee extensors during walking, and this is reflected in the reduced strength of the knee extensors in patients with DSPN.
Vitamin D deficiency causes musculoskeletal dysfunction and has been associated with a reduction in muscle strength, size, and bone density and increased IMNCT (14,27). Muscle weakness and atrophy are prominent in patients with diabetes (1,2,6,7) and have been attributed to vitamin D deficiency (22,23). To our knowledge, the current study is the first to systematically examine differences in muscle function and structure in relation to vitamin D deficiency in patients with type 2 diabetes. Although we show that all patients had insufficient levels of 25OHD, a low level of 25OHD (<25 nmol/L) was not related to a reduction in lower-limb muscle strength or size (15).
The statistical power for the majority of key variables (muscle strength, size, and IMNCT) in this study was 0.83–1, which is high considering the optimal recommendation is 0.8 (40). Some other variables fell below this optimal 0.8 threshold, and for some of these variables, statistical power could have been limiting. Considering that we had such high power for the majority of key variables, the lower power for certain variables may also reflect that no true differences existed between groups in these other variables and would not have been found in a much larger sample. In conclusion, this small but detailed study was adequately powered for the majority of variables examined. Potential confounders, such as differences in BMI and ethnicity between groups, may have had an impact on the findings. However, patients with type 2 diabetes showed both proximal (knee extensor) and distal (ankle plantar flexor) muscle weakness. Proximal muscle weakness was related to a reduction in muscle volume, but distal muscle weakness was not. The latter finding may be a consequence of greater infiltration of distal intramuscular fat. The reduction in muscle strength was related to DSPN but not to low levels of vitamin D.
Article Information
Acknowledgments. The authors thank the staff at the musculoskeletal laboratory of Manchester Metropolitan University and the NIHR/Wellcome Trust Clinical Research Facility of Central Manchester University Hospitals NHS Foundation Trust for providing high-quality service and state-of-the-art facilities to carry out this research.
Funding. This study was funded by National Institutes of Health National Institute of Neurological Disorders and Stroke grant 5R01NS46259-03NINDS and JDRF grant 5-2002-185.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.M.A. contributed to the data research, statistical analysis, and writing of the manuscript. N.D.R., F.L.B., A.J.M.B., and M.J. contributed to the review and revision of the manuscript. R.A.M. contributed to the study design and review and revision of the manuscript. R.A.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Tuttle LJ, Sinacore DR, Cade WT, Mueller MJ. Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys Ther 2011;91:923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther 2008;88:1336–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown SJ, Handsaker JC, Bowling FL, Maganaris CN, Boulton AJ, Reeves ND. Do patients with diabetic neuropathy use a higher proportion of their maximum strength when walking? J Biomech 2014;47:3639–3644 [DOI] [PubMed] [Google Scholar]
- 4.Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol 2010;4:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeves ND, Najafi B, Crews RT, Bowling FL. Aging and type 2 diabetes: consequences for motor control, musculoskeletal function, and whole-body movement. J Aging Res 2013;2013:508756
- 6.Andersen H. Motor dysfunction in diabetes. Diabetes Metab Res Rev 2012;28(Suppl. 1):89–92 [DOI] [PubMed] [Google Scholar]
- 7.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia 1997;40:1062–1069 [DOI] [PubMed] [Google Scholar]
- 8.Andreassen CS, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles–a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI). Diabetologia 2009;52:1182–1191 [DOI] [PubMed] [Google Scholar]
- 9.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther 1994;74:299–308; discussion 309–313 [DOI] [PubMed] [Google Scholar]
- 10.Clark BA, Alloosh M, Wenzel JW, Sturek M, Kostrominova TY. Effect of diet-induced obesity and metabolic syndrome on skeletal muscles of Ossabaw miniature swine. Am J Physiol Endocrinol Metab 2011;300:E848–E857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mersmann F, Bohm S, Schroll A, Boeth H, Duda G, Arampatzis A. Muscle shape consistency and muscle volume prediction of thigh muscles. Scand J Med Sci Sports 2015;25:e208–e213 [DOI] [PubMed] [Google Scholar]
