Abstract
Cationic liposomes (CLs) have been widely examined as vaccine delivery nanoparticles since they can form complexes with biomacromolecules, promote delivery of antigens and adjuvant molecules to antigen-presenting cells (APCs), and mediate cellular uptake of vaccine components. CLs are also known to trigger antigen cross-presentation – the process by which APCs internalize extracellular protein antigens, degrade them into minimal CD8+ T-cell epitopes, and present them in the context of major histocompatibility complex-I (MHC-I). However, the precise mechanisms behind CL-mediated induction of cross-presentation and cross-priming of CD8+ T-cells remain to be elucidated. In this study, we have developed two distinct CL systems and examined their impact on the lysosomal pH in dendritic cells (DCs), antigen degradation, and presentation of peptide:MHC-I complexes to antigen-specific CD8+ T-cells. To achieve this, we have used 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) as the prototypical components of CLs with tertiary amine groups and compared the effect of CLs and anionic liposomes on lysosomal pH, antigen degradation, and cross-presentation by DCs. Our results showed that CLs, but not anionic liposomes, elevated the lysosomal pH in DCs and reduced antigen degradation, thereby promoting cross-presentation and cross-priming of CD8+ T-cell responses. These studies shed new light on CL-mediated cross-presentation and suggest that intracellular fate of vaccine components and subsequent immunological responses can be controlled by rational design of nanomaterials.
Keywords: cationic liposome, nanoparticle, vaccine, cross-presentation, lysosome
Introduction
Cationic liposomes (CLs), composed of positively charged lipids, have received extensive attention as vaccine delivery vehicles.1–5 Wide interest in CLs stems from their unique attributes, including their abilities to form nanocomplexes with anionic plasmid DNAs, peptides, and proteins;1,2 to prolong antigen release at the site of injection (ie, depot effect);6,7 to induce uptake by antigen-presenting cells (APCs) mediated by their ionic interaction with negatively charged cellular membranes;1,8 and to allow the simultaneous delivery of antigen and adjuvant molecules to APCs, including B-cells, for the induction of IgG responses.9–11 Importantly, CLs can also enhance antigen cross-presentation12,13 – the process by which APCs phagocytose extracellular protein antigens, process them intracellularly into peptide epitopes, and present them in the context of major histocompatibility complex-I (MHC-I) on the surfaces of APCs.14,15 Induction of cross-presentation is a critical mode of action for many vaccines in development since effective presentation of MHC-I:peptide complexes on APCs is required for cross-priming (i.e., priming of antigen-specific CD8+ T-cells). Despite these crucial roles that CLs play in cross-presentation by APCs, their precise mechanisms still need to be elucidated.1,8,12,13
Among APCs, dendritic cells (DCs) are the main cell type that exhibits a high efficiency of antigen cross-presentation.14 The specialized role of DCs in cross-presentation is supported by their unique characteristics, including their mildly degradative phagosomes compared to those of other phagocytic cells, such as macrophages; shuttling of endosomal protein antigens to the cytosol for subsequent processing via proteasomes for antigen degradation; and efficient loading of the processed antigen peptides onto MHC-I molecules via either endoplasmic reticulum (ER) or vacuolar endosomal pathways.16–21 Prior studies have also suggested that DCs exhibit a delayed process of acidification and maturation of endolysosomes, which inhibit the activities of lysosomal proteases and deter antigen degradation.21–24 This would allow antigens to be “rescued” from excessive degradation and shuttled to intracellular compartments for complexation with MHC-I molecules.21,25,26
Based on these previous studies, here, we have developed CLs and investigated the impact of CLs on cross-presentation pathways in DCs. Specifically, we have used 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) as the prototypical components of CLs with tertiary amine groups and compared the effect of CLs and anionic liposomes (ALs) on lysosomal pH, antigen degradation, cross-presentation by DCs, and cross-priming of CD8+ T-cells. Our results showed that CLs, but not ALs, increased the cross-presentation of extracellular antigens and the cross-priming of antigen-specific CD8+ T-cells in part by elevating the lysosomal pH and reducing the endolysosomal degradation of protein antigens in DCs.
Materials and methods
Reagents and antibodies
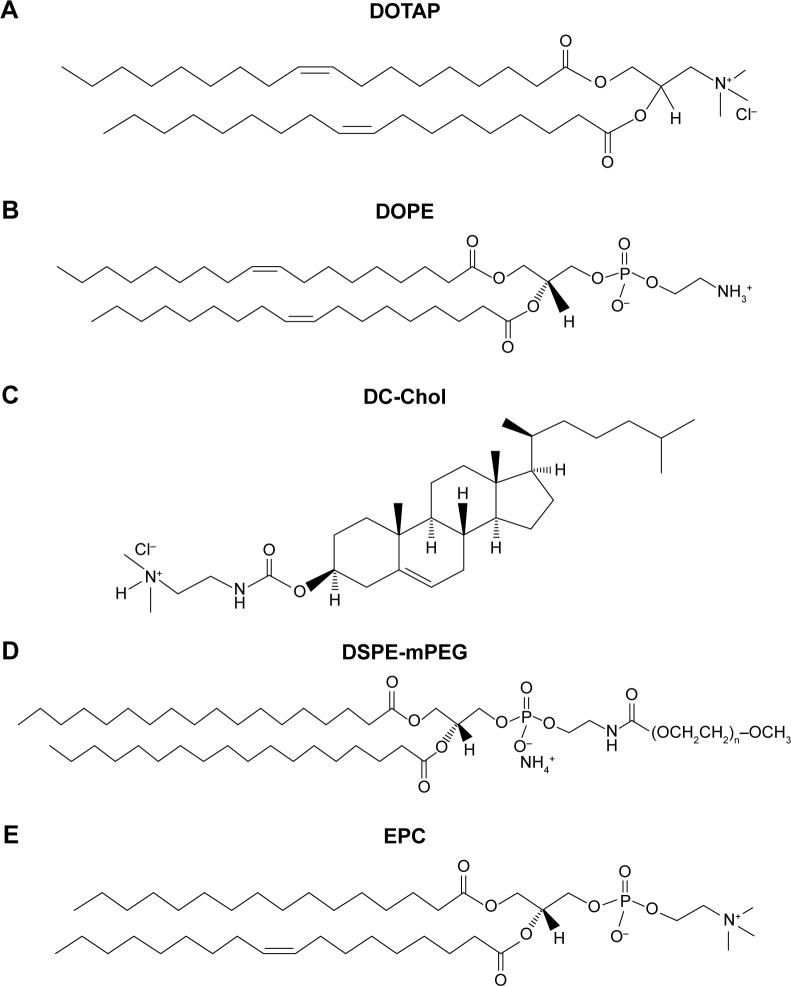
DOTAP, DC-Chol, egg phosphatidylcholine (EPC), cholesterol, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 1,2-distearoyl-sn-glycero-3-phosphoetha-nolamine-N-[methoxy(polyethyleneglycol)-2000] (DSPE-mPEG) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). The structures of these lipids are shown in Figure 1. 4,6-Diamidino-2-phenylindole dihydrochloride (DAPI), carboxyfluorescein diacetate N-succinimidyl ester (CFSE), acridine orange (AO), and chloroquine (CQ) were purchased from Sigma-Aldrich (St Louis, MO, USA). Earle’s balanced salt solution (EBSS) was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Ovalbumin (OVA) was obtained from Worthington (Lakewood, NJ, USA). RPMI 1640 media, fetal bovine serum (FBS), penicillin–streptomycin, β-mercaptoethanol, and ACK lysis buffer were obtained from Life Technologies (Grand Island, NY, USA). Granulocyte macrophage-colony-stimulating factor (GM-CSF) was the product of PeproTech (Rocky Hill, NJ, USA). LysoSensor Green DND-189 was obtained from Invitrogen (Carlsbad, CA, USA). DQ™ OVA was purchased from Molecular Probes (Eugene, OR, USA). Antimouse CD8 antibody (CD8α-APC) was purchased from BD Biosciences (San Jose, CA, USA). A Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Laboratories (Kumamoto, Japan). EasySep™ Mouse CD8+ T-cell Isolation Kit was purchased from STEMCELL Technologies (Vancouver, BC, Canada).
