Abstract
OBJECTIVE
To determine whether, after adjustment for glycemia and other selected covariates, hemoglobin A1c (HbA1c) differed among adults from six Hispanic/Latino heritage groups (Central American, Cuban, Dominican, Mexican, Puerto Rican, and South American) and between Hispanic/Latino and non-Hispanic white adults without self-reported diabetes.
RESEARCH DESIGN AND METHODS
We performed a cross-sectional analysis of data from 13,083 individuals without self-reported diabetes from six Hispanic/Latino heritage groups, enrolled from 2008 to 2011 in the Hispanic Community Health Study/Study of Latinos, and 2,242 non-Hispanic white adults enrolled during the 2007–2012 cycles of the National Health and Nutrition Examination Survey. We compared HbA1c levels among Hispanics/Latinos and between Hispanics/Latinos and non-Hispanic whites before and after adjustment for age, sex, fasting (FPG) and 2-h post–oral glucose tolerance test (2hPG) glucose, anthropometric measurements, and selected biochemical and hematologic variables and after stratification by diabetes status: unrecognized diabetes (FPG ≥7.1 mmol/L or 2hPG ≥11.2 mmol/L), prediabetes (FPG 5.6–7.0 mmol/L or 2hPG 7.8–11.1 mmol/L), and normal glucose tolerance (FPG <5.6 mmol/L and 2hPG <7.8 mmol/L).
RESULTS
Adjusted mean HbA1c differed significantly across all seven groups (P < 0.001). Non-Hispanic whites had significantly lower HbA1c (P < 0.05) than each individual Hispanic/Latino heritage group. Upon stratification by diabetes status, statistically significant differences (P < 0.001) in adjusted mean HbA1c persisted across all seven groups.
CONCLUSIONS
HbA1c differs among Hispanics/Latinos of diverse heritage groups and between non-Hispanic whites and Hispanics/Latinos after adjustment for glycemia and other covariates. The clinical significance of these differences is unknown.
Introduction
Hemoglobin A1c (HbA1c) is a widely used and accepted test for the diagnosis of prediabetes and diabetes and the assessment of glycemic control in patients with diabetes (1). Due to the erythrocytes’ long lifetime and the slow, continuous, and essentially irreversible characteristics of the glycation process, HbA1c reflects the average blood glucose concentration for the preceding 2–3 months (1–4).
Prior studies have suggested and demonstrated that HbA1c may vary across racial/ethnic groups after adjustment for plasma glucose levels (5–12). In individuals without diabetes, two analyses based on the National Health Nutrition and Examination Survey (NHANES) have reported higher HbA1c levels among African Americans and Mexican Americans compared with non-Hispanic whites, and these differences persisted after adjustment for age, sex, and anthropometric, biological, or other covariates (7,8). Another analysis based on NHANES demonstrated differences in the increment in HbA1c with each decade of age among Mexican Americans, non-Hispanic blacks, and non-Hispanic whites (9). In the Diabetes Prevention Program (10), non-Hispanic white individuals with impaired glucose tolerance had baseline HbA1c levels 0.15–0.40% lower than individuals from other racial/ethnic groups (including Hispanics) before and after adjustment for glucose levels and other covariates. Another study of individuals with diabetes reported HbA1c 0.3–0.8% higher among those with ancestry other than non-Hispanic white, despite the fact that individuals from diverse racial/ethnic groups had similar mean plasma glucose levels (11).
The studies cited above reported differences in HbA1c between Hispanics/Latinos and non-Hispanic whites (with or without diabetes) that accounted for a variety of demographic, anthropometric, and biological covariates (7–11). Some of these studies specifically reported differences in HbA1c between non-Hispanic whites and Mexican Americans (7–9). Other studies did not clearly specify the composition of the Hispanic group included or account for differences by Hispanic/Latino heritage (10,11). Considering the diverse genetic admixture and socioeconomic, cultural, geographic, history, and migration patterns that characterize each U.S. Hispanic/Latino group, it cannot be assumed that the previously reported differences in HbA1c compared with non-Hispanic whites apply to all heritage groups. One study suggested that HbA1c may differ between Dominicans and other Hispanics/Latinos with diabetes (heritage groups not specified) (12). However, it is unknown whether this difference is also observed among other Hispanic/Latino heritage groups—independent of glycemic status—and what factors may explain those differences.
Our analysis was based on data from the baseline exam (2008–2011) of the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) and the NHANES 2007–2012 exam cycles. The purpose of this analysis was to compare mean HbA1c levels (before and after adjustment for selected covariates) among six different Hispanic/Latino heritage groups represented in the HCHS/SOL, between Hispanics/Latinos (as a unitary group) from the HCHS/SOL and non-Hispanic whites from NHANES, and between individual Hispanic/Latino heritage groups and non-Hispanic whites from NHANES. In addition, these comparisons would be further stratified by diabetes status category (normal glucose tolerance [NGT], prediabetes, and unrecognized diabetes) based on the American Diabetes Association fasting plasma glucose (FPG) and 2-h post–oral glucose tolerance test plasma glucose (2hPG). Understanding the potential differences in HbA1c levels among different Hispanics/Latinos heritage groups would have important clinical implications in the use of this biomarker as a prediabetes or diabetes diagnostic criterion and in the monitoring of glycemic control in Hispanics/Latinos with diabetes.
Research Design and Methods
Sampling and Examination in the HCHS/SOL
The HCHS/SOL study methods and sampling designs have previously been published (13,14). Briefly, the HCHS/SOL is a longitudinal, population-based study with objectives including description of the prevalence of selected chronic diseases, identifying their risk and/or protective factors, and quantifying incidence of fatal and nonfatal cardiovascular and pulmonary events and all-cause mortality. From March 2008 to June 2011, 16,415 persons, aged 18–74 years at the time of screening, who self-identified as Hispanics/Latinos were examined. Participants were recruited after a multistage probability sampling of the communities in San Diego, CA; Chicago, IL; Miami, FL; and the Bronx, NY. The study was approved by each of the field centers’ and coordinating center's institutional review boards. All enrolled individuals provided signed informed consent. Approximately 93% of participants completed all interviews and tests.
