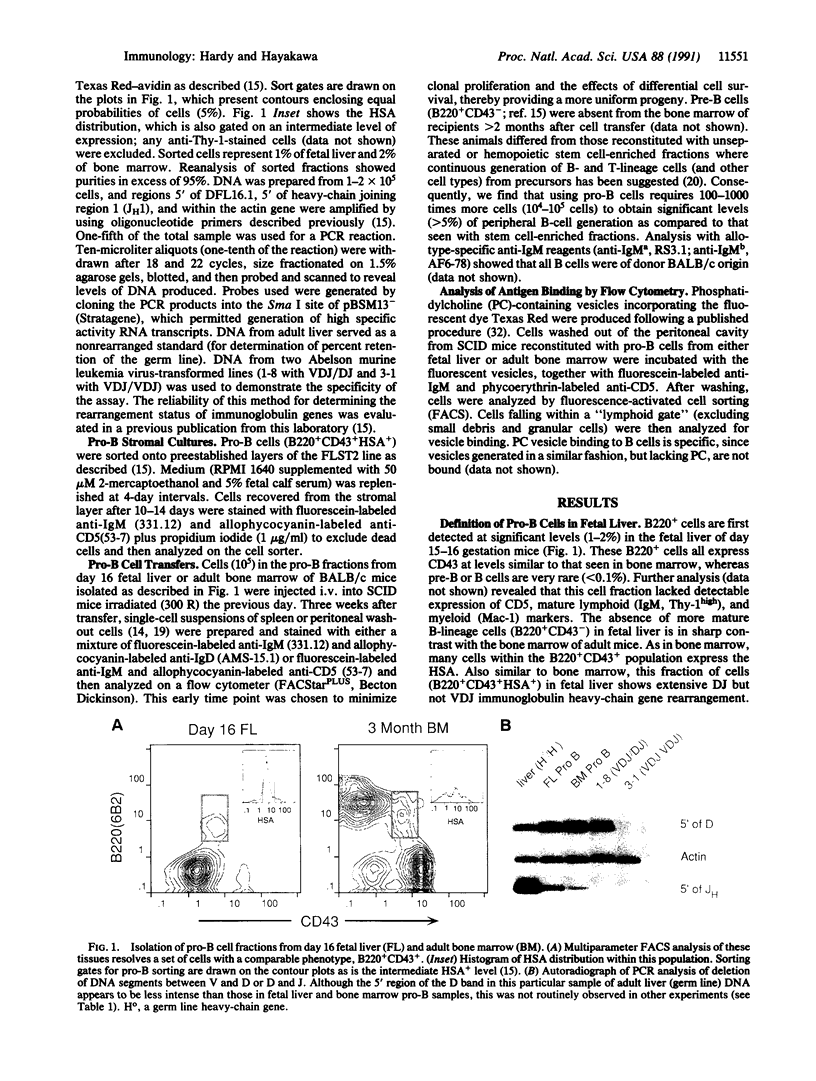
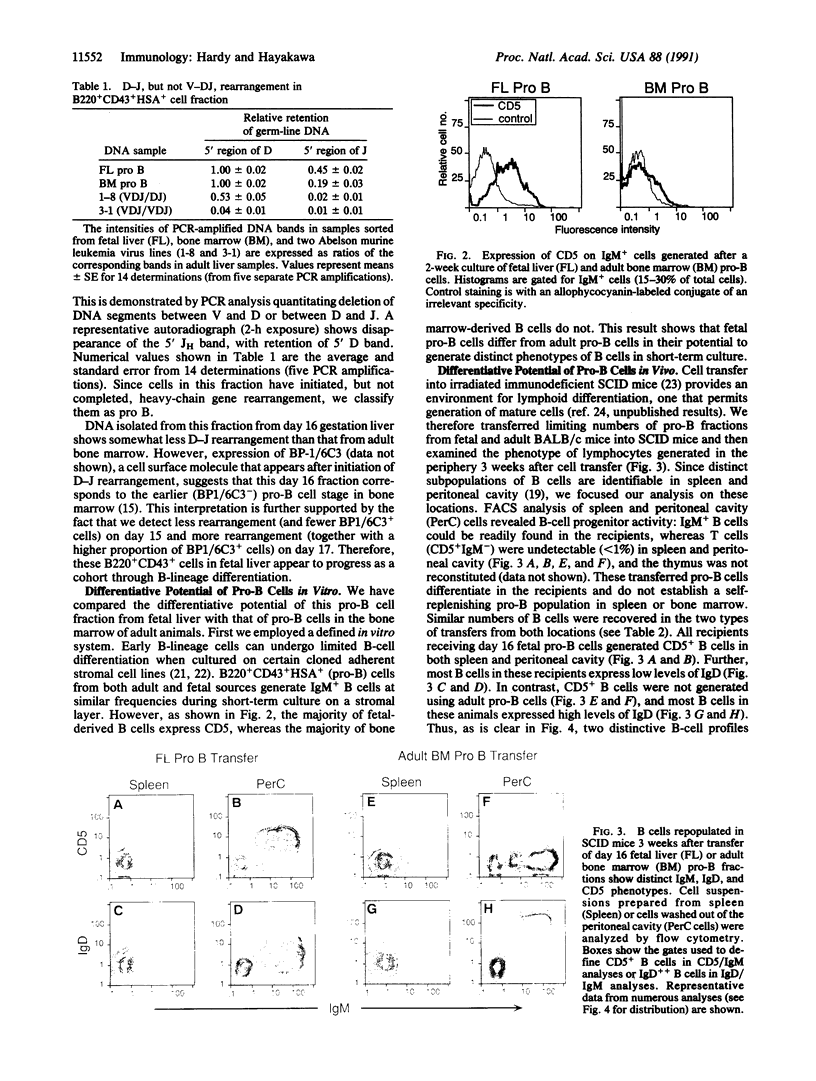
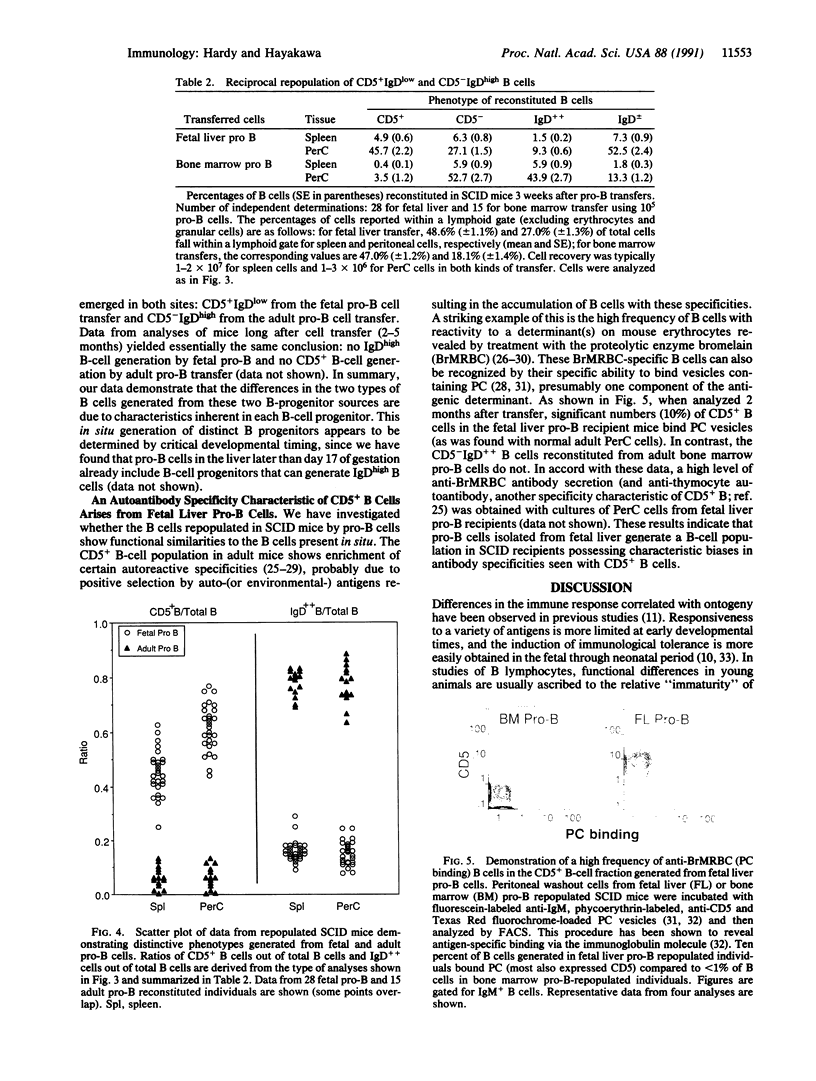
Abstract
B and T lymphocytes are generated from hematopoietic stem cells during both fetal and adult life. A critical unresolved issue is whether the differentiation pathways in lymphopoiesis are the same in fetal and adult animals or whether they differ, similar to the hemoglobin switch in erythropoiesis. We report here that a developmental switch occurs in B lymphopoiesis. We isolated "pro-B" cells (i.e., cells that have initiated, but not completed, heavy-chain gene rearrangement) from fetal and adult sources and investigated their B-cell progeny generated both in vitro and in vivo. Most of the cells from fetal liver, but few from adult bone marrow, expressed CD5. Further, fetal pro-B cells failed to generate cells expressing high levels of IgD in severe combined immunodeficiency mice, whereas adult pro-B cells gave rise to CD5-B cells bearing IgD at levels comparable to the bulk of cells in the spleen of adult mice. Thus, all committed B progenitors in fetal liver of day 16 gestation mice give rise to phenotypically distinct progeny when compared to cells at a comparable differentiation stage in the bone marrow of adult animals. We conclude that the cohort of B-lineage progenitors in early fetal development is committed to a differentiation pathway distinct from that seen in the adult.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Carmack C. E., Shinton S. A., Hayakawa K., Hardy R. R. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J Exp Med. 1990 Jul 1;172(1):371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Denis K. A., Treiman L. J., St Claire J. I., Witte O. N. Long-term cultures of murine fetal liver retain very early B lymphoid phenotype. J Exp Med. 1984 Oct 1;160(4):1087–1101. doi: 10.1084/jem.160.