Abstract
Background
Cancer-induced bone pain (CIBP) is one of the most challenging clinical problems due to a lack of understanding the mechanisms. Recent evidence has demonstrated that activation of microglial G-protein-coupled P2Y12 receptor (P2Y12R) and proinflammatory cytokine production play an important role in neuropathic pain generation and maintenance. However, whether P2Y12R is involved in CIBP remains unknown.
Methods
The purpose of this study was to investigate the role of P2Y12R in CIBP and its molecular mechanisms. Using the bone cancer model inoculated with Walker 256 tumor cells into the left tibia of Sprague Dawley rat, we blocked spinal P2Y12R through intrathecal administration of its selective antagonist MRS2395 (400 pmol/µL, 15 µL).
Results
We found that not only the ionized calcium-binding adapter molecule 1 (Iba-1)-positive microglia in the ipsilateral spinal cord but also mechanical allodynia was significantly inhibited. Furthermore, it decreased the phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) and the production of proinflammatory cytokines interleukin-1β (IL-1β) and interleukin-6 (IL-6), whereas it increased tumor necrosis factor-α (TNF-α).
Conclusion
Taken together, our present results suggest that microglial P2Y12R in the spinal cord may contribute to CIBP by the activation of spinal microglia and p38MAPK pathway, thus identifying a potential therapeutic target for the treatment of CIBP.
Keywords: P2Y12 receptor, cancer-induced bone pain, p38MAPK pathway, cytokines
Video abstract
Introduction
Cancer-induced bone pain (CIBP) is a clinical problem with up to 85% of patients suffering from severe hyperalgesia and tactile allodynia,1 and it can severely compromise the quality of life.2 Currently, there is no effective medicine or method to treat CIBP because molecular mechanisms responsible for the induction and maintenance of bone cancer pain are not fully understood. Recent evidence has demonstrated that glial cells, including microglia in the spinal cord, play important roles in molecular mechanisms of cancer pain.3,4 Activation of glial cells is considered to trigger the production and release of proinflammatory cytokines (interleukin-1β [IL-1β], interleukin-6 [IL-6], and tumor necrosis factor-α [TNF-α]) that contribute to pain hypersensitivity.5
An array of ionotropic (P2X4 and P2X7) and metabotropic (P2Y1, P2Y2, and P2Y12) purinergic receptors are always expressed by microglia and have been proposed to be associated with movement, activation, or paracrine signaling of these cells, and it is necessary for the expansion of the microglia process to phagocytose the injured cells in vivo.6 The P2Y12 receptor (P2Y12R) was initially identified on platelets and subsequently found on microglia in central nervous system (CNS) parenchyma, implicating potential target in both antiplatelet therapy and neuropathic pain. Blocking P2Y12R has been suggested as a possible treatment for neuropathic pain and inhibition of platelet function.7–9 Recently, it has been reported that the activation of p38 mitogen-activated protein kinase (p38MAPK) plays an important role in proinflammatory cytokine production and the progression of neuropathic pain. More recent studies have shown that spontaneous pain and tactile allodynia in female rats with tibial tumors are associated with increased production of phosphorylated-p38MAPK (p-p38MAPK) and proinflammatory cytokines (IL-1β and TNF-α) in the spinal cord of CIBP.10
In light of the importance of this receptor in neuropathic pain and platelet activation, we asked whether microglial P2Y12R plays a similar role in CIBP. Therefore, the purposes of this study are 1) to investigate whether P2Y12R is involved in the establishment of rat CIBP model by inoculating Walker 256 breast cancer cells in the tibia and 2) to examine the effect of P2Y12R antagonist on spinal neuroimmune activity in a CIBP model.
Methods
Animals
All treatment protocols were strictly adhered to the guidelines of the International Society for Pain Research program on animal use. The study was approved by the Jiaxing University’s Institutional Animal Care and Use Committee (IACUC).
Unmated female Sprague Dawley rats (200–250 g) used in the study were provided by Zhejiang Academy of Medical Sciences (Hangzhou, People’s Republic of China). All animals were housed in the animal facility of our hospital under standard conditions with a 12 h light/dark cycle, a constant room temperature of 22–24°C, and humidity of 40–60%. Food and water were available ad libitum. Behavioral studies were conducted in a quiet room between 8 am and 8 pm.
Bone cancer model
Under anesthesia with chloral hydrate (400 mg/kg, intraperi-toneal), the left leg of the rat was shaved and disinfected with iodine. The tibia was pierced using a 25 G needle, and then 5 µL (2´107/mL) suspension of Walker 256 tumor cells was injected slowly into the bone cavity with a 10 µL microsy-ringe. The rats were placed on a hot pad until they returned to normal. The control group did the same surgical treatment as the cancer group, but the same amount of Hank’s solution was injected into the left tibia metaphysic. The normal group (rats) did not receive any treatments.
Intrathecal catheterization
Intrathecal catheterization between L4 and L5 was done immediately after inoculating tumor cells by inserting a PE-10 microtube into the vertebral space as previously described.11 Briefly, an intrathecal catheter (PE-10) filled with sterile saline (~10 µL) was inserted into the intervertebral gap between L4 and L5. After 1 day recovery, 2% lidocaine (20 µL) was injected intrathecally in rats without impaired motor performance. If the rats showed paralysis of the lower limbs within 30 s, it indicated the successful insertion of the catheter. Rats with significant local infection, abnormal activity, paralysis, and catheter loss were excluded. MRS2395 or 5% dimethyl-sulfoxide (DMSO) (v/v, in normal saline) (15 µL) was injected from days 9 to 12 (daily administration) by Hamilton syringe.