In the HCHS/SOL, interviews (including demographic and self-identified Hispanic/Latino heritage group), phlebotomy, processing of biospecimens, and systolic blood pressure (SBP), diastolic blood pressure (DBP), and anthropometric measurements (including BMI) were performed by trained and certified staff following a standard protocol (13). Further detailed information is available at www.cscc.unc.edu/hchs. Participants were asked to consume only water and necessary medications after 10:00 p.m. the night before the baseline visit and to refrain from smoking or physical activity before undergoing the fasting examination procedures. The examination of pregnant women was postponed until 3 months postpartum. Individuals with other chronic diseases or health conditions were not excluded from participating. All participants had FPG and HbA1c measured. After the initial venipuncture, those without self-reported diabetes or FPG ≤150 mg/dL (8.4 mmol/L) underwent a standard 75-g 2-h oral glucose tolerance test (OGTT), from which a 2hPG was obtained. There were no other exclusions for the OGTT.
Sampling and Examination in NHANES
Using a 2-year-cycle multistage probability sampling design, NHANES is conducted to describe health conditions and disease burden among a representative sample of the U.S. civilian noninstitutionalized population. During the 2007–2008, 2009–2010, and 2011–2012 cycles, 29,353 individuals were interviewed and examined (15). Participants’ demographic and anthropometric characteristics, SBP, DBP, and a blood sample were obtained following a standard protocol (16,17). All participants had HbA1c measured. FPG was obtained after an 8- to 24-h fast from individuals who were randomly assigned to the morning examination; a 75-g OGTT was performed only on these individuals to obtain 2hPG. Pregnant women and individuals with cancer, hemophilia, and other selected conditions were included in the examination but did not undergo the OGTT. Further NHANES information is available at http://www.cdc.gov/nchs/nhanes.htm.
Laboratory Methodology
Biospecimens for the select biochemical variables in both the HCHS/SOL and NHANES 2007–2012 cycles were processed and analyzed by the Advanced Research and Diagnostics Laboratory at the University of Minnesota following similar laboratory methodology and quality-control protocols. Plasma glucose was measured in EDTA-anticoagulated plasma using a hexokinase enzymatic method; alanine aminotransferase (ALT), aspartate aminotransferase (AST), and γ-glutamyl aminotransferase (GGT) were measured using an α-ketoglutaratic enzymatic method on a Roche Modular P chemistry analyzer (Roche Diagnostics, Indianapolis, IN). A hemogram (including direct measures of hemoglobin [Hgb] and mean corpuscular volume [MCV]) was measured in EDTA whole blood using a Sysmex XE-2100 instrument (Sysmex America, Inc., Mundelein, IL). HbA1c was measured in EDTA-anticoagulated whole blood using a Tosoh G7 automated, nonporous ion-exchange high-performance liquid chromatography analyzer. Serum insulin was measured using an ELISA assay (Mercodia AB, Uppsala, Sweden) (from 1 October 2006 to 28 October 2009) and a sandwich immunoassay method with the Roche Elecsys 2010 analyzer (Roche Diagnostics) (from 29 October 2009 to 30 June 2011). The glomerular filtration rate was estimated (eGFR) using the MDRD equation.
Definition of Hispanic/Latino Heritage and Non-Hispanic White Categories
In the HCHS/SOL, participants were asked what Hispanic/Latino group best described their heritage (Central American, Cuban, Dominican, Mexican, Puerto Rican, South American, more than one heritage, or other) and, in addition to being of Hispanic/Latino heritage, which other categories they would use to describe themselves (American Indian or Alaskan Native, Asian, Native Hawaiian or Pacific Islander, black or African American, white, more than one race, unknown, or not reported [13, www.cscc.unc.edu/hchs]). Approximately 54% of HCHS/SOL participants responded “unknown/refused” or “multiracial” to the questions about race. Therefore, analyses based on race were not performed within the context of Hispanic/Latino heritage. In the NHANES, participants were asked if they considered themselves to be Hispanic or Latino, and what race or races they considered themselves to be: American Indian or Alaskan Native, Asian, black or African American, native Hawaiian or Pacific Islander, white, other, unknown, or refused (16).
Definition of NGT, Prediabetes, and Unrecognized Diabetes
To account for the possible confounding effects of glycemic treatment on HbA1c, we excluded participants with self-reported diabetes and/or those who were using antihyperglycemia medications from the analysis. Using FPG and 2hPG American Diabetes Association criteria (1,18), we classified the remaining participants as having unrecognized diabetes (FPG ≥7.1 mmol/L or 2hPG ≥11.2 mmol/L), prediabetes (impaired fasting glucose, FPG 5.6–7.0 mmol/L, or impaired glucose tolerance, 2hPG 7.8–11.1 mmol/L), or NGT (FPG <5.6 mmol/L and 2hPG <7.8 mmol/L). We applied the same inclusion and exclusion criteria, definitions of analysis variables, and methods of statistical analyses to the data obtained from both HCHS/SOL and NHANES.
In HCHS/SOL, the total number of enrolled individuals was 16,415 (19). The weighted mean age was 43.2 years (95% CI 43.1–43.3), and ∼21% were born in the U.S. mainland, Puerto Rico, or other U.S. territories. Of the total, 14,071 individuals reported no history of diabetes or use of antihyperglycemia medications. Individuals with an eGFR <60 mL/min/1.73 m2 (N = 471), with age outside of the sampling required range (N = 5), or who self-identified with more than one Hispanic/Latino heritage group (N = 437) were excluded. Also, 75 individuals were excluded due to missing data on HbA1c (N = 51) or Hispanic/Latino heritage group data (N = 24). Therefore, a total of 13,083 HCHS/SOL participants who met all the criteria were included in the analyses.
