Abstract
Purpose
The present study was designed to retrospectively evaluate the prognostic value of the C-reactive protein/albumin (CRP/ALB) ratio in laryngeal squamous cell carcinoma (LSCC).
Methods
One hundred and twenty-nine newly diagnosed LSCC patients admitted between May 2006 and October 2011 were retrospectively reviewed. Their serum CRP and ALB were quantified preoperatively. The relationship between the CRP/ALB ratio and the clinicopathologic features was analyzed. Receiver operating characteristic curve was used to calculate the prognostic value of the CRP/ALB ratio. Then, the Cox proportional hazards model was used in univariate and multivariate analyses to identify significant prognostic factors associated with disease-free survival and overall survival.
Results
The cutoff value for CRP/ALB ratio was 0.047. An elevated CRP/ALB ratio was significantly associated with nodal metastasis, late disease stage, and recurrence. Also, high values of CRP/ALB ratio were significant predictors for poor overall survival and disease-free survival on multivariate analysis.
Conclusion
Pretreatment CRP/ALB ratio may be a significant prognostic marker in LSCC.
Keywords: C-reactive protein/albumin ratio, laryngeal squamous cell carcinoma, prognosis, survival
Introduction
Laryngeal squamous cell carcinoma (LSCC) can commonly be found among head and neck squamous cell carcinomas, and comprises 1%–2% of all malignancies.1 The underlying mechanism of laryngeal carcinogenesis is still uncertain, and reliable diagnostic and prognostic value markers are still insufficient. Few studies have reported that the systemic inflammation plays a significant role in the progression and prognosis of many cancers.2 Also, several inflammation-based prognostic scores, including neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR), have been reported to have prognostic significance in LSCC.3–6
Now, a novel inflammation-based prognostic index, the C-reactive protein/albumin (CRP/ALB) ratio, has been shown to be an independent prognostic marker, and compared with other inflammation-based prognostic markers, it has significant prognostic value in esophageal cancer, nasopharyngeal cancer, and hepatocellular carcinoma.7–9 However, the prognostic significance of the CRP/ALB ratio has not been evaluated in patients with LSCC yet. Hence, we performed a study retrospectively to assess its prognostic value in LSCC patients.
Materials and methods
Patients
We retrospectively reviewed 129 patients with newly diagnosed LSCC admitted between May 2006 and October 2011 from the Department of Otolaryngology, Head and Neck Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University. This study was carried out after ethical approval was granted by the Ethics Committee of Sun Yat-sen Memorial Hospital. All patients gave their written informed consent to review their medical records and for this study. Patients with synchronous cancer, history of cancer, presence of infection or inflammatory conditions, immune disease, and those without pretreatment blood test were excluded from current study. Also, patients who received neoadjuvant radiotherapy or chemotherapy were also excluded from the study. All blood tests were retrieved before the patients received any form of treatment. Quantification of CRP and ALB in serum was undertaken at the laboratories of Sun Yat-sen Memorial Hospital through a standard methodology. All the medical records of the patients as well as their clinical information were reviewed and collected (including age at diagnosis, sex, tumor-node-metastasis [TNM] classification, treatment modalities, pretreatment serum CRP and ALB concentrations, time to recurrence, and death). The CRP/ALB ratio was calculated as serum CRP divided by serum ALB level.7–9 Then, all patients received standard surgery (and radio/chemotherapy, if applicable) according to the National Comprehensive Cancer Network guidelines. Patients with adverse features (eg, extracapsular node spread, positive margins, pT4 primary, N2 or N3 nodal disease, vascular embolism, and perineural invasion) had postoperative radiotherapy or chemoradiotherapy. All patients were followed up for at least 5 years or until October 2016 or until their death.
Statistical analysis
Tumor clinical staging was performed according to the seventh edition of the American Joint Committee on Cancer staging system. Then they were categorized into two subgroups: early stage (stages I and II) and late stage (stages III and IV).
Comparison between the groups was performed using the Mann–Whitney U-test (for normal distribution data) and Student’s t-test (for normal data) for continuous variables and the Chi-square test for categorical variables.
