Abstract
Background
Intravenous contrast-enhanced ultrasonography is a recently developed technique for assessment of tissue perfusion, but has not been used for assessment of skeletal muscle perfusion.
Methods
We studied a 42-year-old woman in whom myonecrosis was suspected due to systemic vasculitis and ischemia. The biceps brachii (right) and quadriceps femoris (vastus medialis) on right-hand side and subsequently left-hand side were imaged. Intravenous bolus of activated perflutren lipid microspheres was injected and B-Flow color mode (brown color) was used within a selected region of interest to image the passage of contrast through muscle parenchyma throughout three cardiac cycles.
Results
Visual interpretation of muscle perfusion was performed based on the maximal intensity of contrast in the muscle, and the speed of contrast replenishment. No deficits were noted in the perfusion pattern. The arterial phase demonstrated stellate vascularity, centrifugal filling, and homogeneous hypervascularity at peak enhancement.
Conclusions
The bolus of contrast resulted in good signal persistence and satisfactory imaging for multiple muscle groups.
Keywords: Muscle perfusion, ultrasonography, lipid microspheres, contrast, myonecrosis, muscle ischemia
INTRODUCTION
Lipid microspheres which are gas-filled micro-bubbles are used as contrast agent in ultrasound-based imaging [1]. The microspheres are distributed through circulation into various tissues proportional to the regional blood flow after intravenous bolus injection [2]. The microspheres generate a hyper-echoic signal due to resonance on ultrasound imaging and release of gas when agitated at frequencies used by the ultrasound imaging systems [3]. Besides the potential role in luminal enhancement of large arteries and cardiac chambers, contrast-enhanced ultrasound has been indicated for quantification of neo-vascularization within the carotid atherosclerotic plaque [4], detection of renal infarction and cortical necrosis [5], quantifying parenchymal perfusion for early detection of graft rejection [6], and assessment of diabetic microangiopathy [7]. A potential role that is not defined is assessment of skeletal muscle perfusion when muscle hypoperfusion, ischemia, or inflammation is suspected. We describe the use of contrast-enhanced ultrasound in a patient in whom myonecrosis was suspected due to systemic vasculitis and ischemia.
CASE DESCRIPTION
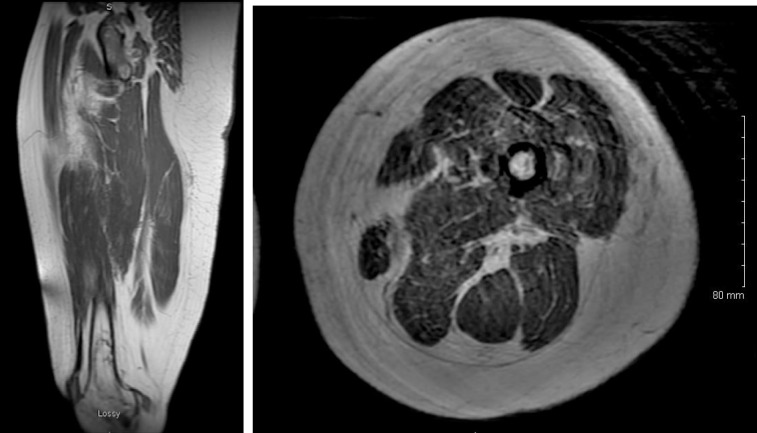
A 42-year-old woman presented with fever, cognitive and speech difficulties, gait abnormalities, and diffuse pain and weakness. She was previously admitted seven months ago with generalized rash. Within two days of admission, the patient had a significant elevation serum creatinine kinase from a baseline value of 44 to 160,000 mg/dl suggesting myonecrosis. Her creatinine was 0.8 mg/dl at admission and increased to 2 mg/dl. Magnetic resonance imaging (MRI) of brain demonstrated bilateral subtle punctate hyperintense foci anteriorly. MRI of thigh muscles (see Figure 1) demonstrated diffuse hyperintense signal suggestive of inflammation and/or ischemia. Chronic bone infarction in distal femoral bones was observed bilaterally. Skeletal muscle perfusion study was performed to evaluate perfusion deficits, which may be consistent with systemic vasculitis.
Figure 1. The antero-posterior (A) and cross-sectional (B) images in T2-weighted images. There is diffuse high T2 signal in thigh muscles, more prominent in the medial and posterior compartments. There is mild fatty infiltration of the muscle with marbled appearance suggestive of mild atrophy.