In NHANES, a total of 29,353 individuals were examined during the 2007–2008, 2009–2010, and 2011–2012 exams (15). Of that total, 13,133 individuals were examined in the morning in the fasting state. Of these, 11,970 reported no history of diabetes or use of antihyperglycemia medications. Individuals with age outside of the HCHS/SOL age range (N = 5,142), with an eGFR <60 mL/min/1.73 m2 (N = 589), who self-identified as other than non-Hispanic white (N = 3,648), who did not undergo an OGTT (N = 347), and with missing HbA1c (N = 2) were excluded. A total of 2,242 non-Hispanic whites (49.7% males) met all the criteria and were included in the analyses.
Statistical Analysis
Weighted means (or percentages) and 95% CIs of characteristics were estimated by Hispanic/Latino heritage group, for everyone in HCHS/SOL, and for non-Hispanic whites in NHANES after merging of the two studies’ data sets. The adjusted conditional marginal means of HbA1c for each Hispanic/Latino heritage group and non-Hispanic whites were obtained from linear regression models that included various covariates: age, sex, BMI, FPG, 2hPG, fasting serum insulin (FSI), Hgb, MCV, SBP, DBP, ALT, AST, and GGT. The selection of these covariates was based on previously documented effect or relationship with HbA1c (10,11,20). We repeated this analysis stratifying by diabetes status categories (NGT, prediabetes, and unrecognized diabetes). We conducted an omnibus test for differences in Hispanic/Latino heritage groups using a Wald F test. For differences that were significant based on the Wald F test, we conducted pairwise testing on every combination of Hispanic/Latino heritage group. The β-coefficients (95% CI) to predict HbA1c and P values were obtained from the merged HCHS/SOL and NHANES samples. We also calculated the mean HbA1c for each HCHS/SOL Hispanic/Latino heritage group in unadjusted linear regression models and with various levels of adjustment to investigate the extent to which confounders might explain unadjusted differences in HbA1c.
The analyses described above were also performed in each data set separately. The results were very similar and have been included in Supplementary Tables 2–4 and Supplementary Fig. 1.
All statistical analyses were performed using SAS, version 9.2, and SAS-callable SUDAAN, version 11.0.1, to account for the complex sampling designs, including unequal probabilities of selection, oversampling, and nonresponse.
Results
Of the 13,083 HCHS/SOL individuals who met the inclusion criteria, 47.9% were men, and the self-identified Hispanic/Latino heritage group breakdown was 40.0% Mexican, 20.8% Cuban, 15.6% Puerto Rican, 10.3% Dominican, 7.9% Central American, and 5.4% South American. Table 1 describes the demographic, anthropometric, and biochemical characteristics of HCHS/SOL participants according to Hispanic/Latino heritage group and non-Hispanic white participants from NHANES. All the characteristics were statistically different (P < 0.05) across groups.
Table 1.
Participants’ characteristics by Hispanic/Latino heritage group from the HCHS/SOL and non-Hispanic whites from NHANES
| Characteristics of participants | HCHS/SOL: March 2008–June 2011 | NHANES 2007–2012: non-Hispanic white (N = 2,242) | ||||||
|---|---|---|---|---|---|---|---|---|
| Dominican (N = 1,220) | Central American (N = 1,485) | Cuban (N = 1,991) | Mexican (N = 5,426) | Puerto Rican (N = 2,016) | South American (N = 945) | All Hispanics/Latinos (N = 13,083) | ||
| Men, % | 38.7 (34.7–42.8) | 47.6 (44.2–51.0) | 52.5 (50.2–54.7) | 46.4 (44.4–48.5) | 51.9 (48.7–55.1) | 45.2 (41.3–49.2) | 47.9 (46.7–49.1) | 49.7 (47.8–51.5) |
| Age, years | 37.2 (35.9–38.4) | 38.2 (37.3–39.2) | 44.3 (43.4–45.3) | 37.0 (36.3–37.7) | 40.1 (39.1–41.1) | 41.5 (40.0–43.0) | 39.1 (38.6–39.5) | 44.0 (43.1–44.9) |
| BMI, kg/m2 | 29.2 (28.6–29.9) | 28.8 (28.4–29.2) | 28.7 (28.4–29.0) | 28.9 (28.6–29.2) | 30.1 (29.6–30.6) | 27.8 (27.4–28.3) | 29.0 (28.9–29.2) | 28.1 (27.8–28.5) |
| HbA1c, % | 5.45 (5.41–5.49) | 5.50 (5.45–5.54) | 5.49 (5.46–5.53) | 5.51 (5.47–5.54) | 5.51 (5.48–5.54) | 5.46 (5.42–5.49) | 5.49 (5.47–5.50) | 5.37 (5.34–5.40) |