Figure 1.
The chemical structures of lipid components used in this study.
Notes: Shown are the chemical structures of lipid components used in this study, including (A) DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; (B) DOPE, dioleoyl-sn-glycero-3-phosphoethanolamine; (C) DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; (D) DSPE-mPEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000]; and (E) EPC, egg phosphatidylcholine.
Abbreviations: DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; DOPE, dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; DSPE-mPEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000]; EPC, egg phosphatidylcholine.
Preparation and characterization of liposomes
CLs and ALs were prepared as we described previously.27,28 DOTAP-CLs, composed of DOTAP/Chol/DSPE-mPEG (10:7.5:1 molar ratio, 7 μmol); DC-Chol-CLs, composed of DC-Chol/DOPE/DSPE-mPEG (10:7.5:1 molar ratio, 7 μmol); and EPC-ALs (ALs), composed of EPC/Chol/DSPE-mPEG (10:7.5:1 molar ratio, 7 μmol), were prepared by the lipid film hydration method. The lipid film was hydrated with 10 mM phosphate-buffered saline (PBS, pH 7.4) and then extruded into unilamellar liposomes with a hand-held extruder (Mini Extruder; Avanti Polar Lipids) using membranes with 200 nm pore sizes (Whatman® Nuclepore™ membrane; Thermo Fisher Scientific) at room temperature. The resultant liposomes were stored at 4°C. Particle size and zeta potential of liposomes were measured using dynamic light scattering (DLS; Zetasizer Nano ZSP; Malvern Instruments, Malvern, UK).
Preparation of bone marrow-derived DCs (BMDCs)
BMDCs were prepared as described previously.29 C57BL/6 mice were housed in a pathogen-free environment and allowed to acclimate for a week before being used in studies. All experiments described in this protocol were approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan and performed according to the established policies and guidelines. Briefly, femur and tibia were isolated from C57BL/6 mice, and cells were collected by flushing the bone marrow with a syringe and passing the cell suspension through a cell strainer (mesh size =40 μm). After centrifugation, cells were seeded into nontissue culture-treated Petri dish at a density of 2×106 cells/dish and cultured in DC culture media (RPMI 1640 supplemented with 10% FBS, 1% penicillin–streptomycin, 50 μM β-mercaptoethanol, and 20 ng/mL GM-CSF) at 37°C with 5% CO2. Culture media were refreshed on days 3, 6, and 8, and BMDCs were used on days 10–12.
CCK-8 assay
Cellular toxicity of liposomes on BMDCs was examined using the CCK-8 assay as described previously.30 Briefly, BMDCs were cultured overnight in 96-well plates at a density of 2×104 cells/well. The cells were then incubated with indicated samples overnight. Next, 10 μL of CCK-8 solution was added to each well. The plates were incubated at 37°C for 2 h in a humidified CO2 incubator. Absorbance was measured at 450 nm with a microplate reader. Cell viability was calculated using the formula ([AE − AB]/[AC − AB]) ×100, where AE, AC, and AB were the absorbance of experimental samples, untreated samples, and controls, respectively. IC50 of the reagents was calculated using GraphPad 5.0 software.
CFSE dilution assay
In vitro cross-presentation of OVA by BMDCs was measured by the CFSE dilution assay with naive OT-I CD8+ T-cells. Briefly, 5×104 BMDCs were pulsed with OVA in the presence or absence of various doses of liposomes or other reagents together with 1 μg/mL of MPLA (TLR4 agonist; Avanti Polar Lipids). After overnight culture, BMDCs were washed three times and coincubated with 5×104 naive OT-I CD8+ T-cells that were isolated from spleens from OT-I transgenic mice with a magnetic CD8+ T-cell-negative selection kit and prelabeled with 1 μM CFSE for 10 min at 37°C. After 72 h of coculture, cells were stained with CD8α-APC and DAPI, and the percentages of live, proliferated OT-I CD8+ T-cells were determined by running flow cytometry (CyAn5™; Beckman Coulter).
AO staining of BMDCs
AO staining was performed as described previously.31,32 Briefly, BMDCs were cultured overnight in 24-well plates at a density of 1×105 per well. After treatment with various reagents overnight or with EBSS for 4 h, BMDCs were stained with AO (2 μg/mL) for 15 min at 37°C. The cells were trypsinized and washed. The fluorescence signal of AO (excitation: 490 nm; emission: 650 nm) in the lysosomal compartments was analyzed by flow cytometric analysis.
LysoSensor fluorescence assay
The effect of liposomes on lysosomal pH was evaluated using the LysoSensor fluorescence assay as described previously.33,34 The cells were cultured overnight in 24-well plates at a density of 1×105 cells/well. After treatment with various reagents for overnight or with EBSS for 4 h, the cells were incubated with LysoSensor Green DND-189 dye (1 μM in a prewarmed medium) at 37°C for 30 min. The cells were trypsinized and washed. Then, LysoSensor fluorescence signal (excitation: 443 nm; emission: 505 nm) was measured by flow cytometric analysis.