Mechanical allodynia (Von Frey test)
The rats were placed in a plastic box (20 cm ×20 cm ×30 cm) on a metal plate and allowed to acclimate to the environment for 30 min. The “paw withdrawal thresholds” (PWTs), the minimum force to evoke a withdrawal response and the tactile threshold, were measured on hind paws ipsilateral to surgery three times at 3 min intervals by electrical mechanical analgesia tester before and every 10 min (last for 70 min) after the application of MRS2395 (400 pmol/µL, 15 µL, intrathecal [i.t.]) or DMSO (5%, 15 µL, i.t.) on day 9 postinoculation and, then, once a day from days 9 to 12. The average was considered as a value to mechanical stimuli. The investigator was unaware to the test group. All the PWTs as raw data were given in grams.
Immunohistochemistry
On day 12 postinoculation, after Von Frey testing, the rats were deeply anesthetized with 4% chloral hydrate (600 mg/kg), and then the aorta was warmed with warm saline, followed by injection of cold 4% paraformaldehyde (pH 7.2–7.4). After perfusion, the rat L4–L6 spinal cord segments were removed and fixed in the same fixative for 2–4 h, after which they were soaked in 30% sucrose solution. Frozen sections with a thickness of 30 mm were cut using a microtome (Leica CM1900), and five nonadjacent sections were randomly selected for immunohistochemical staining. Sections were incubated in 3% H2O2 for 10 min and then with primary antibodies for the anti-ionized calcium-binding adapter molecule 1 (Iba-1) (1:100, goat; Abcam, Cambridge, UK) and p-p38MAPK (1:500, rabbit; Cell Signaling Technology, Denver, CO, USA) overnight at 4°C. Then, the sections were washed three times with phosphate buffer saline (PBS) and incubated with an antigoat or antirabbit second antibody for 30 min at room temperature.
The primary antibody was omitted and incubated directly with the secondary antibody, resulting in negative staining of Iba-1 and p-p38MAPK. The color development was carried out with 0.06% diaminobenzidine. Under a microscope, 0.01 M PBS solution was used to terminate the color reaction. The sections were placed on a glass slide, dried, dehydrated, cleared, and finally covered with cover slips. The sections were observed under fluorescence microscopy (C7070wz; Olympus Corporation, Tokyo, Japan), and images were captured. Five regions were randomly selected from the fixed size (an area of 200 µm ×300 µm) of the spinal dorsal horn (I–IV layers) in each image and evaluated using Image-Pro Plus. The average number of Iba-1-immunoreactive (Iba-1-IR) and p-p38MAPK-positive cells was determined (n=4). All results were expressed as mean ± SD.
Western blot analysis
Rats were first deeply anesthetized and decapitated, and L4–L6 segments of spinal dorsal horn were cut out quickly and disposed with protease and phosphatase to make it homogenized. The homogenate was centrifuged at 13,000× g at 4°C for 10 min. The supernatant was used for Western blot analyses. Protein concentration in the homogenate was measured using the bicinchoninic acid (BCA) kit and used to calculate volume for equal protein loading. After transferring, the polyvinylidene fluoride membranes were exposed to specific antibodies against P2Y12R (1:500, rabbit antimouse P2Y12R antibody; Merck & Co., Kenilworth, NJ, USA), Iba-1 (1:1000, rabbit antimouse Iba-1 antibody; Merck & Co.), p-p38 MAPK (1:1000, rabbit anti-mouse p-p38 MAPK antibody; Merck & Co.), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:20,000, rabbit antimouse GAPDH antibody; Sigma-Aldrich, St Louis, MO, USA) overnight at 4°C. After extensive washing with tris buffered saline with Tween®, the suspension was incubated with a dilution of horseradish peroxidase labeled goat antirabbit immunoglobulin G antibody with a ratio of 1:5000 for 1 h at room temperature. Following extensive washing, the signal was detected using Western Lightning ECL and measured by the optical densitometry on Image-Pro Plus6.0 using β-actin as a control.
Cytokine analysis
To determine IL-1β, IL-6, and TNF-α levels, the L4–L6 segments of the spinal cord were collected 4 h after intrathecal injection on day 12 postinoculation. The tissues were washed twice with PBS and cut into pieces with sterile scissors. After being milled, the tissues were digested with 1% collagenase for 25 min at 37°C. The supernatant was collected after centrifugation (12,000× g, 10 min). The samples were then boiled at 95°C for 10 min before being processed for Western blot analysis. Primary antibodies anti-IL-1β, anti-IL-6, and anti-TNF-α (rabbit; Abcam), together with the antirabbit secondary antibody, were used to detect the cytokines produced in these tissues.
Statistical analysis
All results are presented as mean ± SD. Statistical tests were performed using SPSS (Version 11.5). The changes of the PWTs and the immunohistochemistry staining of Iba-1 and p-p38MAPK in all groups were analyzed by two-way analysis of variance (ANOVA) followed by least significant difference (LSD) (when homogeneity of variance) or Tamhane’s T2 (when heterogeneity of variance) post hoc test. Unpaired T-test was used for blot analysis. The production of IL-6, IL-1β, or TNF-α cytokines was analyzed by one-way ANOVA and then compared by LSD post hoc test. Figures were prepared using Adobe Illustrator CS (Version 11.0; Adobe Systems, San Jose, CA, USA). For all experiments, P<0.05 indicates statistically significant difference.