PROCEDURE
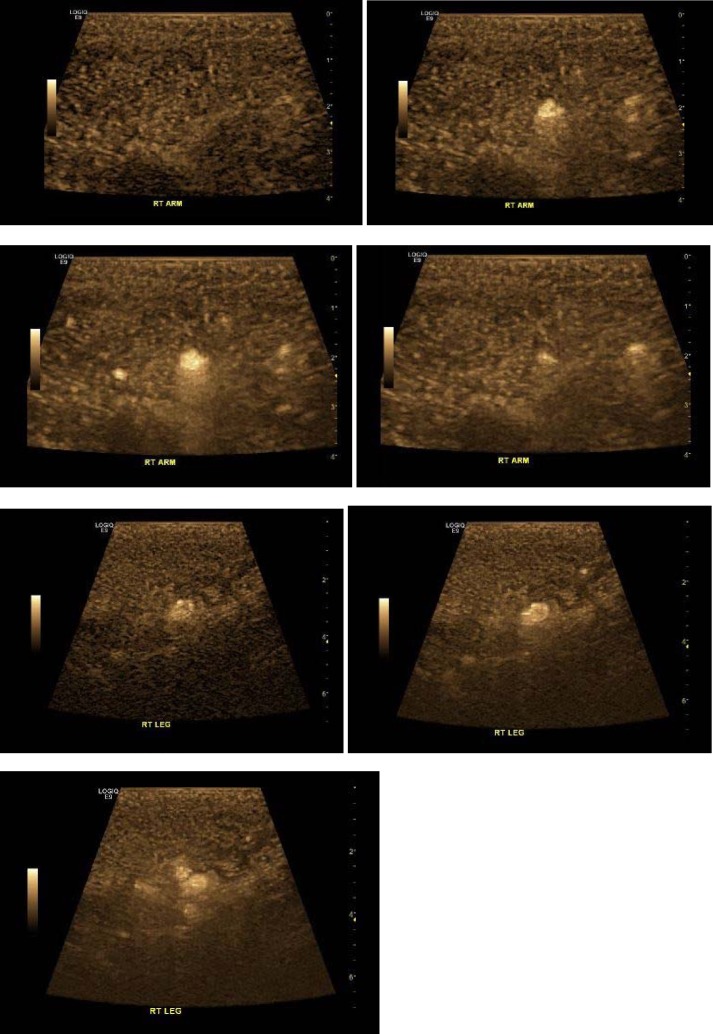
A 9L-D H40442LM transducer (bandwidth 3–8 MHz, FOV 43 mm) and LOGIQ E9 ultrasound machine (GE Healthcare, Wauwatosa, WI, USA) were used to image the muscle tissue. The biceps brachii (right) was imaged in the anterior compartment of the arm. A section was selected that demonstrated multiple arterial flow channels on color flow imaging view of flow in a region. The diluted IV bolus method was used to administer activated DEFINITY (Perflutren Lipid Microsphere) Injectable Suspension (Lantheus Medical Imaging, Inc.). A 10-cc syringe filled with 1.3 ml of activated DEFINITY diluted with 8.7 ml of preservative-free saline was gently hand agitated to evenly distribute microspheres. The injection was performed over 45 s. A B-Flow mode was used as soon as contrast was visualized within the arteries. B-Flow Color (brown color) was displayed within a selected region of interest for easier display of smaller vessels and minimizing of tissue motion artifacts. The passage of contrast was visualized through muscle parenchyma in region of interest that was visualized throughout three cardiac cycles (see Figure 2).
Figure 2. Arterial phase contrast-enhanced ultrasound images obtained with maximum-intensity-projection technique show sequential images obtained in arterial phase of contrast-enhanced ultrasonography in biceps brachii (right A–D) and quadriceps femoris (vastus medialis, right E–G). The images show stellate vascularity, centrifugal filling, and homogeneous hypervascularity at peak enhancement.
Visual interpretation of muscle perfusion was performed based on the maximal intensity of contrast in the muscle, and the speed of contrast replenishment [8]. No deficits were noted in the perfusion pattern. The arterial phase demonstrated stellate vascularity, centrifugal filling, and homogeneous hypervascularity at peak enhancement. Two other regions of interest were imaged (see Figures 5 and 6). The quadriceps femoris (vastus medialis) were imaged on right-hand side and subsequently left-hand side. There was some obscuration of signal due to subcutaneous edema. No deficits were noted in the perfusion pattern. The bolus resulted in good signal persistence and satisfactory imaging for approximately 5 min after bolus dose of contrast.
DISCUSSION
The concept of studying perfusion using contrast-enhanced ultrasound is based on distribution of an agent that has the same intra-vascular rheology of red blood cells. The ultrasound enhances the micro-bubble signal (while suppressing the soft tissue signal), and can produce direct, real-time, 2-D visualization of blood vessel flow. The technique allows visualization of small-sized arteries, which cannot be adequately imaged using the current methodologies of Duplex ultrasound, positron emission tomography, and contrast-enhanced computed tomography (CT) and MRI scans [9]. The variable transit time of contrast through the diseased vessels can render the opacification inadequate during acquisition of CT and MRI scans [10]. The continuous imaging provided by ultrasound allows visualization of contrast opacification in a dynamic manner through various phases.