DQ™ OVA assay
The DQ-OVA assay was performed as described previously35 to study antigen processing and presentation induced by liposomes. Briefly, BMDCs (1×106) were treated overnight with different doses of liposomes. After extensive washing with PBS, the cells were incubated with 10 μg/mL DQ-OVA for 15 min at 37°C. The cells were washed, and the green fluorescence of DQ-OVA was monitored by flow cytometric analysis. BMDCs incubated with DQ-OVA at 4°C were used as the control.
Statistical analysis
Data were analyzed using GraphPad 5.0 software. Comparison between the two groups was performed using Student’s nonpaired t-test, and comparison among three or more groups was performed using one-way analysis of variance (ANOVA) with Dunnett’s posttest or Bonferroni’s posttest. A P-value of <0.05 was considered statistically significant (*P<0.05, **P<0.01, ***P<0.001; not significant; P>0.05).
Results
Characterization of CLs
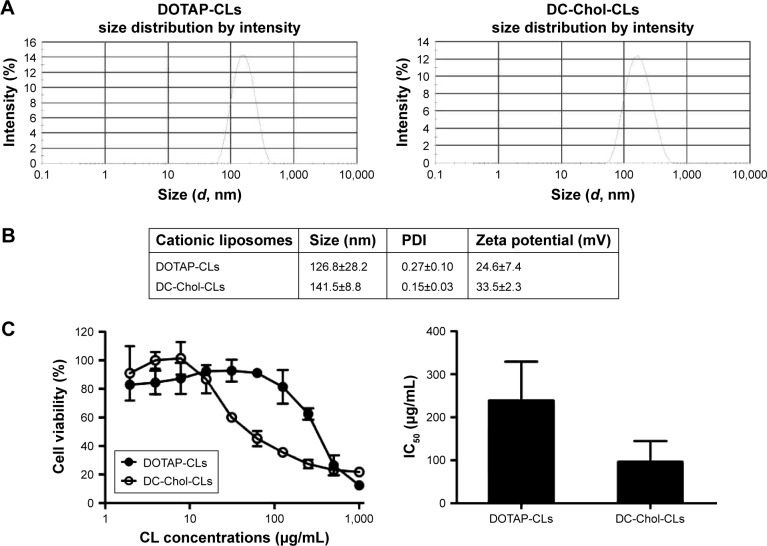
We have developed CLs containing DOTAP or DC-Chol as the prototypical cationic lipid components and performed in vitro characterizations to understand how CLs impact antigen cross-presentation by DCs. Using the lipid thin film method, we synthesized DOTAP-CLs composed of DOTAP/Chol/DSPE-mPEG (10:7.5:1 molar ratio) and DC-Chol-CLs composed of DC-Chol/DOPE/DSPE-mPEG (10:7.5:1 molar ratio; Figure 1). The DLS data showed that DOTAP-CLs and DC-Chol-CLs were 130±28 and 140±8.8 nm in diameter with polydispersity indexes of 0.27±0.10 and 0.15±0.03, respectively (Figure 2A and B). Due to the presence of tertiary amino groups on DOTAP and DC-Chol, these CLs exhibited positive zeta potential of 25±7.4 nm and 34±2.3 mV, respectively (Figure 2B). We assessed the cytotoxicity of CLs on BMDCs exposed to increasing concentrations of DOTAP-CLs and DC-Chol-CLs. During the overnight culture, DOTAP-CLs and DC-Chol-CLs significantly reduced the viability of BMDCs in a dose-dependent manner, with the IC50 values of 240 and 97 μg/mL, respectively (Figure 2C).
Figure 2.
Characterization of CLs and their cytotoxicity in BMDCs.
Notes: (A) Shown are representative, intensity-based size distributions of DOTAP-CLs and DC-Chol-CLs as determined by DLS. (B) The average size, PDI, and zeta potential of CLs are shown. Data are expressed as mean ± SD (n =9). (C) CLs at a series of concentrations were incubated with BMDCs overnight, and cellular viability was measured using the CCK-8 assay. Left panel: shown are the representative cell viability data. Right: the IC50 values of DOTAP-CLs and DC-Chol-CLs on BMDCs are shown. Data represent the mean ± SD from at least three repeated experiments with n =3.
Abbreviations: BMDCs, bone marrow-derived dendritic cells; CCK-8, Cell Counting Kit-8; CLs, cationic liposomes; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; DLS, dynamic light scattering; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; PDI, polydispersity index.
