Abstract
OBJECTIVE
We assessed whether the apolipoprotein ε4 (APOE4) genotype affects the relationship of variability in long-term glycemic control (measured by HbA1c SD of multiple measurements) with white matter hyperintensities (WMHs) in elderly patients with type 2 diabetes (T2D).
RESEARCH DESIGN AND METHODS
WMH volume was generated from structural T1 and fluid-attenuated inversion recovery MRI in each subject. The analysis included 124 subjects; 27 (21.8%) had one or more APOE4 alleles.
RESULTS
HbA1c variability was associated with significantly higher WMH in APOE4 carriers (r = 0.47, P = 0.03), controlling for age, sex, mean HbA1c, number of follow-up years, and a composite of cardiovascular risk factors, but not in noncarriers (r = −0.04, P = 0.71; P for interaction = 0.050).
CONCLUSIONS
The results suggest that the APOE4 genotype affects the relationship of long-term glycemic control with WMH load so that APOE4 carriers may be more vulnerable to the insults of poor control.
Introduction
Type 2 diabetes (T2D) has been associated with cognitive decline and dementia (1). Neuroimaging studies have examined the neural correlates of cognitive impairment in T2D, revealing white matter hyperintensities (WMHs), which are believed to reflect ischemic injury, and cortical and subcortical atrophy, which may underlie observed cognitive changes (2).
The mechanisms by which long-term deleterious T2D processes affect the brain are unknown. Variability in HbA1c (percent of glycated hemoglobin) has been associated with cognitive decline (3) and T2D complications (4) beyond the effects of high mean HbA1c.
The apolipoprotein ε4 (APOE4) allele is a major risk factor for cognitive decline and dementia (5). We have recently reported that higher HbA1c levels are associated with lower cognitive performance in APOE4 carriers but not in noncarriers, suggesting higher vulnerability of APOE4 carriers to the effects of poor glycemic control on cognition (6). Furthermore, the association of the APOE4 and WMH in elderly patients is controversial, with some studies reporting an association of the APOE4 allele with increasing WMHs and cerebral microbleeds (7) and others failing to find such association (8).
Evidence indicating that the APOE genotype modifies the relationship of glycemic control with WMH in T2D could provide insight into the mechanisms underlying the association between APOE and risk for cognitive decline and identify specific groups at particularly high risk. In this study, we investigated the interrelationships of the APOE genotype with long-term variability of glycemic control and WMH. We hypothesized that poor glycemic control is more strongly associated with WMHs in APOE4 carriers.
Research Design and Methods
Participants were recruited from the Israel Diabetes and Cognitive Decline (IDCD) study, for which long-term information on HbA1c exists. IDCD is a collaboration of the Icahn School of Medicine at Mount Sinai (New York, NY), Sheba Medical Center (Tel HaShomer, Israel), and Maccabi Health Services (MHS) (Tel Aviv, Israel). The IDCD study design has been previously described in detail (9). Briefly, community-dwelling Israeli elderly individuals with T2D (≥65 years old) were recruited from the MHS diabetes registry (Supplementary Fig. 1). Participants had complete APOE genotyping, demographic, and T2D-related characteristic data. Criteria for enrollment into the IDCD study were having T2D; having normal cognition on entry; being free of any neurological (e.g., Parkinson disease, stroke), psychiatric (e.g., schizophrenia), or other diseases (e.g., alcohol or drug abuse) that might affect cognition; having an informant; being fluent in Hebrew; and living in the area of Tel Aviv. The study was approved by Mount Sinai, Sheba, and MHS institutional review board committees. Participants provided signed informed consent.
For each participant, HbA1c variability was defined as the SD from the average level across 17.92 (9.22) HbA1c measurements. DNA was extracted from blood samples. APOE genotypes were determined at Polymorphic DNA Technologies (Alameda, CA) and dichotomized as APOE4 carriers and noncarriers.
Randomly selected participants from the IDCD cohort were invited to undergo MRI. MRI scans were performed at the Sheba diagnostic imaging department on a 3-T scanner (Signa HDxt 16V02; GE Medical Systems, Waukesha, WI). High-resolution (1-mm3) images were acquired by using a three-dimensional fast spoiled gradient echo T1-weighted sequence (repetition time 7.3 s, echo time 2.7 s, flip angle 20°, inversion time 450 ms). Next, a T2-weighted fluid-attenuated inversion recovery sequence (repetition time 9,500 ms, echo time 123 ms, axial slice width and gap 3 and 0.4 mm, field of view 22 cm, 64 × 64 matrix, flip angle 90°) was acquired.
WMH Segmentation
We used Statistical Parameter Mapping version 8 software (www.fil.ion.ucl.ac.uk/spm) and its VBM8 Lesion Segmentation Toolbox (LST), following previously described methods (10). The LST automated method for quantifying white matter damage is reliable and has been shown to have a high degree of agreement with manual delineation of WMH in fluid-attenuated inversion recovery images (10). The default LST settings were used with the exception of κ (k), a value indicating the threshold for the initial lesion mask. Visual inspection of the probability maps across participants by using various k values, to maximize sensitivity while reducing false positive results, indicated that a k = 0.15 was the optimal value for our sample images. This procedure generated one binary lesion image per participant from which a total lesion volume (in milliliters) map was calculated.