Results
Effects of intrathecal injection of MRS2395 on mechanical allodynia in CIBP
To examine the effects of MRS2395 on bone cancer-induced pain behaviors, vehicle (DMSO) or MRS2395 was intrathecally administered in the cancer or sham groups. As shown in Figure 1A, the PWTs did not change in the sham + DMSO and sham + 2,2-Dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester (MRS) groups (P>0.05, n=8/group). In contrast, the PWTs were signifi-cantly decreased in the cancer + DMSO group on day 9 postinoculation (P<0.01, n=8/group; Figure 1A). The PWTs following single intrathecal injection of MRS2395 began to increase from 20 min, reached a peak at 30 min, and lasted until 60 min. The PWTs at 20 min (32.76±7.46), 30 min (33.99±6.07), and 40 min (30.55±6.41) following the administration of MRS2395 were significantly higher compared to the cancer group (26.24±5.73, P<0.05 for 20 min; 25.30±2.96, P<0.01 for 30 min; 26.15±4.07, P<0.05 for 40 min; n=8/group; Figure 1A). The results show that the use of MRS2395 can reduce the mechanical allodynia. Thresholds of cancer + DMSO group and cancer + MRS group were obviously reduced than that of sham + DMSO group on days 9–12 postinoculation (P<0.01); compared to thresholds of cancer + DMSO group, thresholds of cancer + MRS group were increased obviously at 30 min post-MRS2395 intrathecal injection on day 9 postinoculation (P<0.01). However, the thresholds of cancer + MRS group had a slight enhancement during days 10–12 postinoculation that did not reach statistical significance (P>0.05, Figure 1B).
Figure 1.
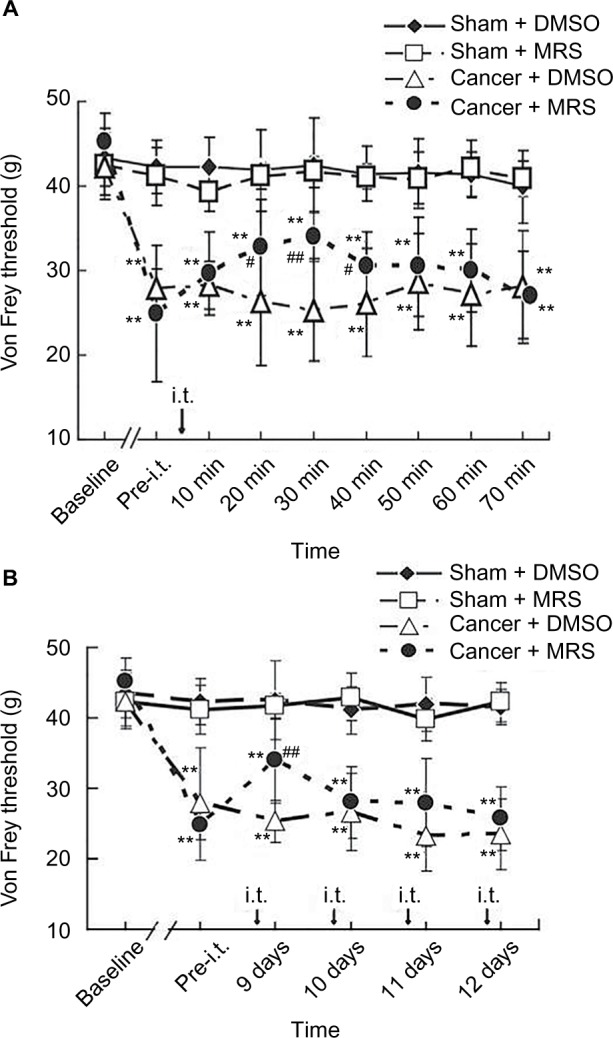
Inhibitory effects of MRS2395 on mechanical allodynia induced by bone cancer.
Notes: Von Frey test was used to determine the tactile sensitivity using the application of ascending force intensities. The PWTs were measured on hind paws ipsilateral to surgery three times at 3 min intervals by electrical mechanical analgesia tester (A) before and every 10 min (last for 70 min) after the administration of MRS2395 (400 pmol/µL, 15 µL, i.t.) or DMSO (5%, 15 µL, i.t.) on day 9 postinoculation and (B) once a day from days 9 to 12 after administration. All of the PWTs are given as raw data in grams. (A) PWTs on the ipsilateral side of bone cancer were significantly but transiently increased by single intrathecal injection of MRS2395 (400 pmol/µL) at day 9 postinoculation but were still lower than those in the sham group, indicating a partial alleviation of mechanical allodynia after the administration of MRS2395. **P<0.01, vs sham group and #P<0.05, ##P<0.01 vs cancer group. (B) The PWTs on the ipsilateral side of bone cancer were partly increased after intrathecal injection of MRS2395 (400 pmol/µL, 15 µL, once daily) from days 9 to 12 postinoculation, but there was no significance between the cancer + DMSO group and the cancer + MRS group. Eight rats were used in each group.
Abbreviations: I.t., intrathecal; PWTs, paw withdrawal thresholds; DMSO, dimethylsulfoxide; MRS, 2,2-Dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester.
CIBP increases P2Y12R expressions in spinal dorsal horn
To study the effects of CIBP on P2Y12R expression, we measured the relative densitometry of immunoblots of P2Y12R protein in the L4–L6 spinal cord from normal groups, sham groups, and cancer groups. As shown in Figure 2A and B, the expression of P2Y12R in the L4–L6 spinal cord was increased in bone cancer rats (n=4, #P<0.05 vs normal groups, *P<0.05 vs sham groups). These data suggested that CIBP led to a significant increase in P2Y12R expression in the L4–L6 spinal cord.