| HbA1c, mmol/mol | 36.1 (35.6–36.6) | 36.6 (36.1–37.1) | 36.6 (36.2–37.0) | 36.7 (36.4–37.1) | 36.7 (36.4–37.1) | 36.2 (35.8–36.6) | 36.5 (36.3–36.7) | 35.2 (34.9–35.5) |
| FPG, mmol/L | 5.16 (5.10–5.21) | 5.33 (5.26–5.39) | 5.37 (5.32–5.42) | 5.35 (5.29–5.41) | 5.27 (5.22–5.32) | 5.28 (5.22–5.33) | 5.31 (5.28–5.34) | 5.34 (5.31–5.38) |
| 2hPG, mmol/L | 6.27 (6.11–6.42) | 6.52 (6.37–6.67) | 6.78 (6.64–6.92) | 6.50 (6.40–6.60) | 6.32 (6.19–6.45) | 6.56 (6.36–6.77) | 6.49 (6.43–6.55) | 6.07 (5.98–6.17) |
| FSI, pmol/L | 65.2 (61.7–68.8) | 78.6 (75.4–81.8) | 78.3 (75.0–81.7) | 75.4 (72.3–78.6) | 76.9 (73.0–80.8) | 66.4 (62.6–70.3) | 75.0 (73.3–76.6) | 67.0 (63.2–70.9) |
| Hgb, g/dL | 13.5 (13.4–13.7) | 14.0 (13.9–14.2) | 14.2 (14.1–14.3) | 14.0 (13.9–14.0) | 13.9 (13.8–14.0) | 13.8 (13.7–13.9) | 14.0 (13.9–14.0) | 14.6 (14.49–14.7) |
| MCV, fL | 88.0 (87.6–88.5) | 88.8 (88.3–89.2) | 90.4 (90.0–90.7) | 89.0 (88.7–89.3) | 89.0 (88.6–89.4) | 89.7 (89.2–90.2) | 89.2 (89.0–89.3) | 89.9 (89.5–90.2) |
| SBP, mmHg | 119.6 (118.3–121.0) | 119.7 (118.6–120.8) | 122.8 (121.8–123.7) | 115.6 (114.9–116.2) | 120.3 (119.4–121.3) | 117.5 (116.1–118.9) | 118.5 (118.1–119.0) | 117.5 (116.7–118.4) |
| DBP, mmHg | 73.6 (72.8–74.4) | 72.7 (71.9–73.5) | 74.9 (74.3–75.6) | 69.5 (69.0–70.0) | 73.2 (72.6–73.9) | 70.1 (69.1–71.1) | 71.9 (71.5–72.2) | 70.0 (69.1–70.9) |
| ALT, units/L | 22.7 (21.4–24.0) | 28.7 (27.3–30.0) | 26.8 (25.7–27.8) | 30.2 (28.9–31.4) | 27.7 (25.9–29.5) | 26.8 (25.0–28.7) | 28.0 (27.4–28.6) | 25.2 (24.5–25.8) |
| AST, units/L | 22.1 (21.2–22.9) | 24.4 (23.7–25.1) | 23.4 (23.0–23.9) | 25.3 (24.5–26.1) | 25.9 (24.6–27.1) | 24.0 (22.9–25.1) | 24.6 (24.1–25.0) | 24.9 (24.4–25.3) |
| GGT, units/L | 25.5 (23.8–27.1) | 35.2 (32.2–38.1) | 33.2 (30.4–35.9) | 32.5 (31.0–34.1) | 36.3 (33.3–39.3) | 28.7 (25.8–31.5) | 32.3 (31.3–33.3) | 25.4 (24.3–26.6) |
Data are means (95% CI). Data were weighted and adjusted to the 2010 U.S. Census. All variables were significantly different (P < 0.05) across all seven racial/heritage groups.
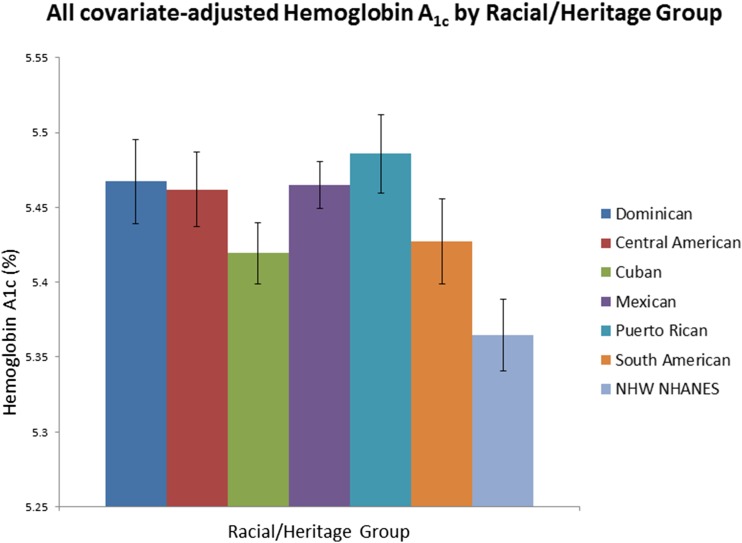
Figure 1 illustrates all covariate–adjusted mean (95% CI) HbA1c levels by racial/ethnic group. The all covariate–adjusted mean HbA1c was significantly different among Hispanic/Latino heritage groups (Wald F test, P < 0.001). Based on pairwise comparisons, individuals of Cuban heritage had significantly (P < 0.05) lower adjusted mean HbA1c levels (5.42% [95% CI 5.40–5.44]) compared with other individual Hispanic/Latino heritage groups, except those of South American heritage (5.43% [95% CI 5.40–5.46]). Based on pairwise comparisons, non-Hispanic whites had significantly lower (5.36% [95% CI 5.34–5.39]) adjusted mean HbA1c levels compared with each Hispanic/Latino heritage group (each P < 0.05). We added diabetes status categories (based on FPG and 2hPG) to the adjustment model, and the differences across the seven groups persisted and remained statistically significant (data not shown).
Figure 1.
Error bars represent 95% CIs. The covariates included sex, age, BMI, FPG, 2hPG, FSI, Hgb, MVC, SBP, DBP, ALT, AST, and GGT. The HCHS/SOL data were collected from March 2008 to June 2011, and NHANES data were collected from 2007 to 2012. NHW, non-Hispanic white.
In Table 2, we examined the differences in all covariate–adjusted mean HbA1c levels by diabetes status category. Within the NGT category, the all covariate–adjusted mean HbA1c level differed significantly according to Hispanic/Latino heritage groups (Wald F test, P < 0.001). Pairwise comparisons showed that this difference was attributable to significant differences (P < 0.05) in HbA1c of Cuban versus Mexican and Puerto Rican heritage groups (adjusted mean HbA1c difference 0.04–0.05% [National Glycohemoglobin Standardization Program (NGSP) units]), and Puerto Rican vs. South American heritage groups (adjusted mean HbA1c difference 0.04% [NGSP units]). The all covariate–adjusted mean HbA1c of non-Hispanic whites differed significantly from that of individual Hispanic/Latino heritage group (difference 0.05–0.10% [NGSP units], each P < 0.05).
Table 2.