- 12.Handsfield GG, Meyer CH, Hart JM, Abel MF, Blemker SS. Relationships of 35 lower limb muscles to height and body mass quantified using MRI. J Biomech 2014;47:631–638 [DOI] [PubMed] [Google Scholar]
- 13.Nakai R, Azuma T, Sudo M, Urayama S, Takizawa O, Tsutsumi S. MRI analysis of structural changes in skeletal muscles and surrounding tissues following long-term walking exercise with training equipment. J Appl Physiol (1985) 2008;105:958–963 [DOI] [PubMed] [Google Scholar]
- 14.Bignotti B, Cadoni A, Martinoli C, Tagliafico A. Imaging of skeletal muscle in vitamin D deficiency. World J Radiol 2014;6:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes MR, Ribeiro PA, Ledur P, Souza GC, Clausell N, Schaan BD. Vitamin D insufficiency is associated with lower physical function in patients with heart failure and diabetes. J Diabetes Res 2014;2014:320930 [DOI] [PMC free article] [PubMed]
- 16.Gordon PL, Doyle JW, Johansen KL. Association of 1,25-dihydroxyvitamin D levels with physical performance and thigh muscle cross-sectional area in chronic kidney disease stage 3 and 4. J Ren Nutr 2012;22:423–433 [DOI] [PMC free article] [PubMed]
- 17.Heath KM, Elovic EP. Vitamin D deficiency: implications in the rehabilitation setting. Am J Phys Med Rehabil 2006;85:916–923 [DOI] [PubMed]
- 18.Young KA, Snell-Bergeon JK, Naik RG, et al. Vitamin D deficiency and coronary artery calcification in subjects with type 1 diabetes. Diabetes Care 2011;34:454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rejnmark L. Effects of vitamin D on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis 2011;2:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceglia L, Niramitmahapanya S, da Silva Morais M, et al. A randomized study on the effect of vitamin D₃ supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab 2013;98:E1927–E1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott D, Sanders KM, Ebeling PR. Vitamin D, muscle function, and falls in older adults: does reduced deposition of intramuscular adipose tissue influence the relationship? J Clin Endocrinol Metab 2013;98:3968–3970 [DOI] [PubMed] [Google Scholar]
- 22.Alam U, Najam O, Al-Himdani S, et al. Marked vitamin D deficiency in patients with diabetes in the UK: ethnic and seasonal differences and an association with dyslipidaemia. Diabet Med 2012;29:1343–1345 [DOI] [PubMed]
- 23.Riek AE, Oh J, Bernal-Mizrachi C. Vitamin D regulates macrophage cholesterol metabolism in diabetes. J Steroid Biochem Mol Biol 2010;121:430–433 [DOI] [PubMed] [Google Scholar]
- 24.Tesfaye S, Boulton AJ, Dyck PJ, et al.; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care 2015;38:1138–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes 2004;53:1543–1548 [DOI] [PubMed] [Google Scholar]
- 27.Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2014;2:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892 [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–379 [DOI] [PubMed] [Google Scholar]
- 30.Brown SJ, Handsaker JC, Bowling FL, Boulton AJ, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care 2015;38:1116–1122 [DOI] [PubMed] [Google Scholar]
- 31.Handsaker JC, Brown SJ, Bowling FL, Maganaris CN, Boulton AJ, Reeves ND. Resistance exercise training increases lower limb speed of strength generation during stair ascent and descent in people with diabetic peripheral neuropathy. Diabet Med. 26 June 2015 [Epub ahead of print]. DOI: 10.1111/dme.12841 [DOI] [PubMed]
- 32.Andersen H. Motor neuropathy. Handb Clin Neurol 2014;126:81–95 [DOI] [PubMed] [Google Scholar]
- 33.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci 1995;50:64–67 [DOI] [PubMed] [Google Scholar]
- 34.Berciano J, Gallardo E, Fernández-Torre JL, González-Quintanilla V, Infante J. Magnetic resonance imaging of lower limb musculature in acute motor axonal neuropathy. J Neurol 2012;259:1111–1116 [DOI] [PubMed] [Google Scholar]
- 35.Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 2006;55:806–812 [DOI] [PubMed] [Google Scholar]
- 36.Giacomozzi C, D’Ambrogi E, Cesinaro S, Macellari V, Uccioli L. Muscle performance and ankle joint mobility in long-term patients with diabetes. BMC Musculoskelet Disord 2008;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer MD, Seynnes OR, di Prampero PE, et al. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol 2008;104:401–407 [DOI] [PubMed] [Google Scholar]
- 38.Pisot R, Narici MV, Simunic B, et al. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol 2008;104:409–414 [DOI] [PubMed] [Google Scholar]
- 39.Seynnes OR, Maganaris CN, de Boer MD, di Prampero PE, Narici MV. Early structural adaptations to unloading in the human calf muscles. Acta Physiol (Oxf) 2008;193:265–274 [DOI] [PubMed] [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. New York, Routledge, 1988, p. 273–406 [Google Scholar]