Figure 2.
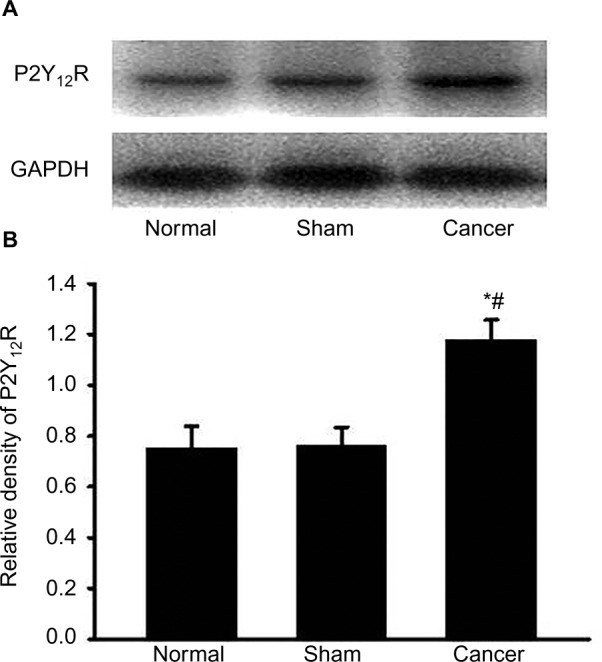
Cancer-induced bone pain increases P2Y12R expressions in spinal dorsal horn.
Notes: The levels of P2Y12R in the L4–L6 spinal cord were determined using the Western blot technique. (A) Gel panels show products from the L4–L6 spinal cord taken from normal rats, sham rats, and bone cancer rats 9 days after surgery. GAPDH was determined as loading control. (B) Normalized data of immune blot densitometry showed that the relative level of P2Y12R protein, which was normalized to GAPDH, was significantly increased in the L4–L6 spinal cord from bone cancer rats compared to normal rats (n=4; #P<0.05, unpaired T-test) and sham rats (n=4; *P<0.05, unpaired T-test).
Abbreviations: P2Y12R, P2Y12 receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
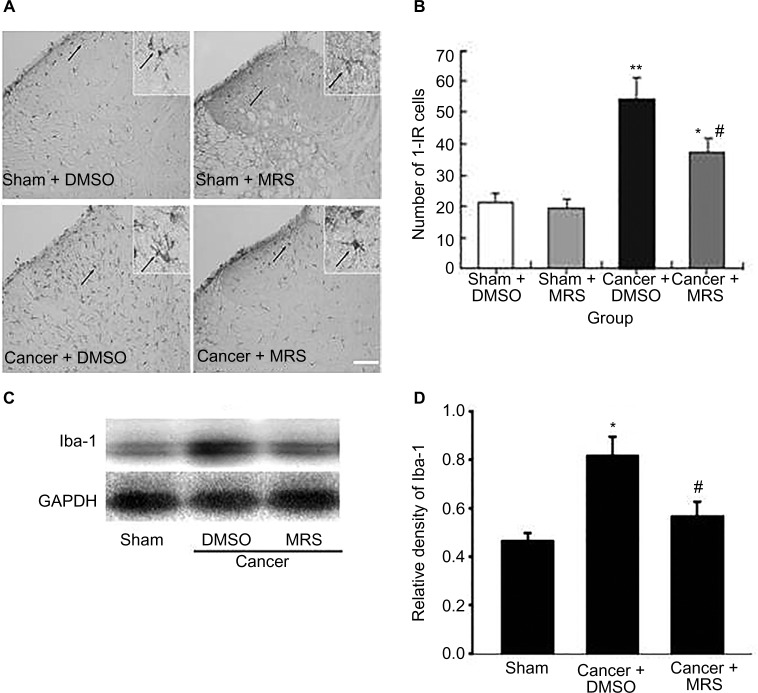
Effects of intrathecal injection of MRS2395 on the activation of spinal microglia induced by bone cancer
To examine whether microglia activation was associated with P2Y12R, we analyzed by Western blot (Figure 3) and immunohistochemistry (Figure 3A and B). The expression of the microglial markers was examined in the L4–L6 spinal cord of sham rats and bone cancer rats that received administration of the P2Y12R antagonist MRS2395 or DMSO. Western blot analysis revealed that the level of Iba-1 was upregulated in cancer group and partially inhibited by MRS2395 (P<0.05) (Figure 3C and D). The morphology of microglia in the sham + DMSO and sham + MRS2395 groups showed that they were nonactivated with small cell bodies and more ramifications (Figure 3A).
Figure 3.
Activation of microglia in the spinal dorsal horn induced by bone cancer and the inhibitory action of MRS2395.
Notes: (A) The staining of Iba-1-IR cells has been carried out for the sham + DMSO, sham + MRS2395, cancer + DMSO, and cancer + MRS2395 groups. The sham groups showed the morphology of lighter staining and more ramifications, suggesting inactivated microglia. The cancer + DMSO group showed deeper staining of Iba-1 and less ramification, suggesting activated microglia, and this activation was inhibited by MRS2395. (B) Comparison of average number of activated microglia in all groups. (C) Gel panels show products from the L4–L6 spinal cord taken from sham rats and bone cancer rats 9 days after administering with DMSO, MRS2395 using the Western blot. GAPDH was used as loading control. (D) Averaged data of immune blot densitometry showed that the relative level of Iba-1 protein, which was normalized to GAPDH, increased in bone cancer rats compared to sham + DMSO and sham + MRS2395 rats and partially suppressed by MRS2395. *P<0.05, **P<0.01 vs sham group and #P<0.05.