All covariate–adjusted mean HbA1c levels* by racial/heritage group and diabetes status category
| Diabetes status categories† | HCHS/SOL: March 2008–June 2011 |
NHANES 2007–2012: non-Hispanic white (N = 2,242) | ||||||
|---|---|---|---|---|---|---|---|---|
| Dominican (N = 1,220) | Central American (N = 1,485) | Cuban (N = 1,991) | Mexican (N = 5,426) | Puerto Rican (N = 2,016) | South American (N = 945) | All Hispanics/Latinos (N = 13,083) | ||
| NGT‡ |
823; 5.33 (5.30–5.36)# |
919; 5.33 (5.30–5.36)# |
1,187; 5.31 (5.28–5.34)§‖# |
3,274; 5.35 (5.33–5.37)# |
1,256; 5.35 (5.33–5.38)¶# |
607; 5.32 (5.29–5.35)# |
8,066; 5.34 (5.32–5.35) |
1,329; 5.26 (5.24–5.28) |
| Prediabetes‡ |
327; 5.66 (5.61–5.72)# |
460; 5.62 (5.58–5.66)# |
663; 5.56 (5.53–5.59)**††§# |
1,742; 5.62 (5.59–5.64)# |
613; 5.61 (5.56–5.67)# |
290; 5.58 (5.53–5.63)# |
4,095; 5.60 (5.59–5.62) |
797; 5.47 (5.43–5.51) |
| Unrecognized diabetes‡ | 70; 6.33 (6.20–6.46)# | 106; 6.44 (6.37–6.56)# | 141; 6.19 (6.06–6.32)‖†† | 410; 6.30 (6.21–6.40)# | 147; 6.41 (6.27–6.55)# | 48; 6.19 (5.95–6.43) | 922; 6.29 (6.23–6.36) | 116; 6.05 (5.93–6.18) |
Data are N; means in NGSP units (95% CI).
*Adjusted for sex, age, BMI, FPG, 2hPG, FSI, Hgb, MCV, SBP, DBP, ALT, AST, and GGT.
†Glycemic categories include NGT (FPG <5.6 mmol/L and 2hPG <7.8 mmol/L), prediabetes (FPG 5.6–7.0 mmol/L or 2hPG 7.8–11.1 mmol/L), and unrecognized diabetes (FPG ≥7.1 mmol/L or 2hPG ≥11.2 mmol/L).
‡The Wald F test differences across all groups were statistically significant (P < 0.001).
§Pairwise comparison was statistically significant (P < 0.05) compared with the Mexican heritage group.
‖Pairwise comparison was statistically significant (P < 0.05) compared with the Puerto Rican heritage group.
¶Pairwise comparison was statistically significant (P < 0.05) compared with the South American heritage group.
#Pairwise comparison was statistically significant (P < 0.05) compared with non-Hispanic whites.
**Pairwise comparison was statistically significant (P < 0.05) compared with the Dominican heritage group.
††Pairwise comparison was statistically significant (P < 0.05) compared with the Central American heritage group.
Within the prediabetes category, the all covariate–adjusted mean HbA1c differed significantly according to Hispanic/Latino heritage group (Wald F test, P < 0.001). The pairwise comparisons showed that this difference was attributable to significant differences (P < 0.05) in the HbA1c levels between the Cuban and the Central American, Dominican, and Mexican heritage groups (difference 0.06–0.10% [NGSP units]). The all covariate–adjusted mean HbA1c also differed significantly between non-Hispanic whites and each Hispanic/Latino heritage group (difference 0.09–0.19% [NGSP units], each P < 0.05).
Within the unrecognized diabetes category, the all covariate–adjusted mean HbA1c level differed significantly among Hispanics/Latinos (Wald F test, P < 0.001). This difference was attributable to significant differences between the Cuban and the Central American and Puerto Rican heritage groups (difference 0.22–0.25% [NGSP units], P < 0.05). The difference in all covariate–adjusted HbA1c was significant between non-Hispanic whites and Central American, Dominican, Mexican, and Puerto Rican heritage groups (difference 0.25–0.39% [NGSP units], each P < 0.05).
In a separate analysis, we compared the all covariate–adjusted mean HbA1c level of the HCHS/SOL (unitary group), the HCH/SOL Mexican heritage group, and the NHANES non-Hispanic whites and performed a parallel analysis comparing the NHANES Mexican Americans, NHANES Hispanics (unitary group), and NHANES non-Hispanic whites. The differences between HCHS/SOL and NHANES non-Hispanic whites were similar, statistically significant, and in the same direction as the differences between NHANES Mexican American/Hispanic and non-Hispanic whites (data not shown).
We estimated the β of the regression model including all covariates associated with HbA1c for the combined HCHS/SOL and NHANES data without stratification by glycemic category (Supplementary Table 1). A β indicates the difference in HbA1c level for each category of categorical variables (e.g., heritage, sex) or for each one-unit-higher level of continuous variables (e.g., BMI, FPG). The variable is considered statistically significant when the CI does not contain zero. Age, sex, age * sex interaction term, BMI, FPG, and 2hPG were associated with HbA1c across all seven groups. When the regression model was evaluated in HCHS/SOL and NHANES separately (Supplementary Table 2), the same associations were observed, except the age * sex interaction, and FSI was associated with HbA1c among Hispanics/Latinos but not among non-Hispanic whites.
Table 3 illustrates the model of adjustments of mean HbA1c according to covariates included in the combined model and by racial/heritage group. The largest changes in mean HbA1c occurred after adjustment for age, FPG, and 2hPG.
Table 3.