CLs enhance antigen cross-presentation by BMDCs
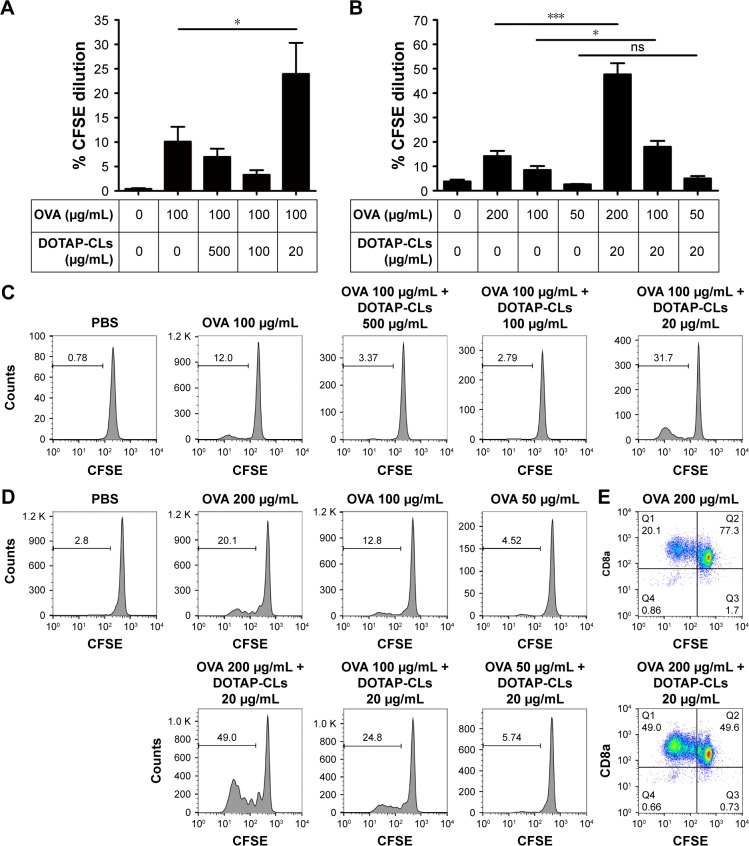
We then examined the ability of BMDCs to promote antigen cross-presentation and cross-priming of antigen-specific CD8+ T-cells. Throughout our studies, we used endotoxin-free chicken OVA as a model protein antigen since OVA has been widely used in initial vaccine development studies as well as in studies examining the mechanisms of cross-presentation.12,19–21,36,37 BMDCs were pulsed with soluble OVA in the presence or absence of CLs. After overnight incubation, BMDCs were cocultured with CFSE-labeled naive OVA-specific CD8+ T-cells isolated from OT-I transgenic mice that only have CD8+ T-cells expressing T-cell receptor recognizing OVA-derived epitope, SIINFEKL, complexed with MHC-I H-2Kb molecules. After 3 days of coculture, we performed flow cytometric analysis to quantify CFSE dilution, which is proportional to the proliferation of OT-I T-cells. We first fixed the OVA concentration at 100 μg/mL and examined the impact of adding various concentrations of DOTAP-CLs (Figure 3). When BMDCs were pulsed with 100 μg/mL of OVA in the presence of 20 μg/mL of DOTAP-CLs, we observed significantly enhanced cross-priming and expansion of OT-I CD8+ T-cells (P<0.05, Figure 3A and C). However, increasing the DOTAP-CL concentration >20 μg/mL did not improve cross-priming, potentially due to the low viability of BMDCs exposed to high dose of DOTAP-CLs (Figure 2C). We then evaluated the effect of 20 μg/mL of DOTAP-CLs on the cross-presentation of various concentrations of OVA. As evidenced by extensive CFSE dilution, BMDCs pulsed with 200 or 100 μg/mL of OVA in the presence of 20 μg/mL of DOTAP-CLs exhibited a significantly enhanced cross-presentation and cross-priming of OT-I CD8+ T-cells (Figure 3B, D, and E).
Figure 3.
DOTAP-CLs promote antigen cross-presentation by BMDCs.
Notes: (A) The percentage of OT-I CD8+ T-cells that proliferated after coculture with BMDCs pulsed with OVA in the presence of different doses of DOTAP-CLs. (B) The percentage of OT-I CD8+ T-cells that proliferated after coculture with BMDCs pulsed with OVA in the presence or absence of 20 μg/mL DOTAP-CLs. (C) Representative flow cytometric histograms of (A) are shown. (D) Representative flow cytometric histograms and (E) scatter plots from (B) are shown. The data were analyzed by one-way ANOVA, followed by a multiple comparison post-test. *P<0.05; ***P<0.001. Data represent the mean ± SD from at least three repeated experiments with n =4–6 for (A) and n =2–4 for (B).
Abbreviations: ANOVA, analysis of variance; BMDCs, bone marrow-derived dendritic cells; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CLs, cationic liposomes; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; ns, not significant; OVA, ovalbumin; PBS, phosphate-buffered saline.
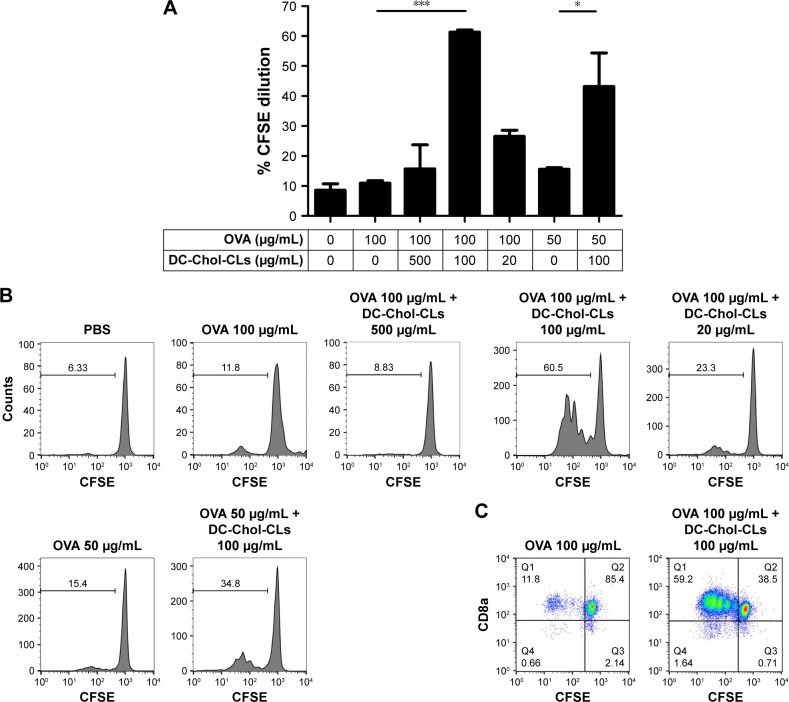
To extend our findings to other CL systems, we performed similar studies using DC-Chol-CLs. In the presence of DC-Chol-CLs at 100 μg/mL, BMDCs pulsed with 100 μg/mL or even 50 μg/mL of OVA exhibited significantly increased cross-presentation and cross-priming of OT-I CD8+ T-cells (P<0.001, Figure 4A–C). However, DC-Chol-CLs at a higher concentration of 500 μg/mL and a lower concentration of 20 μg/mL failed to enhance cross-priming with 100 μg/mL of OVA (Figure 4A and B). In the absence of OVA, BMDCs pulsed with only DOTAP-CLs or DC-Chol-CLs did not induce any detectable proliferation of OT-I CD8+ T-cells (Figure S1). Overall, these results demonstrate that CLs promote antigen cross-presentation by DCs and mediate the cross-priming of CD8+ T-cells in an antigen-dependent manner.
Figure 4.
DC-Chol-CLs promote antigen cross-presentation by BMDCs.
Notes: (A) The percentage of OT-I CD8+ T-cells that proliferated after coculture with BMDCs pulsed with OVA in the presence of different doses of DC-Chol-CLs. (B) Representative flow cytometric histograms and (C) scatter plots from (A) are shown. The data were analyzed by one-way ANOVA, followed by a multiple comparison post-test. *P<0.05; ***P<0.001. Data represent the mean ± SD from three repeated experiments with n =2–3.