Abbreviations: Iba-1, ionized calcium-binding adapter molecule 1; Iba-1-IR, Iba-1-immunoreactive; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; DMSO, dimethylsulfoxide; MRS, 2,2-Dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester.
Microglia were significantly activated in the cancer + DMSO group, exhibiting the characteristics of swollen cell bodies and retracted processes (Figure 3A). This activation was obviously suppressed by MRS2395 (Figure 3A). The number of Iba-1-positive cells (37.35±3.34) in the cancer + MRS2395 group was significantly lower than that in the cancer + DMSO group (54.25±7.16, P<0.05) but was still higher than that in the sham + MRS group (21.35±2.53, P<0.05; Figure 3B), indicating that the activation of microglia was partially inhibited by MRS2395.
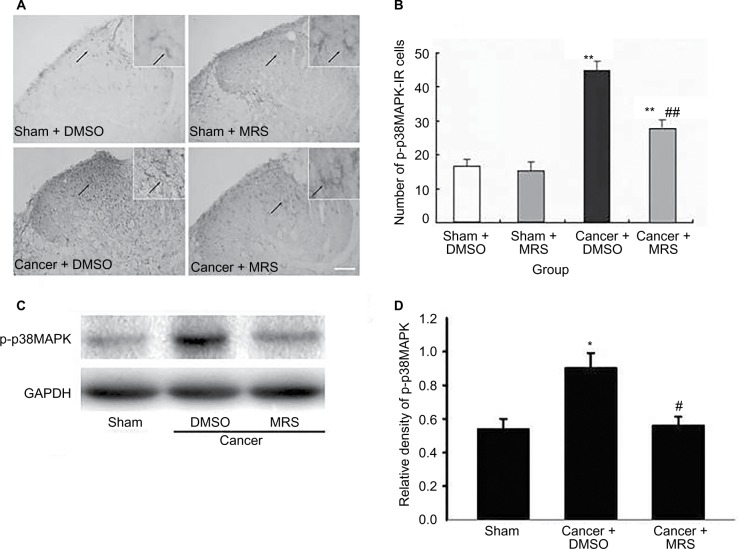
Effects of intrathecal injection of MRS2395 on p-p38MAPK expression in the spinal cord induced by bone cancer
It has been reported that nerve injury can activate the cascade of p38MAPK and the activation of p38MAPK in microglial cells in the spinal cord can cause neuropathic pain. To verify whether p38MAPK phosphorylation was regulated by P2Y12R in CIBP, the expression of p-p38MAPK protein in dorsal horn was examined by Western blot and immunohistochemistry using anti-p-p38MAPK antibody. Western blot analysis revealed that the level of p-p38MAPK was increased in the cancer group and partially suppressed by MRS2395 (P<0.05) (Figure 4).
Figure 4.
Effects of intrathecal injection of MRS2395 on p-p38MAPK in the spinal cord-induced by bone cancer.
Notes: (A) The staining of p-p38MAPK-IR cells has been carried out for the sham + DMSO, sham + MRS2395, cancer + DMSO, and cancer + MRS groups. It showed lighter staining of p-p38MAPK and more ramifications in sham groups, suggesting inactivated microglia. In cancer + DMSO group, deeper staining of p-p38MAPK and less ramification were demonstrated for activated microglia, while this activation was inhibited by MRS2395. (B) Comparison of average number of activated microglia in all groups. (C) Gel panels show products from the L4–L6 spinal cord taken from sham rats and bone cancer rats 9 days after administering with DMSO, MRS2395 using the Western blot. GAPDH was used as loading control. (D) Averaged data of immune blot densitometry showed that the relative level of p-p38MAPK protein, which was normalized to GAPDH, increased in bone cancer rats compared to sham + DMSO and sham + MRS2395 rats (n=4) and partially suppressed by MRS2395 (n=4). *P<0.05, **P<0.01 vs sham group and #P<0.05, ##P<0.01 vs cancer group.
Abbreviations: p-p38MAPK, phosphorylated-p38 mitogen-activated protein kinase; p-p38MAPK-IR, p-p38MAPK immunoreactivity; DMSO, dimethylsulfoxide; MRS, 2,2-Dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In addition, we found that immunoreactive cells migrate into the dorsal horn of the spinal cord, especially in the superficial dorsal horn. The morphology of p-p38MAPK-positive cells in sham groups showed that the cells were more inactivated with less numbers and smaller cell bodies, while they were significantly activated in the cancer + DMSO group with more numbers and swollen cell bodies (Figure 4A). Furthermore, this activation was significantly inhibited by MRS2395 (Figure 4A). As shown in Figure 4B, the number of the p-p38MAPK-positive cells was significantly upregulated in the cancer group and partially suppressed by MRS2395 (P<0.01; n=4/group).