Unadjusted and adjusted mean HbA1c levels by racial/ethnic heritage group
| Adjustment variables | HCHS/SOL: March 2008–June 2011 |
NHANES 2007–2012: non-Hispanic white (N = 2,242) | ||||||
|---|---|---|---|---|---|---|---|---|
| Dominican (N = 1,220) | Central American (N = 1,485) | Cuban (N = 1,991) | Mexican (N = 5,426) | Puerto Rican (N = 2,016) | South American (N = 945) | All Hispanics/Latinos (N = 13,083) | ||
| Unadjusted HbA1c (%) |
5.45 (5.41–5.49) |
5.50 (5.45–5.54) |
5.49 (5.46–5.53) |
5.51 (5.47–5.54) |
5.51 (5.48–5.54) |
5.46 (5.42–5.49) |
5.49 (5.48–5.51) |
5.37 (5.34–5.40) |
| Sex |
5.45 (5.41–5.49) |
5.50 (5.45–5.54) |
5.49 (5.46–5.53) |
5.51 (5.47–5.54) |
5.51 (5.47–5.54) |
5.46 (5.42–5.49) |
5.49 (5.48–5.51) |
5.37 (5.34–5.40) |
| Age |
5.51 (5.47–5.55) |
5.54 (5.50–5.58) |
5.46 (5.43–5.49) |
5.57 (5.53–5.60) |
5.53 (5.50–5.56) |
5.46 (5.43–5.49) |
5.52 (5.51–5.54) |
5.34 (5.32–5.37) |
| Age * sex interaction** |
5.51 (5.47–5.55) |
5.54 (5.50–5.58) |
5.46 (5.43–5.49) |
5.57 (5.53–5.60) |
5.53 (5.50–5.55) |
5.46 (5.43–5.49) |
5.52 (5.51–5.54) |
5.34 (5.32–5.37) |
| BMI (kg/m2) |
5.44 (5.40–5.48) |
5.49 (5.45–5.54) |
5.49 (5.45–5.52) |
5.50 (5.47–5.53) |
5.48 (5.45–5.51) |
5.47 (5.43–5.51) |
5.49 (5.47–5.50) |
5.38 (5.35–5.41) |
| FPG and 2hPG (mg/dL) |
5.47 (5.44–5.49) |
5.45 (5.42–5.48) |
5.43 (5.41–5.45) |
5.44 (5.43–5.46) |
5.49 (5.47–5.52) |
5.43 (5.40–5.46) |
5.45 (5.44–5.46) |
5.37 (5.35–5.40) |
| FSI (μU/mL) |
5.46 (5.42–5.50) |
5.48 (5.44–5.53) |
5.48 (5.44–5.51) |
5.50 (5.47–5.53) |
5.50 (5.47–5.53) |
5.46 (5.42–5.50) |
5.49 (5.47–5.50) |
5.38 (5.35–5.41) |
| Hgb (g/dL) |
5.44 (5.40–5.49) |
5.50 (5.45–5.54) |
5.49 (5.46–5.53) |
5.50 (5.47–5.54) |
5.50 (5.47–5.54) |
5.46 (5.42–5.49) |
5.49 (5.47–5.51) |
5.37 (5.34–5.40) |
| MCV (fL) |
5.44 (5.39–5.48) |
5.49 (5.45–5.54) |
5.50 (5.46–5.53) |
5.50 (5.47–5.54) |
5.50 (5.47–5.53) |
5.46 (5.42–5.49) |
5.49 (5.47–5.51) |
5.37 (5.35–5.40) |
| SBP and DBP (mmHg) |
5.44 (5.40–5.48) |
5.49 (5.44–5.53) |
5.46 (5.42–5.49) |
5.52 (5.49–5.56) |
5.49 (5.46–5.52) |
5.46 (5.43–5.50) |
5.49 (5.47–5.51) |
5.38 (5.35–5.41) |
| ALT, AST, GGT (units/L) |
5.46 (5.42–5.50) |
5.48 (5.44–5.53) |
5.48 (5.44–5.51) |
5.49 (5.46–5.53) |
5.50 (5.47–5.53) |
5.45 (5.42–5.49) |
5.48 (5.47–5.50) |
5.38 (5.35–5.41) |
| All covariates | 5.47 (5.44–5.50) | 5.46 (5.44–5.49) | 5.42 (5.40–5.44) | 5.46 (5.45–5.48) | 5.49 (5.46–5.51) | 5.43 (5.40–5.46) | 5.46 (5.45–5.47) | 5.36 (5.34–5.39) |
Data presented as means in NGSP units (95% CI).
**This model included age, sex, and an interaction term for age * sex.
Conclusions
The observations described in this analysis both confirm previous findings and offer new light on population-based differences in HbA1c. Compared with Hispanics/Latinos (as a group), non-Hispanic whites demonstrated significantly lower adjusted mean HbA1c levels within each glycemic category. The difference in adjusted mean HbA1c level between non-Hispanic whites and Hispanics/Latinos ranged from 0.08 to 0.24% (NGSP units) across diabetes status categories. Mean adjusted HbA1c levels also varied among Hispanic/Latino heritage groups, with differences ranging from 0.04 to 0.25% (NGSP units) across diabetes status categories. The largest difference in mean adjusted HbA1c (between non-Hispanic whites and Hispanics/Latinos and among Hispanic/Latino heritage groups or across the seven groups) was observed in the unrecognized diabetes category. Neither the difference in HbA1c between non-Hispanic whites and Hispanics/Latinos nor the differences among the Hispanic/Latino heritage groups could be explained by controlling for age, sex, BMI, plasma glucose, or hematologic or biochemical covariates. To the best of our knowledge, these are new findings that have not been described and compared before in a large sample of U.S. Hispanic/Latino adults of diverse heritage groups.
Our results confirm that there are statistically significant differences in mean HbA1c levels between Hispanics/Latinos (as a unitary group) and non-Hispanic whites with NGT, prediabetes, and unrecognized diabetes after adjustment for age, sex, BMI, FPG, 2hPG, FSI, Hgb, MCV, SBP, and hepatic enzymes, as previously demonstrated in NHANES (9), the Diabetes Prevention Program (10), and by Herman et al. (11). In addition, we observed that HbA1c had a direct relationship with age, as demonstrated in the Framingham Offspring Study and the NHANES (21).