Abbreviations: ANOVA, analysis of variance; BMDCs, bone marrow-derived dendritic cells; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CLs, cationic liposomes; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; OVA, ovalbumin; PBS, phosphate-buffered saline.
CLs promote the alkalization of lysosomes in DCs
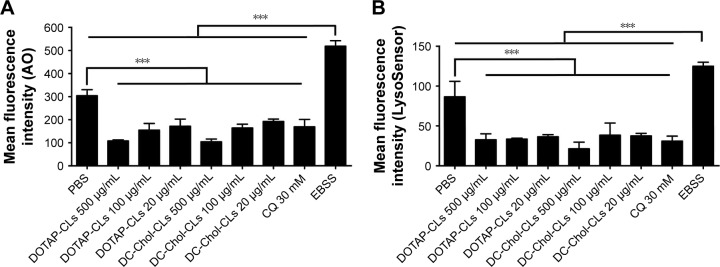
Intrigued by the results presented earlier, we sought to define how CLs induced antigen cross-presentation by DCs. As amine-functionalized moieties are known for their buffering capacities at basic pH, we asked whether CLs interfered with the acidification of lysosomes. To achieve this, we used the AO and LysoSensor dye to evaluate the lysosomal pH of BMDCs after CLs treatment. AO is a nucleic acid dye that enters acidic compartments, such as lysosomes, and upon protonation at acidic pH, the sequestered AO becomes fluorescent with the maximum signal at 490 nm excitation/650 nm emission.31,32 Similarly, the LysoSensor dye becomes fluorescent in acidic environments with the maximum signal at 443 nm excitation/505 nm emission.38 BMDCs pulsed with DOTAP-CLs and DC-Chol-CLs at concentrations ranging from 20 to 500 μg/mL exhibited a significantly decreased fluorescence intensity of AO and LysoSensor dye compared with PBS-treated BMDCs (P<0.001, Figure 5A and B). On the other hand, EBSS, which is a lysosome-acidifying agent, increased the fluorescence signal. Taken all together, these results suggested that CLs interfered with the acidification process of lysosomes in DCs, leading to alkalization of lysosomes.
Figure 5.
CLs interfere with the acidification of lysosomal pH in BMDCs.
Notes: (A) The mean fluorescence intensity of BMDCs stained with AO after CL treatment. After BMDCs were treated with indicated groups overnight, BMDCs were stained by AO, followed by the measurement of AO signal with flow cytometric analysis. (B) The mean fluorescence intensity of BMDCs stained with LysoSensor Green DND-189 dye after CL treatment. After BMDCs were treated with indicated groups overnight, BMDCs were stained by LysoSensor, followed by the measurement of LysoSensor signal with flow cytometric analysis. The data were analyzed by one-way ANOVA, followed by a multiple comparison post-test. ***P<0.001. Data represent the mean ± SD from at least three repeated experiments with n =3–4.
Abbreviations: ANOVA, analysis of variance; AO, acridine orange; BMDCs, bone marrow-derived dendritic cells; CLs, cationic liposomes; CQ, chloroquine; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; EBSS, Earle’s balanced salt solution; PBS, phosphate-buffered saline; SD, standard deviation.
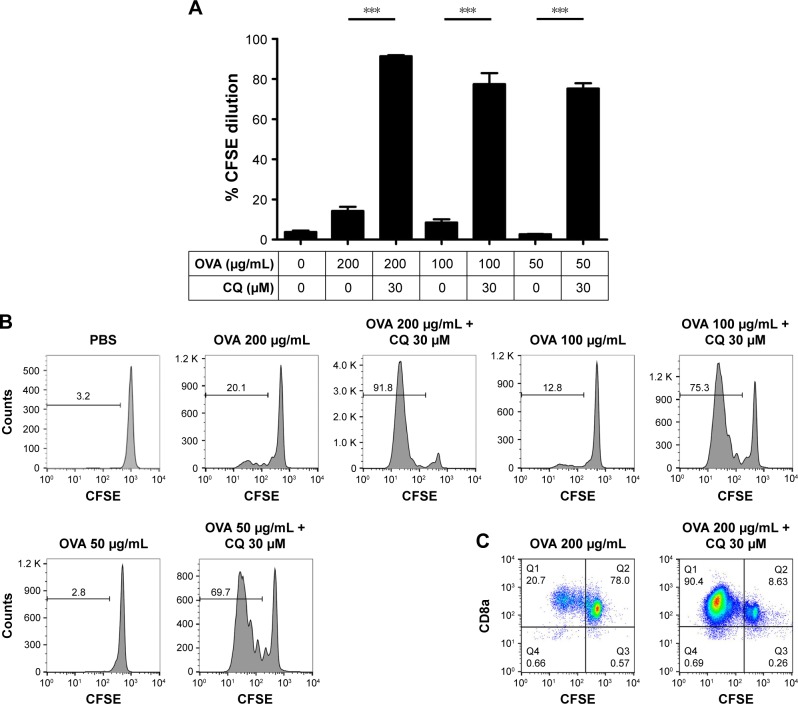
We validated our assays using a well-established control group, namely CQ, which is a lysosomotropic agent that accumulates in acidic cellular compartments, such as endosomes and lysosomes, and increases the lysosomal pH.25 As expected, CQ treatment increased the lysosomal pH of BMDCs, as reflected by a significantly decreased AO and LysoSensor dye fluorescence (P<0.001, Figure 5A and B). Furthermore, CQ significantly increased the cross-presentation of OVA at various concentrations ranging from 50 to 200 μg/mL (P<0.001, Figure 6A–C). Overall, our results indicated that CQ as well as CLs, including DOTAP-CLs and DC-Chol-CLs, interfered with acidification of lysosomes and promoted antigen cross-presentation and cross-priming of antigen-specific CD8+ T-cells.
Figure 6.
CQ enhances antigen cross-presentation by BMDCs.
Notes: (A) The percentage of OT-I CD8+ T-cells that proliferated after coculture with BMDCs pulsed with OVA and 30 μM CQ. (B) Representative flow cytometric histograms and (C) scatter plots from (A) are shown. The data were analyzed by one-way ANOVA, followed by a multiple comparison post-test. ***P<0.001. Data represent the mean ± SD from three repeated experiments with n =2–4.
Abbreviations: ANOVA, analysis of variance; BMDCs, bone marrow-derived dendritic cells; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CQ, chloroquine; OVA, ovalbumin; PBS, phosphate-buffered saline.