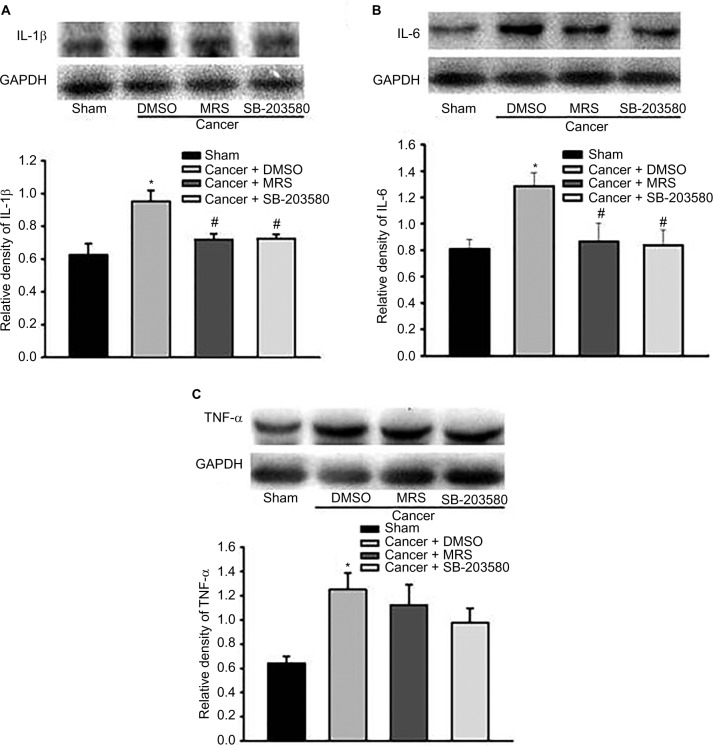
Effects of intrathecal injection of MRS2395 and SB-203580 on proinflammatory cytokines’ production in spinal dorsal horn induced by bone cancer
In order to fully reveal the impacts of MRS2395 intervention on spinal nociceptive responses, the expression of proinflammatory cytokines in the spinal L4–L6 fragments was observed by Western blot. An inhibitor of p38MAPK, SB-203580, has been included in this study. It showed a significant upregulation of proinflammatory cytokines in the cancer + DMSO group, ie, IL-1β (P<0.001, n=4; Figure 5A), IL-6 (P<0.001, n=4; Figure 5B), and TNF-α (P<0.05, n=4; Figure 5C), compared to that in the sham groups. It also showed a significant decrease in IL-1β (P<0.01, n=4; Figure 5A) and IL-6 (P<0.001, n=4; Figure 5B) production in the MRS2395-treated cancer group and SB-203580-treated cancer group, but not TNF-α, compared to that in the DMSO-treated bone cancer pain rats.
Figure 5.
Pretreatment with P2Y12R inhibitor MRS2395 and p38MAPK inhibitor SB-203580 suppresses the expression of IL-1β and IL-6, but not TNF-α, in the L4–L6 spinal cord from bone cancer rats using the Western blot.
Notes: The production of IL-1β (A), IL-6 (B), or TNF-α (C) was increased in the cancer + DMSO group than in the sham groups. The increased expression of IL-1β and IL-6 was suppressed by intrathecal injection of MRS2395 (n=4) and SB-203580 (n=4) except TNF-α. *P<0.05 vs sham group and #P<0.05 vs cancer + DMSO group.
Abbreviations: IL-1β, interleukin-1β; IL-6, interleukin-6; P2Y12R, P2Y12 receptor; p38MAPK, p38 mitogen-activated protein kinase; TNF-α, tumor necrosis factor-α; DMSO, dimethylsulfoxide; MRS, 2,2-Dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
In this study, we provide evidence for the first time that the activation of P2Y12R in spinal microglial plays an important role in the development and progression of CIBP, using immunohistochemical, pharmacological, and Western blot approaches. The new findings are as follows: 1) CIBP induced an increase in the expression of P2Y12R in spinal cord; 2) the intrathecal infusion of the P2Y12R antagonist MRS2395 suppressed the activation of microglia in CIBP; 3) the inhibition of P2Y12R decreased the expression of pro-inflammatory cytokines; and 4) the intrathecal infusion of the P2Y12R antagonist MRS2395 partly attenuated mechanical allodynia in CIBP.
The P2Y12R was initially found expressed on platelets and has a formal name “P2ADP receptor” because it is activated by ADP.12 Later, this receptor was cloned from the rat platelet cDNA library and was renamed “P2Y12R”.13 P2Y12R has been reported to be expressed constitutively in spinal microglia.7,14,15 This study extended these findings by showing the dynamic upregulation of spinal microglia in CIBP. In addition, intrathecal administration of MRS2395 (an inhibitor of P2Y12R) inhibited the microglial activation. The main reason for the decreased number of microglia in the ipsilateral spinal cord was considered to result from the inhibition of P2Y12R. The increased expression of spinal microglia in the immunohistochemistry for Iba-1 suggested that P2Y12R was simultaneously expressed in CIBP by our data. P2Y12R-mediated microglial functions (eg, process movement, chemotaxis, and morphological changes) were considered to be indispensable to the development of mechanical allodynia in CIBP. Mice with P2Y12 deficiency (P2Y12−/−) displayed impaired tactile allodynia after nerve injury without any change in basal mechanical sensitivity.8 These results, along with P2Y12R localization that highly limits to microglia, suggested that activation of P2Y12R in the spinal cord was an important cause of allodynia in CIBP.