Statistical Analysis
The skewedness and kurtosis were 1.49 and 1.61 for HbA1c variability, respectively, and 1.70 and 3.16 for WMH, respectively, suggesting nonnormal distributions of the variables. Thus, we applied square-root transformation to both variables, which improved skewedness and kurtosis for HbA1c to 0.96 and 0.12 and to 0.70 and −0.05 for WMH, so the normalized variables were used in all analyses. We assessed the relationship of long-term glycemic variability with WMHs by using a general linear model analysis controlling for age, sex, number of follow-up years in the registry (a surrogate of duration of T2D), and mean HbA1c level. We also controlled for a cardiovascular risk component derived from the first principal component of factor analyses, which comprised systolic and diastolic blood pressure, LDL and HDL cholesterol, and creatinine. The interaction of APOE4 genotype with HbA1c was assessed. We then repeated this analysis by using partial correlations stratified by APOE genotype (comparing APOE4 allele carriers and noncarriers), controlling for the same factors. Fisher z transformation was used to compare these partial correlations.
Differences between APOE genotyping on sociodemographic or medical characteristics were evaluated by Student t test and Pearson χ2 test. P = 0.05 (two-sided) was used to determine statistical significance. For analysis, we used SPSS version 19.0 software (IBM Corporation, Chicago, IL).
Results
Participants (n = 124; 27 APOE4 carriers [21.8%] and 97 noncarriers [78.2%]) had mean age of 71.50 (3.79) years. The number of years in the MHS registry was 8.61 (2.45), ranging from 3 to 18 years. Mean HbA1c level was 6.70 (0.85) and mean HbA1c SD level was 0.563 (0.476). The majority of participants were male (61.4%). APOE4 carriers did not differ significantly from noncarriers on any demographic or medical characteristics (age P = 0.82, sex P = 0.99, HbA1c P = 0.30, HbA1c SD P = 0.45, years in the registry P = 0.62, number of HbA1c measurements P = 0.41) or on WMH volume (APOE4 carriers 11.06 [11.27], APOE4 noncarriers 12.95 [12.55], P = 0.48). Mean levels of HbA1c correlated with HbA1c SD (r = 0.57, P < 0.001).
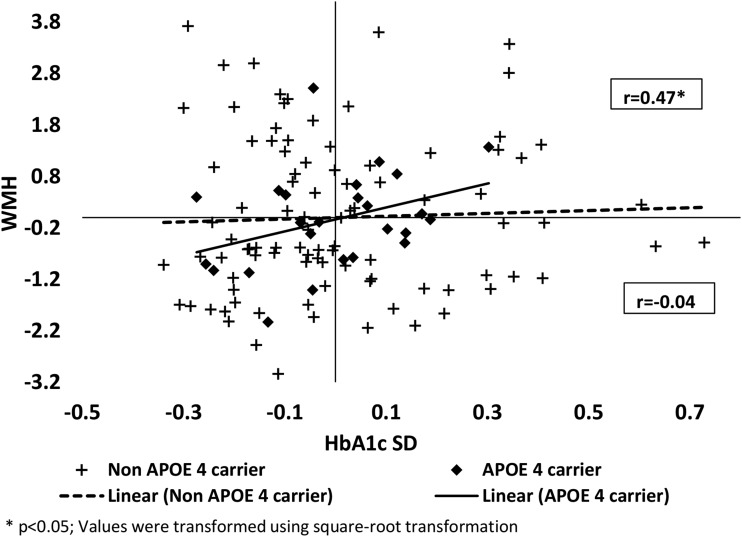
In the overall sample, HbA1c variability was not associated with WMHs (r = 0.11, P = 0.24). However, we found a significant interaction of APOE4 genotype with HbA1c on WMH (F [1,112] = 3.94, P = 0.050), controlling for age, sex, years in the registry, mean HbA1c, and related cardiovascular factors. In secondary analyses, the association between HbA1c variability and WMH volume differed significantly between APOE4 carriers and noncarriers. In the stratified analysis, APOE4 carrier WMH volume was significantly associated with HbA1c variability (r = 0.47, P = 0.03). No association was found in the APOE4 noncarriers (r = −0.04, P = 0.71). Comparison of these correlations using Fisher z transformation showed significant differences between the APOE4 carriers and noncarriers (P = 0.01) (Fig. 1).
Figure 1.
A significant association of HbA1c SD with WMH in APOE4 carriers (r = 0.47, P = 0.03) and no association in noncarriers (r = −0.04, P = 0.71). P = 0.01 for the difference between the two partial correlations.
Conclusions
The findings indicate that in patients with T2D, long-term glycemic control variability is associated with higher WMH load in APOE4 carriers but not in APOE4 noncarriers, suggesting that APOE4 carriers are potentially more susceptible to the deleterious effects of poor glycemic control on the brain. Consistent with these results, APOE4 carriers do not have more WMHs than noncarriers among T2D patients with dementia (8). Also in line with the current findings, in elderly subjects without dementia, the association of white matter integrity with T2D (reflecting higher HbA1c levels compared with non-T2D) is exacerbated among APOE4 carriers (11).