ALs have no measurable effect on antigen cross-presentation
To examine the impact of the surface charge of liposomes on antigen cross-presentation, we prepared ALs and performed similar studies as described earlier. EPC-ALs, composed of EPC/Chol/DSPE-mPEG (10:7.5:1 molar ratio, 7 μmol), were 132±26 nm in diameter with a polydispersity index of 0.20±0.05 and a negative zeta potential value of −23.4±1.6 mV (Figure 7A and B). In contrast to CLs that exhibited cytotoxicity at concentrations >100 μg/mL, we did not observe any significant reduction in the viability of BMDCs incubated with 2–1,000 μg/mL of ALs (Figure 7C). Next, we investigated whether ALs had any impact on antigen cross-presentation. Interestingly, ALs at concentrations ranging from 20 to 500 μg/mL had no statistically significant effect on the cross-presentation of 100 μg/mL of OVA (Figure 7D and E). We also evaluated the effect of ALs on the lysosomal pH of BMDCs. AL-treated BMDCs exhibited similar levels of AO and LysoSensor Green DND-189 signals as PBS-treated BMDCs (Figure 7F and G). Overall, ALs did not alter lysosomal pH in BMDCs or had any measurable effect on the cross-presentation of antigens or cross-priming of antigen-specific CD8+ T-cells.
Figure 7.
Characterization of anionic liposomes (ALs).
Notes: (A) Shown is a representative, intensity-based size distribution of ALs as determined by DLS. (B) The average size, PDI, and zeta potential of ALs are shown. (C) After BMDCs were incubated overnight with a series of ALs’ concentrations, cellular viability was measured using the CCK-8 assay. ALs had no detectable effect on cross-presentation of OVA. (D) The percentage of OT-I CD8+ T-cells that proliferated after coculture with BMDCs pulsed with OVA and different doses of ALs. (E) Representative flow cytometric histograms of (D) are shown. The effect of ALs on the lysosomal pH of BMDCs was measured by (F) AO staining and (G) LysoSensor Green DND-189 dye staining, followed by flow cytometric analysis. The data were analyzed by one-way ANOVA, followed by a multiple comparison post-test. ***P<0.001. Data represent the mean ± SD from at least three repeated experiments with n =9 for (A), n =2–3 for (D), and n =2–4 for (F and G).
Abbreviations: ALs, anionic liposomes; ANOVA, analysis of variance; AO, acridine orange; BMDCs, bone marrow-derived dendritic cells; CCK-8, Cell Counting Kit-8; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CQ, chloroquine; DLS, dynamic light scattering; PBS, phosphate-buffered saline; PDI, polydispersity index; OVA, ovalbumin.
DQ-OVA assay
We next studied the effect of CLs and ALs on antigen processing in BMDCs using DQ-OVA, a self-quenched conjugate of OVA that exhibits bright green fluorescence upon proteolytic degradation.35 Compared with PBS treatment, BMDCs treated with DOTAP-CLs and DC-Chol-CLs significantly decreased DQ-OVA fluorescence, indicating the inhibition of DQ-OVA degradation (P<0.001, Figure 8). In contrast, BMDCs treated with ALs had similar levels of fluorescence as PBS-treated BMDCs, indicating antigen processing and degradation. In summary, these results suggested that CLs, but not ALs, reduced the extent of antigen processing and degradation within lysosomes of BMDCs, which led to enhanced antigen cross-presentation and cross-priming of antigen-specific CD8+ T-cells.
Figure 8.
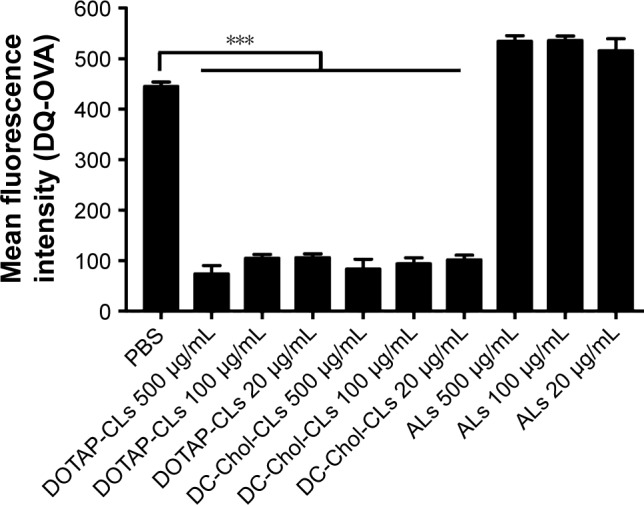
CLs decrease antigen degradation in BMDCs.
Notes: After overnight treatment of different doses of CLs or ALs, BMDCs were incubated for 15 min at 37°C with 10 μg/mL DQ-OVA. The green fluorescence of DQ-OVA was monitored by flow cytometric analysis. BMDCs incubated with DQ-OVA at 4°C were used as control group. The data were analyzed by one-way ANOVA, followed by a multiple comparison post-test. ***P<0.001. Data represent the mean ± SD from three repeated experiments with n =2–4.
Abbreviations: ALs, anionic liposomes; ANOVA, analysis of variance; BMDCs, bone marrow-derived dendritic cells; CLs, cationic liposomes; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; PBS, phosphate-buffered saline; OVA, ovalbumin.
Discussion
CLs have shown great potential as effective vaccine delivery systems.1–5 However, the precise mechanisms of how CLs promote cross-presentation and cross-priming of antigen-specific CD8+ T-cells remain to be elucidated. Previous studies have shown that CLs composed of DOTAP and DOPC or dimethyldioctadecylammonium (DDA) enhanced the cross-presentation in a dose-dependent manner,3,8 while DCs pulsed with low doses of protein antigen in CLs containing TLR3 or TLR9 agonists cross-primed CD8+ T-cell responses.2 These studies have presented the potential mechanisms of CL-mediated enhancement in cross-presentation, including an increased uptake of antigens and codelivery of antigens and adjuvants to DCs.1,8–11 Extending from these studies, here, we have examined the impact of CLs on lysosomal pH in DCs and antigen processing and degradation. Using two distinct CL systems, namely DOTAP-CLs and DC-Chol-CLs, we have demonstrated that CLs with weakly basic tertiary amine head groups elevated the lysosomal pH in DCs and reduced the extent of antigen degradation, which promoted antigen cross-presentation by DCs and the cross-priming of antigen-specific CD8+ T-cells.