Important strengths of our study include the diversity of Hispanic/Latino heritage groups and the large sample size—in both the HCHS/SOL and the NHANES—which provides more reliable estimates for HbA1c, the ability to exclude individuals who had conditions that might impact the relationship between HbA1c and the selected covariates, and adequate power to assess statistical differences among Hispanic/Latino heritage groups and non-Hispanic whites according to diabetes status categories.
The evaluation of differences in HbA1c levels should also be cautiously interpreted from a racial/ethnic perspective. The terms “non-Hispanic white” and “Hispanic/Latino” give the impression that these two demographic groups are distinct races or ethnicities and that each Hispanic/Latino heritage group constitutes a separate racial or ethnic entity. These interpretations assume that race is a purely biological classification of human ancestry/origins, and not a sociocultural construct (22); fail to acknowledge that Hispanics/Latinos could be of any race; and could mislead the interpretation of the observations by attributing differences exclusively to ancestry. On the other hand, the genetics of Hgb glycation have not been fully described but are currently being studied (23–25). Genetic analyses of HbA1c and other select variables assessed during the baseline examination are currently in progress and will be published separately.
The differences in covariate-adjusted mean HbA1c levels among Hispanics/Latinos or between Hispanics/Latinos and non-Hispanic whites could be interpreted in the light of mediators that were not evaluated. For instance, conditions that increase or decrease red cell turnover, including Hgb variants and hemoglobinopathies (26), history of blood transfusion (27) or blood loss, or other blood or coagulation disorders were not evaluated as part of the baseline examination in the HCHS/SOL. Differences in red blood cell morphology (28), red cell life span (29–31) or Hgb glucose affinity and glycation (28,31,32), iron deficiency and iron deficiency anemia (33), and obstructive sleep apnea (34,35), among other factors, may have also mediated the observed differences. Some of these factors may not be intrinsically related to a specific Hispanic/Latino genetic or cultural heritage but may depend on cumulative nutritional, socioeconomic, and medical history (36) and on local community resources or exposures. In addition, although both the reliability and repeatability of the HbA1c assay in the study were high, the analyses presented in this report only represent a one-time assessment correlation and not repeated measurements over time.
Existing HbA1c assays can only detect levels within one decimal place of percent unit based on the NGSP (37). Hence, the clinical implications of the differences in HbA1c to two decimal places observed in our analysis are unknown. The precision of the observed statistically significant differences in adjusted mean HbA1c levels in the NGT and prediabetes categories would not be detected in a clinical assay, and a diagnostic or therapeutic decision might not be pursued. In contrast, adjusted mean HbA1c level in the unrecognized diabetes category would be detected and considered sufficient to prompt diagnostic or therapeutic decisions.
Similarly, the clinical significance of the observed differences in HbA1c in our analysis for long-term diabetes-related complications is unknown. In the UK Prospective Diabetes Study (UKPDS), every 1% NGSP unit reduction in HbA1c was associated with a 37% reduction in microvascular complications and 43% reduction in amputations and mortality associated with peripheral vascular disease (38). In the Diabetes Control and Complications Trial (DCCT), a sustained difference in HbA1c of 1.9% NGSP units between patients with type 1 diabetes on intensive insulin therapy and patients on conventional insulin therapy was associated with a 76% risk reduction in the onset of retinopathy and 34% risk reduction in the onset of microalbuminuria (39). Further reduction in HbA1c to the normal range (e.g., 6.0%) was expected to be associated with additional risk reduction in the onset and progression of retinopathy (40). However, the clinical significance of the smaller differences in HbA1c (e.g., 0.25% and 0.39%) observed between Hispanics/Latinos and non-Hispanic whites with unrecognized diabetes in this study is unknown. These differences are not the result of an intervention to reduce glycemic levels and may not reflect differences in glycemia, since mean HbA1c levels were adjusted for FPG and 2hPG. Since HCHS/SOL participants have been followed annually and a second examination is being conducted, assessment of risk for diabetes and macro- and microvascular complications associated with baseline and follow-up HbA1c will be possible in the future.
The findings presented in this analysis suggest that statistically significant differences in HbA1c exist between Hispanics/Latinos and non-Hispanic whites and among different Hispanic/Latino heritage groups. The mechanisms and clinical implications behind these differences need to be further investigated. Understanding the relationship between HbA1c and nonglycemic factors and incorporating them into the interpretation of HbA1c tests may prove valuable to the diagnosis of diabetes and monitoring glycemic control of Hispanics/Latinos.
Supplementary Material
Article Information
Acknowledgments. The authors thank Drs. Paul Sorlie (National Heart, Lung, and Blood Institute, National Institutes of Health [NIH]), Kiang Liu (Feinberg School of Medicine, Northwestern University), and Remington Nevin (Johns Hopkins Bloomberg School of Public Health) for reviewing the manuscript. The authors also thank the more than 250 staff members across the Field Centers, whose dedication and ceaseless energy made the recruitment and baseline examination a success. Also, the authors thank the community board members and other community partners for their vision and feedback and thank the more than 16,000 participants examined at the baseline examination.
Funding. The first phase of the HCHS/SOL was carried out as a collaborative study, supported by contracts from the National Heart, Lung, and Blood Institute, to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The collaborative and co-funded NIH institutes, centers, and offices for the study include the National Institute on Minority Health and Health Disparities, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the NIH Office of Dietary Supplements.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the NIH; or the U.S. Department of Health and Human Services.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.L.A.-S., G.H., N.S., and G.A.T. participated in data collection. M.L.A.-S., L.L.H., M.A., A.M., B.T., and C.C.C. proposed and performed the data analysis. M.L.A.-S. wrote the manuscript. L.L.H., M.A., A.M., E.W., B.T., G.H., Y.T., N.S., A.L.G., L.C.G., and G.A.T. reviewed the manuscript. M.L.A.-S., A.M., and C.C.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-2579/-/DC1.