Consulting a rat model of bone cancer pain reported by Medhurst et al,16,17 intrathecal administration of MRS2395 alleviates pain hypersensitive behaviors. The PWTs started to increase from 20 min, peaked at 30 min, and lasted for 40 min following intrathecal injection of MRS2395 on day 9 postinoculation, indicating a transient inhibitory action. Kobayashi et al7 reported that the time course of P2Y12R upregulation in neuropathic pain was different for CIBP in microglia; it peaked at day 3 after nerve injury and returned to normal after 30 days, suggesting that the expression of nucleotides receptors in microglia cells was regulated by different regulatory mechanisms. The mechanism suggested that cancer pain was composed of nerve injury-induced neuropathic pain and proinflammation pain.18 An absence of microglia activation was a characteristic of the spinal cord central sensitization in mouse models of bone cancer pain.19,20 Furthermore, it has been demonstrated that microglia are the early responders to glial cells, and then followed by the activation of astrocytes, which in turn keep such long-term pathological states maintained as persistent pain.21 Activation of spinal microglia and astrocytes has been demonstrated by several teams to contribute significantly to the maintenance and development of neuropathic pain and inflammatory.22 It is suggested here that the activation of microglia and astrocyte extensively related to long-term hyperalgesia. This study provides more convincing evidence that spinal microglia have a strong association with the development of cancer pain. It is difficult to control bone cancer pain because pathological reconstruction has emerged from CNS, namely central sensitization. To suppress the formation of spinal sensitization at early station, it may be feasible to restrain the activation of microglia. Most importantly, the inhibitory effect of MRS2395 on bone cancer cell-induced pain hypersensitivity in rats makes the drug a promising candidate for clinical application.
We hypothesized that the alleviation of pain mediated by MRS2395 in bone cancer pain may use a complex downstream signaling pathway, namely p38MAPK pathway. Now studies have shown that the activation of p38MAPK induced by bone cancer can be significantly inhibited by intrathecal injection of MRS2395, which was consistent with the previous reports. As one of the most important members of the MAPK family, p38 was also found activated in bone cancer pain models,23–25 in addition to inflammatory pain and neuropathic pain models.26–28 Many works have shown that p38 played important roles in the development of bone pain-related and bone cancer-related processes. For example, p38 has been found positively regulating the osteoclastic activity and plays an important role in the metastasis and growth of bone cancer cells.29 Recently, Cottrell et al demonstrated that p38 inhibition was a therapeutic strategy for managing fracture pain.30 In addition, P2Y12R antagonist MRS2395 and antisense knockdown of P2Y12R expression suppressed the phosphorylation of p38MAPK in spinal microglia after partial sciatic nerve ligation.7 In contrast, it has been demonstrated that the P2X7 purinoceptor and p38MAPK, both selectively expressed by activated spinal microglia, may also be required to maintain pain behavior in CIBP.31
The activation of spinal microglia has been demonstrated in bone cancer pain. Additionally, it may contribute to the hypersensitivity by releasing IL-1β and TNF-α.5,10,20 A recent study suggested important roles of proinflammatory cytokines for tumor growth and bone cancer-associated pain.5 The increased proinflammatory cytokine production by glial cells has been now considered as an important marker for the identification of glial activation in chronic pain.32 In this study, the expression of IL-1β and IL-6 was significantly inhibited in the cancer group at the 12th day after intrathecal injection of MRS2395, with a mild increase in TNF-α. Therefore, peripheral inflammation has profound effects on the nervous system, and cytokines seem to be major players for such effects. The expression of inflammatory cytokines is dissimilar, reflecting the different mechanism in the pathogenesis of bone cancer pain. IL-1β is secreted by mono-nuclear cells, skin cells, fibroblasts, endothelial cells, and so on, playing a very important role in the local immunity. IL-6 is the main mediator in infection and tissue damage. TNF-α is early released by mononuclear macrophages under stress, initiating other cytokines and involving in immune adjustment, fever, and inflammation. It needs further study on their specific interactions in bone cancer pain. Thus, the inhibition of proinflammatory cytokine expression by MRS2395 may be at least partially attributed to the antiallodynic effect of MRS2395 in bone cancer pain. In the behavioral study, allodynia induced by bone cancer was significantly inhibited after MRS2395 intervention. Therefore, we speculate that the P2Y12R might correlate with bone cancer-induced allodynia. It has been proposed that the descending pain facilitation can partially underlie pain hypersensitivity. This study demonstrates that intrathecal injection of MRS2395 can reverse this facilitation by inhibiting microglial activation without disrupting pain memory. This is significantly different from the analgesic effects of morphine in neurons.
Conclusion
The P2Y12R can induce the activation of spinal microglia in CIBP through p38MAPK signaling pathway. Consequently, the activation of spinal microglia p38MAPK signaling pathway in CIBP may lead to increased production of pro-inflammatory cytokines or other effectors into the nociceptive network, resulting in the manifestation of cancer pain behaviors. Taken together, P2Y12R plays a critical role in spinal microglia activation in CIBP and represents a potential therapeutic target for cancer pain management.