To delineate the potential mechanism(s) of CL-mediated promotion of antigen cross-presentation, we have used two lysosomal pH indicators, AO and LysoSensor dye,31,32,38 and shown that CLs increased the lysosomal pH in DCs (Figure 5). Since lysosomal proteases are active at low pH but not at alkaline pH,39 we used the DQ-OVA assay to interrogate whether the increased lysosomal pH influenced antigen processing and degradation. Our results have indicated that CLs interfered with or retarded antigen processing and degradation in DCs (Figure 8). Furthermore, we have performed control studies with ALs whose lipid components were the same as DOTAP-CLs, except for DOTAP replaced with EPC. ALs at all concentrations tested in our experiments failed to change the lysosomal pH, even at 500 μg/mL (Figure 7F and G), and ALs had no measurable effect on the extent of antigen cross-presentation (Figure 7D and E). We have also validated our assays using CQ, a lysosomotropic agent,25 which increased the lysosomal pH (Figure 5) and promoted DC cross-presentation and cross-priming of CD8+ T-cells (Figure 6). Taken together, treatment of DCs with CLs, but not ALs, increased the lysosomal pH, which reduced antigen degradation in the endolysosomal compartments and promoted cross-presentation and cross-priming. These results highlight the critical role of cationic lipids with weak bases in the induction of cross-presentation.
The key factors of vaccine nanocarriers known to affect DC cross-presentation include the physicochemical properties of nanomaterials that modulate internalization pathways and intracellular fate of antigens in APCs; the protection of antigens from protease-mediated degradation; and the ligand modification on the surfaces of nanoparticles that influence APC targeting.4,40,41 Our data demonstrate that the ability of nanomaterials to regulate lysosomal pH of the target APCs plays a major role in antigen cross-presentation and elicitation of CD8+ T-cell response. Based on our data presented here as well as other previous reports suggesting endolysosomal destabilization triggered by CLs and lipophilic cationic drugs,42,43 we speculate that CL-mediated alkalization of lysosomal pH in DCs reduces the extent or kinetics of antigen degradation, which leads to CL-induced disruption of endolysosomal membranes, cytosolic delivery of protein antigens, and ultimately, promotion of antigen cross-presentation and cross-priming of antigen-specific CD8+ T-cells. To address these issues, future studies will need to further delineate the underlining mechanisms of CL-mediated cross-presentation and cross-priming and to validate the results in vivo.
One of the major limitations of CL-based antigen delivery systems is their cytotoxicity at high concentrations. Indeed, we observed that DOTAP-CLs with IC50 =240 μg/mL in BMDCs were able to promote cross-presentation at the liposomal concentration of 20 μg/mL, but not 100 or 500 μg/mL approaching or exceeding the IC50 value (Figure 3), whereas DC-Chol-CLs with IC50 =97 μg/mL induced cross-presentation at 100 μg/mL, but not 500 μg/mL (Figure 4). Notably, to address cytotoxicity issues associated with CLs, we have recently reported the reduction of CL cytotoxicity in part by complexing or shielding CLs with biopolymers.44 Another caveat of our studies is their limitation to in vitro assays. To validate our results in a physiological condition, future preclinical studies should be directed to interrogate the effects of CL species and their doses on DC cytotoxicity, intracellular processing of antigens and cross-presentation – parameters that need to be taken into account for the design and development of CL-based vaccine delivery systems.
Conclusion
Our study sheds new light on the mechanisms of CL-mediated antigen cross-presentation in DCs. CLs with weak bases elevate the lysosomal pH in DCs and reduce antigen degradation, thereby promoting antigen cross-presentation and cross-priming of CD8+ T-cell responses. Nanomaterials with defined physicochemical properties can be designed to control the endolysosomal pH, protease activity, antigen processing, and cross-presentation in DCs. Rational design of vaccine delivery platforms may offer a promising strategy for improving and facilitating vaccine development.
Supplementary material
CLs or CQ alone had no effect on the percentage accumulation of terminally divided OT-I CD8+ T-cells.
Notes: The percentage accumulation of terminally divided OT-I CD8+ T-cells after treatment of CLs or CQ alone on DCs. OVA treatment was used as a decent positive control. Data represent the mean ± SD from at least three repeated experiments with n=2–4.
Abbreviations: CLs, cationic liposomes; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CQ, chloroquine; DCs, dendritic cells; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; OVA, ovalbumin.
Acknowledgments
This study was supported in part by NIH 1R01-AI127070, NIH 1R01-EB022563, NIH UL1TR000433, and China Scholarship Fund. JJM is a young Investigator supported by the Melanoma Research Alliance (348774), NSF CAREER Award (1553831), and DoD/CDMRP Peer Reviewed Cancer Research Program (W81XWH-16-1-0369). Opinions interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the article apart from those disclosed.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Christensen D, Korsholm KS, Andersen P, Agger EM. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2011;10(4):513–521. doi: 10.1586/erv.11.17. [DOI] [PubMed] [Google Scholar]
- 2.Zaks K, Jordan M, Guth A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 3.Agger EM, Rosenkrands I, Hansen J, et al. Cationic liposomes formulated with synthetic mycobacterial cordfactor (CAF01): a versatile adjuvant for vaccines with different immunological requirements. PLoS One. 2008;3(9):e3116. doi: 10.1371/journal.pone.0003116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014;31(10):2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuai R, Ochyl LJ, Schwendeman A, Moon JJ. Lipid-based nanoparticles for vaccine applications. In: Jo H, Jun HW, Shin H, editors. Biomedical Engineering: Convergence Technologies. Amsterdam: Elsevier BV; 2015. [Google Scholar]
- 6.Henriksen-Lacey M, Bramwell VW, Christensen D, Agger EM, Andersen P, Perrie Y. Liposomes based on dimethyldioctadecylammonium promote a depot effect and enhance immunogenicity of soluble antigen. J Control Release. 2010;142(2):180–186. doi: 10.1016/j.jconrel.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Henriksen-Lacey M, Christensen D, Bramwell VW, et al. Comparison of the depot effect and immunogenicity of liposomes based on dimethyldioctadecylammonium (DDA), 3beta-[N-(N′,N′-Dimethylaminoethane) carbomyl] cholesterol (DC-Chol), and 1,2-Dioleoyl-3-trimethylammonium propane (DOTAP): prolonged liposome retention mediates stronger Th1 responses. Mol Pharm. 2011;8(1):153–161. doi: 10.1021/mp100208f. [DOI] [PubMed] [Google Scholar]