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K. Use of the scid mouse transplantation system in studies of lymphocyte differentiation. Curr Top Microbiol Immunol. 1989;152:169–172. doi: 10.1007/978-3-642-74974-2_20. [DOI] [PubMed] [Google Scholar]
- Enver T., Raich N., Ebens A. J., Papayannopoulou T., Costantini F., Stamatoyannopoulos G. Developmental regulation of human fetal-to-adult globin gene switching in transgenic mice. Nature. 1990 Mar 22;344(6264):309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gulley M. L., Ogata L. C., Thorson J. A., Dailey M. O., Kemp J. D. Identification of a murine pan-T cell antigen which is also expressed during the terminal phases of B cell differentiation. J Immunol. 1988 Jun 1;140(11):3751–3757. [PubMed] [Google Scholar]
- Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Semin Hematol. 1981 Jan;18(1):39–62. [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991 May 1;173(5):1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Carmack C. E., Shinton S. A., Riblet R. J., Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989 May 15;142(10):3643–3651. [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986 Oct;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Parks D. R., Herzenberg L. A. Demonstration of B-cell maturation in X-linked immunodeficient mice by simultaneous three-colour immunofluorescence. Nature. 1983 Nov 17;306(5940):270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Kishimoto T., Hayakawa K. Differentiation of B cell progenitors in vitro: generation of surface IgM+ B cells, including Ly-1 B cells, from Thy-1- asialoGM1+ cells in newborn liver. Eur J Immunol. 1987 Dec;17(12):1769–1774. doi: 10.1002/eji.1830171214. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Allison J. P. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988 Sep 29;335(6189):443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Carmack C. E., Hyman R., Hardy R. R. Natural autoantibodies to thymocytes: origin, VH genes, fine specificities, and the role of Thy-1 glycoprotein. J Exp Med. 1990 Sep 1;172(3):869–878. doi: 10.1084/jem.172.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986 Apr;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta K., Kina T., MacNeil I., Uchida N., Peault B., Chien Y. H., Weissman I. L. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990 Sep 7;62(5):863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ito K., Bonneville M., Takagaki Y., Nakanishi N., Kanagawa O., Krecko E. G., Tonegawa S. Different gamma delta T-cell receptors are expressed on thymocytes at different stages of development. Proc Natl Acad Sci U S A. 1989 Jan;86(2):631–635. doi: 10.1073/pnas.86.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Press J. L. The B cell specificity repertoire: its relationship to definable subpopulations. Transplant Rev. 1975;24:41–83. doi: 10.1111/j.1600-065x.1975.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Mercolino T. J., Arnold L. W., Haughton G. Phosphatidyl choline is recognized by a series of Ly-1+ murine B cell lymphomas specific for erythrocyte membranes. J Exp Med. 1986 Jan 1;163(1):155–165. doi: 10.1084/jem.163.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercolino T. J., Arnold L. W., Hawkins L. A., Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988 Aug 1;168(2):687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercolino T. J., Locke A. L., Afshari A., Sasser D., Travis W. W., Arnold L. W., Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989 Jun 1;169(6):1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Sieburg C. E., Whitlock