Contrast-enhanced ultrasound has been used to study perfusion of myocardium [11–13] and liver [14, 15] in previous studies. We used maximum-intensity-projection techniques, tracking the course of the micro-bubbles through arterial and capillary phases of opacification within skeletal muscles during multiple cardiac cycles. Other studies have used the contrast refill-kinetics using replenishment flashes for study of tissue perfusion [11–13]. Such techniques involve a continuous intravenous infusion of ultrasound contrast agent, and ultrasound acquisition is set for low power (power-pulse inversion and pulse inversion techniques 12–25 Hz) for real-time perfusion imaging with minimal detection of background tissue signal [16]. Acquisition of signal at low power also preserves the micro-bubbles for several minutes. Flash-replenishment technique is a two-phase process with first phase involving destruction of micro-bubbles in the scan volume by high-power burst and second phase observing reperfusion at a low-power acquisition [11–13, 16].
The administration of ultrasound micro-bubble contrast has a very good safety profile. A study from 28 Italian centers found 20 adverse events from 23,188 investigations related to intravenous administration of ultrasound micro-bubble contrast [17]. Two events were considered serious, and none was fatal. We describe the potential use of contrast-enhanced ultrasound for characterizing skeletal muscle perfusion, which may be of value in patients with myopathy related to vasculopathy and ischemia.
Ethical standards and patient consent
For this type of study, ethical approval and patient consent are not required.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
None.
REFERENCES
- Sirsi S, Borden M. Microbubble compositions, properties and biomedical applications. Bubble Sci Eng Technol. 2009;1:3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzen FW, Bassingthwaighte JB. Blood flow distributions by microsphere deposition methods. Cardiovasc Res. 2000;45:13–21. doi: 10.1016/s0008-6363(99)00252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong N, et al. Basic acoustic properties of microbubbles. Echocardiography. 2002;19:229–240. doi: 10.1046/j.1540-8175.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- van den Oord SC, et al. Effect of carotid plaque screening using contrast-enhanced ultrasound on cardiovascular risk stratification. Am J Cardiol. 2013;111:754–759. doi: 10.1016/j.amjcard.2012.11.033. [DOI] [PubMed] [Google Scholar]
- McArthur C, Baxter GM. Current and potential renal applications of contrast-enhanced ultrasound. Clin Radiol. 2012;67:909–922. doi: 10.1016/j.crad.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Grzelak P, et al. Perfusion of kidney graft pyramids and cortex in contrast-enhanced ultrasonography in the determination of the cause of delayed graft function. Ann Transplant. 2011;16:48–53. [PubMed] [Google Scholar]
- Duerschmied D, et al. Contrast ultrasound perfusion imaging of lower extremities in peripheral arterial disease: a novel diagnostic method. Eur Heart J. 2006;27:310–315. doi: 10.1093/eurheartj/ehi636. [DOI] [PubMed] [Google Scholar]
- Dijkmans PA, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol. 2006;48:2168–2177. doi: 10.1016/j.jacc.2006.05.079. [DOI] [PubMed] [Google Scholar]
- Miller A, et al. An approach to the diagnosis and management of systemic vasculitis revised version with tracked changes removed. Clin Exp Immunol. 2010;160:143–160. doi: 10.1111/j.1365-2249.2009.04078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling AN, et al. Technical considerations for lower limb multidetector computed tomographic angiography. Vasc Med. 2011;16:131–143. doi: 10.1177/1358863X10388347. [DOI] [PubMed] [Google Scholar]
- Zoppellaro G, et al. Simultaneous assessment of myocardial perfusion, wall motion, and deformation during myocardial contrast echocardiography: a feasibility study. Echocardiography. 2016;33:889–895. doi: 10.1111/echo.13190. [DOI] [PubMed] [Google Scholar]
- Du GQ, et al. Measurement of myocardial perfusion and infarction size using computer-aided diagnosis system for myocardial contrast echocardiography. Ultrasound Med Biol. 2015;41:2466–2477. doi: 10.1016/j.ultrasmedbio.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, et al. Analysis of myocardial perfusion or myocardial function for detection of regional myocardial abnormalities. An echocardiographic multicenter comparison study using myocardial contrast echocardiography and 2D echocardiography. Eur J Echocardiogr. 2007;8:438–448. doi: 10.1016/j.euje.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Ding H, et al. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285–297. doi: 10.7863/jum.2005.24.3.285. [DOI] [PubMed] [Google Scholar]
- Jang HJ, et al. Enhancement patterns of hepatocellular carcinoma at contrast-enhanced US: comparison with histologic differentiation. Radiology. 2007;244:898–906. doi: 10.1148/radiol.2443061520. [DOI] [PubMed] [Google Scholar]
- Schlosser T, et al. Feasibility of the flash-replenishment concept in renal tissue: which parameters affect the assessment of the contrast replenishment? . Ultrasound Med Biol. 27:937–944. doi: 10.1016/s0301-5629(01)00397-0. [DOI] [PubMed] [Google Scholar]
- Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]