Glycemic control variability was calculated as the SD of numerous measures of HbA1c per patient, thus comprising a long-term glycemic control measurement. Glycemic control variability has been associated with T2D microvascular complications, such as nephropathy and microalbuminuria (4,12), independently of mean HbA1c in T2D. Both hyperglycemia (13) and hypoglycemia (14) have been associated with increased dementia risk. Thus, HbA1c variability may better capture the contributions to brain disease of periods of dangerously high or low HbA1c levels because if a risk factor includes both very high and low levels, the average of a mixture of extremes could mimic optimality.
Previous studies examined the association of T2D and glycemic control with WMH, showing inconsistent results. By using volumetric assessment rather than visual rating scales, an increase of WMH associated with T2D was found (15). Additionally, WMHs were associated with elevated levels of HbA1c in elderly subjects without dementia (16). Other studies did not find such associations (17). This inconsistency may be explained by the diversity of WMH quantification methodology (2) or, as implied from the current results, by interactions with disease factors such as HbA1c variability and APOE genotype.
Study strengths are the random selection of participants from the IDCD study, a cohort representative of elderly patients with T2D in Israel; the highly valid diagnosis of T2D; and the availability of long-term data on HbA1c and covariates. The primary limitation of the study is that the results, although robust and significant, are based on a relatively modest sample size of APOE4 carriers, thus requiring confirmation in future studies.
In summary, the results indicate modification by APOE genotype of the association between HbA1c variability and WMH in patients with T2D. This suggests that a common underlying mechanism for glycemic variability and the APOE genotype play a role in WMH load, leading to differential brain vulnerability of patients with T2D to poor glycemic control.
Supplementary Material
Article Information
Funding. This research was conducted while I.C. was a New Investigator Award in Alzheimer Disease recipient from the American Federation for Aging Research and was supported by National Institute on Aging grants P50-AG-05138 (to M.S.) and R01-AG-034087 and R21-AG-043878 (to M.S.B.) as well as the Helen Bader Foundation, the Leroy Schecter Foundation, and the Irma T. Hirschl Scholar Award (to M.S.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L. researched data and wrote the manuscript. R.R.-S. and M.S.B. researched data, contributed to the discussion, and reviewed the manuscript. A.H. researched data and reviewed the manuscript. R.P., G.T., L.R., R.M., and H.H. researched data. T.K., I.C., L.G., J.S., M.S., S.C.J., and B.B.B. contributed to the discussion and reviewed the manuscript. I.C., M.S., and M.S.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-2331/-/DC1.
References
- 1.Li J, Shao YH, Gong YP, Lu YH, Liu Y, Li CL. Diabetes mellitus and dementia - a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 2014;18:1778–1789 [PubMed] [Google Scholar]
- 2.Lee JH, Choi Y, Jun C, et al. . Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinol Metab (Seoul) 2014;29:112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravona-Springer R, Moshier E, Schmeidler J, et al. . Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis 2012;30:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugawara A, Kawai K, Motohashi S, et al. . HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia 2012;55:2128–2131 [DOI] [PubMed] [Google Scholar]
- 5.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy [published correction appears in Nat Rev Neurol 2013. doi:10.1038/nmeurol.201.32]. Nat Rev Neurol 2013;9:106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravona-Springer R, Heymann A, Schmeidler J, et al. . The ApoE4 genotype modifies the relationship of long-term glycemic control with cognitive functioning in elderly with type 2 diabetes. Eur Neuropsychopharmacol 2014;24:1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schilling S, DeStefano AL, Sachdev PS, et al. . APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology 2013;81:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirono N, Yasuda M, Tanimukai S, Kitagaki H, Mori E. Effect of the apolipoprotein E epsilon4 allele on white matter hyperintensities in dementia. Stroke 2000;31:1263–1268 [DOI] [PubMed] [Google Scholar]
- 9.Beeri MS, Ravona-Springer R, Moshier E, et al. . The Israel Diabetes and Cognitive Decline (IDCD) study: design and baseline characteristics. Alzheimers Dement 2014;10:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt P, Gaser C, Arsic M, et al. . An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage 2012;59:3774–3783 [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Fratiglioni L, Laukka EJ, et al. . Effects of vascular risk factors and APOE ε4 on white matter integrity and cognitive decline. Neurology 2015;84:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penno G, Solini A, Zoppini G, et al.; Renal Insufficiency and Cardiovascular Events (RIACE) Study Group . Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: a cross-sectional analysis of the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Cardiovasc Diabetol 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care 2004;27:2335–2340 [DOI] [PubMed] [Google Scholar]
- 14.Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ. Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab 2009;297:E194–E201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jongen C, van der Grond J, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JP; Utrecht Diabetic Encephalopathy Study Group . Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia 2007;50:1509–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 2005;237:251–257 [DOI] [PubMed] [Google Scholar]
- 17.de Bresser J, Tiehuis AM, van den Berg E, et al.; Utrecht Diabetic Encephalopathy Study Group . Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.