- 8.Korsholm KS, Agger EM, Foged C, et al. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121(2):216–226. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao X, Wang RY, Qiu Q, Alter HJ, Shih JW. Enhanced hepatitis C virus NS3 specific Th1 immune responses induced by co-delivery of protein antigen and CpG with cationic liposomes. J Gen Virol. 2004;85(pt 6):1545–1553. doi: 10.1099/vir.0.79896-0. [DOI] [PubMed] [Google Scholar]
- 10.Jaafari MR, Badiee A, Khamesipour A, et al. The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine. 2007;25(32):6107–6117. doi: 10.1016/j.vaccine.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol. 2013;10(2):103–106. doi: 10.1038/cmi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varypataki EM, van der Maaden K, Bouwstra J, Ossendorp F, Jiskoot W. Cationic liposomes loaded with a synthetic long peptide and poly(I:C): a defined adjuvanted vaccine for induction of antigen-specific T cell cytotoxicity. AAPS J. 2015;17(1):216–226. doi: 10.1208/s12248-014-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maji M, Mazumder S, Bhattacharya S, et al. A lipid based antigen delivery system efficiently facilitates MHC class-I antigen presentation in dendritic cells to stimulate CD8(+) T cells. Sci Rep. 2016;6:27206. doi: 10.1038/srep27206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heath WR, Belz GT, Behrens GM, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 15.Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Presentation of phagocytosed antigens by MHC class I and II. Traffic. 2013;14(2):135–152. doi: 10.1111/tra.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425(6956):397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 17.Rock KL. The ins and outs of cross-presentation. Nat Immunol. 2003;4(10):941–943. doi: 10.1038/ni1003-941. [DOI] [PubMed] [Google Scholar]
- 18.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 19.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9(5):558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 20.Alloatti A, Kotsias F, Pauwels AM, et al. Toll-like receptor 4 engagement on dendritic cells restrains phago-lysosome fusion and promotes cross-presentation of antigens. Immunity. 2015;43(6):1087–1100. doi: 10.1016/j.immuni.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 22.Lennon-Dumenil AM, Bakker AH, Maehr R, et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J Exp Med. 2002;196(4):529–540. doi: 10.1084/jem.20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil-Torregrosa BC, Lennon-Dumenil AM, Kessler B, et al. Control of cross-presentation during dendritic cell maturation. Eur J Immunol. 2004;34(2):398–407. doi: 10.1002/eji.200324508. [DOI] [PubMed] [Google Scholar]
- 24.Savina A, Peres A, Cebrian I, et al. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity. 2009;30(4):544–555. doi: 10.1016/j.immuni.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Accapezzato D, Visco V, Francavilla V, et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med. 2005;202(6):817–828. doi: 10.1084/jem.20051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cebrian I, Visentin G, Blanchard N, et al. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147(6):1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Sun J, Li H, et al. Lyophilized HER2-specific PEGylated immunoliposomes for active siRNA gene silencing. Biomaterials. 2010;31(9):2655–2664. doi: 10.1016/j.biomaterials.2009.11.112. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Chen H, Yu Y, et al. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials. 2013;34(38):10084–10098. doi: 10.1016/j.biomaterials.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 29.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 30.Su X, Song H, Niu F, et al. Co-delivery of doxorubicin and PEGylated C16-ceramide by nanoliposomes for enhanced therapy against multidrug resistance. Nanomedicine (Lond) 2015;10(13):2033–2050. doi: 10.2217/nnm.15.50. [DOI] [PubMed] [Google Scholar]
- 31.Yue W, Hamai A, Tonelli G, et al. Inhibition of the autophagic flux by salinomycin in breast cancer stem-like/progenitor cells interferes with their maintenance. Autophagy. 2013;9(5):714–729. doi: 10.4161/auto.23997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmgren MG. Acridine orange as a probe for measuring pH gradients across membranes: mechanism and limitations. Anal Biochem. 1991;192(2):316–321. doi: 10.1016/0003-2697(91)90542-2. [DOI] [PubMed] [Google Scholar]
- 33.Yang K, Lu Y, Xie F, et al. Cationic liposomes induce cell necrosis through lysosomal dysfunction and late stage autophagic flux inhibition. Nanomedicine (Lond) 2016;11(23):3117–3137. doi: 10.2217/nnm-2016-0289. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Wu Y, Jin S, et al. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano. 2011;5(11):8629–8639. doi: 10.1021/nn202155y. [DOI] [PubMed] [Google Scholar]
- 35.Spadaro F, Lapenta C, Donati S, et al. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119(6):1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 36.Moon JJ, Suh H, Bershteyn A, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10(3):243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houde M, Bertholet S, Gagnon E, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425(6956):402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 38.Lin HJ, Herman P, Kang JS, Lakowicz JR. Fluorescence lifetime characterization of novel low-pH probes. Anal Biochem. 2001;294(2):118–125. doi: 10.1006/abio.2001.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 40.Fan Y, Moon JJ. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines (Basel) 2015;3(3):662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Feng SS, Guo Y. Antibody engineering promotes nanomedicine for cancer treatment. Nanomedicine (Lond) 2010;5(8):1141–1145. doi: 10.2217/nnm.10.94. [DOI] [PubMed] [Google Scholar]
- 42.Wattiaux R, Jadot M, Warnier-Pirotte MT, Wattiaux-De Coninck S. Cationic lipids destabilize lysosomal membrane in vitro. FEBS Lett. 1997;417(2):199–202. doi: 10.1016/s0014-5793(97)01283-0. [DOI] [PubMed] [Google Scholar]
- 43.Kornhuber J, Henkel AW, Groemer TW, et al. Lipophilic cationic drugs increase the permeability of lysosomal membranes in a cell culture system. J Cell Physiol. 2010;224(1):152–164. doi: 10.1002/jcp.22112. [DOI] [PubMed] [Google Scholar]
- 44.Fan Y, Sahdev P, Ochyl LJ, Akerberg JJ, Moon JJ. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release. 2015;208:121–129. doi: 10.1016/j.jconrel.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CLs or CQ alone had no effect on the percentage accumulation of terminally divided OT-I CD8+ T-cells.
Notes: The percentage accumulation of terminally divided OT-I CD8+ T-cells after treatment of CLs or CQ alone on DCs. OVA treatment was used as a decent positive control. Data represent the mean ± SD from at least three repeated experiments with n=2–4.
Abbreviations: CLs, cationic liposomes; CFSE, carboxyfluorescein diacetate N-succinimidyl ester; CQ, chloroquine; DCs, dendritic cells; DC-Chol, 3β-[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane; OVA, ovalbumin.