Acknowledgments
This study was supported by funding from the Natural Science Foundation of Zhejiang Province (LY16H090016 and LY17H090019), the Zhejiang Provincial and Ministerial Cultivation Plan for Medicine and Health (2015PY010), the National Natural Science Foundation of China (81341035 and 81171057), the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (2012-RC-22), Zhejiang Province, Zhejiang north regional, the construction projects of Anesthesia Discipline Special Disease Center, and Jiaxing City, the construction projects of Key Laboratory of Nerve and Pain Medicine, and construction funding of Medicine Key Discipline of Pain Therapy, Integrated Traditional and Western Medicine of Zhejiang Province (2012-XK-A31). The authors acknowledge the technical support from the Central Laboratory of the First Hospital of Jiaxing. We also thank Professor Renshan Ge, a native speaker, the former head of the Endocrinology Lab of the CBR in Population Council (USA) for English language editing.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M. Translational medicine: cancer pain mechanisms and management. Br J Anaesth. 2008;101(1):87–94. doi: 10.1093/bja/aen100. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Wang LN, Yao M, Yang JP, et al. Cancer-induced bone pain sequentially activates the ERK/MAPK pathway in different cell types in the rat spinal cord. Mol Pain. 2011;7:48. doi: 10.1186/1744-8069-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao M, Chang XY, Chu YX, et al. Antiallodynic effects of propentofylline elicited by interrupting spinal glial function in a rat model of bone cancer pain. J Neurosci Res. 2011;89(11):1877–1886. doi: 10.1002/jnr.22711. [DOI] [PubMed] [Google Scholar]
- 5.Geis C, Graulich M, Wissmann A, et al. Evoked pain behavior and spinal glia activation is dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer pain. Neuroscience. 2010;169(1):463–474. doi: 10.1016/j.neuroscience.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia. 2007;55(8):873–884. doi: 10.1002/glia.20509. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi K, Yamanaka H, Fukuoka T, Dai Y, Obata K, Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J Neurosci. 2008;28(11):2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tozaki-Saitoh H, Tsuda M, Miyata H, Ueda K, Kohsaka S, Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J Neurosci. 2008;28(19):4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyu D, Glenn JR, White AE, et al. Mode of action of P2Y(12) antagonists as inhibitors of platelet function. Thromb Haemost. 2011;105(1):96–106. doi: 10.1160/TH10-07-0482. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Yang J, Wang L, et al. Tibia tumor-induced cancer pain involves spinal p38 mitogen-activated protein kinase activation via TLR4-dependent mechanisms. Brain Res. 2010;1346:213–223. doi: 10.1016/j.brainres.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Zhang YQ, Ji GC, Wu GC, Zhao ZQ. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99(3):525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 12.Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273(4):2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- 13.Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki Y, Hoshi M, Akazawa C, et al. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44(3):242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi K, Fukuoka T, Yamanaka H, et al. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498(4):443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- 16.Medhurst SJ, Walker K, Bowes M, et al. A rat model of bone cancer pain. Pain. 2002;96(1–2):129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 17.Yao M, Yang JP, Wang LN, et al. Feasibility of establishment of rat model of bone cancer pain by using Walker 256 cells cultured in vitro or in vivo. Zhonghua Yi Xue Za Zhi. 2008;88(13):880–884. [PubMed] [Google Scholar]
- 18.Schwei MJ, Honore P, Rogers SD, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19(24):10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honore P, Rogers SD, Schwei MJ, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98(3):585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 20.Hald A, Nedergaard S, Hansen RR, Ding M, Heegaard AM. Differential activation of spinal cord glial cells in murine models of neuropathic and cancer pain. Eur J Pain. 2009;13(2):138–145. doi: 10.1016/j.ejpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20(2):467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 22.Liang L, Wang Z, Lu N, Yang J, Zhang Y, Zhao Z. Involvement of nerve injury and activation of peripheral glial cells in tetanic sciatic stimulation-induced persistent pain in rats. J Neurosci Res. 2010;88(13):2899–2910. doi: 10.1002/jnr.22439. [DOI] [PubMed] [Google Scholar]
- 23.Zwerina J, Hayer S, Redlich K, et al. Activation of p38MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum. 2006;54(2):463–472. doi: 10.1002/art.21626. [DOI] [PubMed] [Google Scholar]
- 24.Dong H, Tian YK, Xiang HB, Tian XB, Jin XG. The cellular location and significance of p38alpha/beta isoforms in the lumbar spinal cord of the bone cancer pain rats. Zhonghua Yi Xue Za Zhi. 2007;87(1):53–57. [PubMed] [Google Scholar]
- 25.Svensson CI, Medicherla S, Malkmus S, et al. Role of p38 mitogen activated protein kinase in a model of osteosarcoma-induced pain. Pharmacol Biochem Behav. 2008;90(4):664–675. doi: 10.1016/j.pbb.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Almela P, Garcia-Nogales P, Romero A, Milanes MV, Laorden ML, Puig MM. Effects of chronic inflammation and morphine tolerance on the expression of phospho-ERK 1/2 and phospho-P38 in the injured tissue. Naunyn Schmiedebergs Arch Pharmacol. 2009;379(3):315–323. doi: 10.1007/s00210-008-0356-x. [DOI] [PubMed] [Google Scholar]
- 27.Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;213(2):257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen YR, Suter MR, Ji RR, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia contributes to incision-induced mechanical allodynia. Anesthesiology. 2009;110(1):155–165. doi: 10.1097/ALN.0b013e318190bc16. [DOI] [PubMed] [Google Scholar]
- 29.Schultz RM. Potential of p38 MAP kinase inhibitors in the treatment of cancer. Prog Drug Res. 2003;60:59–92. doi: 10.1007/978-3-0348-8012-1_2. [DOI] [PubMed] [Google Scholar]
- 30.Cottrell JA, Meyenhofer M, Medicherla S, Higgins L, O’Connor JP. Analgesic effects of p38 kinase inhibitor treatment on bone fracture healing. Pain. 2009;142(1–2):116–126. doi: 10.1016/j.pain.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Hansen RR, Nielsen CK, Nasser A, et al. P2´7 receptor-deficient mice are susceptible to bone cancer pain. Pain. 2011;152(8):1766–1776. doi: 10.1016/j.pain.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30(11):581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]