References
- 1.American Diabetes Association 2. Classification and Diagnosis of Diabetes. Diabetes Care 2016;39(Suppl. 1):S13–S22 [DOI] [PubMed] [Google Scholar]
- 2.Kilpatrick ES. Glycated haemoglobin in the year 2000. J Clin Pathol 2000;53:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn R, Fonseca V. Translating the A1C Assay. Diabetes Care 2008;31:1704–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab 2012;97:1067–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziemer DC, Kolm P, Weintraub WS, et al. . Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med 2010;152:770–777 [DOI] [PubMed] [Google Scholar]
- 7.Saaddine JB, Fagot-Campagna A, Rolka D, et al. . Distribution of HbA(1c) levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care 2002;25:1326–1330 [DOI] [PubMed] [Google Scholar]
- 8.Menke A, Rust KF, Savage PJ, Cowie CC. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distributions in U.S. population subgroups: NHANES 2005-2010. Ann Epidemiol 2014;24:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson MB, Schriger DL. Effect of age and race/ethnicity on HbA1c levels in people without known diabetes mellitus: implications for the diagnosis of diabetes. Diabetes Res Clin Pract 2010;87:415–421 [DOI] [PubMed] [Google Scholar]
- 10.Herman WH, Ma Y, Uwaifo G, et al.; Diabetes Prevention Program Research Group . Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman WH, Dungan KM, Wolffenbuttel BHR, et al. . Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1,5-anhydroglucitol in over 2000 patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:1689–1694 [DOI] [PubMed] [Google Scholar]
- 12.Getaneh A, Andres R, Brillon DJ, Findley SE. Hemoglobin A(₁c) criterion for diabetes diagnosis among Hispanic and non-Hispanic populations. Endocr Pract 2011;17:210–217 [DOI] [PubMed] [Google Scholar]
- 13.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, et al. . Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavange LM, Kalsbeek WD, Sorlie PD, et al. . Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol 2010;20:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES Response Rates and Population Totals [Internet]. Hyattsville, MD, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available from http://www.cdc.gov/nchs/nhanes/response_rates_CPS.htm. Accessed 15 November 2014
- 16.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: Plan and Operations, 1999-2010. Vital Health Stat 2013;1:1–37 [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics. 2011-2012 National Health and Nutrition Examination Survey [Internet]. Hyattsville, MD, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available from http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/manuals11_12.htm. Accessed 15 November 2014
- 18.American Diabetes Association Standards of Medical Care in Diabetes: Classification and Diagnosis of Diabetes. Diabetes Care 2015;38(Suppl. 1):S8–S16 [DOI] [PubMed] [Google Scholar]
- 19.Daviglus ML, Talavera GA, Avilés-Santa ML, et al. . Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA 2012;308:1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarmul JA, Pignone M, Pletcher MJ. Interpreting hemoglobin A1c in combination with conventional risk factors for prediction of cardiovascular risk. Circ Cardiovasc Qual Outcomes 2015;8:501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pani LN, Korenda L, Meigs JB, et al. . Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001-2004. Diabetes Care 2008;31:1991–1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C. “Race” and “ethnicity” in biomedical research: how do scientists construct and explain differences in health? Soc Sci Med 2009;68:1183–1190 [DOI] [PubMed] [Google Scholar]
- 23.Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes 2001;50:2858–2863 [DOI] [PubMed] [Google Scholar]
- 24.Cohen RM, Snieder H, Lindsell CJ, et al. . Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006;29:1739–1743 [DOI] [PubMed] [Google Scholar]
- 25.Soranzo N, Sanna S, Wheeler E, et al.; WTCCC . Common variants at 10 genomic loci influence hemoglobin A₁(C) levels via glycemic and nonglycemic pathways. Diabetes 2010;59:3229–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacks DB. Hemoglobin variants and hemoglobin A1c analysis: problem solved? Clin Chem 2003;49:1245–1247 [DOI] [PubMed] [Google Scholar]
- 27.Spencer DH, Grossman BJ, Scott MG. Red cell transfusion decreases hemoglobin A1c in patients with diabetes. Clin Chem 2011;57:344–346 [DOI] [PubMed] [Google Scholar]
- 28.Veeranna V, Zalawadiya SK, Panaich SS, Ramesh K, Afonso L. The association of red cell distribution width with glycated hemoglobin among healthy adults without diabetes mellitus. Cardiology 2012;122:129–132 [DOI] [PubMed] [Google Scholar]
- 29.Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ. Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 1990;33:208–215 [DOI] [PubMed] [Google Scholar]
- 30.Cohen RM, Franco RS, Khera PK, et al. . Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood 2008;112:4284–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuttall FQ, Gannon MC, Swaim WR, Adams MJ. Stability over time of glycohemoglobin, glucose, and red blood cell survival in hematologically stable people without diabetes. Metabolism 2004;53:1399–1404 [DOI] [PubMed] [Google Scholar]
- 32.Clark SLD, Santin AE, Bryant PA, Holman R, Rodnick KJ. The initial noncovalent binding of glucose to human hemoglobin in nonenzymatic glycation. Glycobiology 2013;23:1250–1259 [DOI] [PubMed] [Google Scholar]
- 33.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care 2010;33:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shpirer I, Rapoport MJ, Stav D, Elizur A. Normal and elevated HbA1C levels correlate with severity of hypoxemia in patients with obstructive sleep apnea and decrease following CPAP treatment. Sleep Breath 2012;16:461–466 [DOI] [PubMed] [Google Scholar]
- 35.Priou P, Le Vaillant M, Meslier N, et al.; IRSR Sleep Cohort Group . Independent association between obstructive sleep apnea severity and glycated hemoglobin in adults without diabetes. Diabetes Care 2012;35:1902–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers HF. Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. J Behav Med 2009;32:9–19 [DOI] [PubMed] [Google Scholar]
- 37.Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) Steering Committee . Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem 2011;57:205–214 [DOI] [PubMed] [Google Scholar]
- 38.Stratton IM, Adler AI, Neil HA, et al. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 40.The Diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44:968–983 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.