C. A., Weissman I. L. Isolation of two early B lymphocyte progenitors from mouse marrow: a committed pre-pre-B cell and a clonogenic Thy-1-lo hematopoietic stem cell. Cell. 1986 Feb 28;44(4):653–662. doi: 10.1016/0092-8674(86)90274-6. [DOI] [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Nakamoto B., Agostinelli F., Manna M., Lucarelli G., Stamatoyannopoulos G. Fetal to adult hemopoietic cell transplantation in humans: insights into hemoglobin switching. Blood. 1986 Jan;67(1):99–104. [PubMed] [Google Scholar]
- Pardoll D. M., Fowlkes B. J., Bluestone J. A., Kruisbeek A., Maloy W. L., Coligan J. E., Schwartz R. H. Differential expression of two distinct T-cell receptors during thymocyte development. Nature. 1987 Mar 5;326(6108):79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- Pearse M., Gallagher P., Wilson A., Wu L., Fisicaro N., Miller J. F., Scollay R., Shortman K. Molecular characterization of T-cell antigen receptor expression by subsets of CD4- CD8- murine thymocytes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6082–6086. doi: 10.1073/pnas.85.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell C. A., Mercolino T. J., Grdina T. A., Arnold L. W., Haughton G., Clarke S. H. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur J Immunol. 1989 Jul;19(7):1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Bunch C., Kelly S., Gunn Y., Breckon G. Control of haemoglobin switching by a developmental clock? Nature. 1985 Jan 24;313(6000):320–323. doi: 10.1038/313320a0. [DOI] [PubMed] [Google Scholar]
- Zanjani E. D., Lim G., McGlave P. B., Clapp J. F., Mann L. I., Norwood T. H., Stamatoyannopoulos G. Adult haematopoietic cells transplanted to sheep fetuses continue to produce adult globins. Nature. 1982 Jan 21;295(5846):244–246. doi: 10.1038/295244a0. [DOI] [PubMed] [Google Scholar]