Receiver operating characteristic (ROC) curves were plotted to generate the optimal cutoff point for overall survival (OS). Survival curves were analyzed according to the Kaplan–Meier method and compared with the log-rank test to evaluate the differences between survival rates among different groups. Multivariable analysis using a Cox proportional hazards model was performed to find the prognostic factors associated with OS and disease-free survival (DFS) based on those factors that proved to be significant in the univariate analysis. A two-tailed P-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS v22.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
A total of 129 patients were enrolled in this study, in which males were predominant (92.2%). The median age of these patients was 61 (range: 29–82) years. According to the seventh edition of the American Joint Committee on Cancer TNM staging system, 42 (32.6%) patients were classified as stage I, 38 (29.5%) as stage II, 32 (24.8%) as stage III, and 17 (13.2%) as stage IV. Eighty-five (65.89%) patients underwent surgery alone and 44 (34.41%) underwent surgery with radiotherapy or radiochemotherapy postoperatively.
The median of CRP value was 5.214 mg/L (range: 0.232–69.285) and that of ALB value was 40.100 g/L (range: 30.900–47.900). The median CRP/ALB ratio was 0.374 (range: 0.006–2.020). ROC curves were plotted based on OS, and the area under the curve was used to determine the discriminative ability of the CRP/ALB ratio (Figure 1). The optimal CRP/ALB ratio cutoff value for OS was 0.047, and the patients were divided into two subgroups based on this cutoff value (<0.047, n=73; ≥0.047, n=56).
Figure 1.
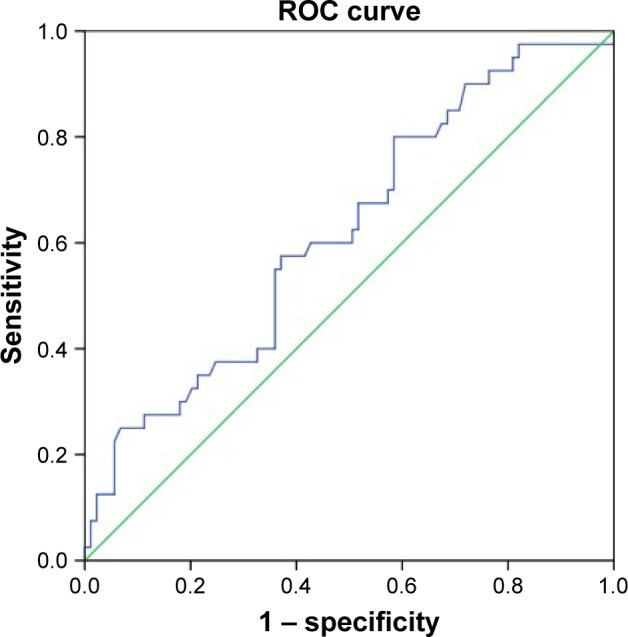
ROC curve analysis of the CRP/ALB ratio for survival status among the 129 patients with LSCC.
Notes: The optimal cutoff value was 0.047 according to the ROC analysis (area 0.62 [95% CI, 0.52–0.72], P<0.05). Diagonal segments are produced by ties. Green line represents reference line. Blue line represents the curve for CRP/ALB ratio.
Abbreviations: CI, confidence interval; CRP/ALB, C-reactive protein/albumin; LSCC, laryngeal squamous cell carcinoma; ROC, receiver operating characteristic.
Table 1 shows the associations between CRP/ALB ratio and clinicopathologic characteristics. A higher CRP/ALB ratio was shown to have association with nodal metastasis (P<0.01), a late disease stage (P<0.05), and cancer recurrence (P<0.05). No significance in this ratio was observed for any of the other characteristics.
Table 1.
Patient demographics and clinical characteristics
| Characteristics | Total n, (%) | CRP/ALB ratio
|
P-value | |
|---|---|---|---|---|
| <0.047 n, (%) | ≥0.047 n, (%) | |||
| Total | 129 | 73 | 56 | |
| Age, years | 0.53 | |||
| ≤60 | 64 (49.6) | 38 (52.1) | 26 (46.4) | |
| >60 | 65 (50.4) | 35 (47.9) | 30 (53.6) | |
| Sex | 0.30 | |||
| Male | 119 (92.2) | 66 (90.4) | 53 (94.6) | |
| Female | 10 (7.8) | 7 (9.6) | 3 (5.4) | |
| Smoking status | 0.96 | |||
| Nonsmokers or ex-smokers | 51 (39.6) | 29 (39.7) | 22 (39.2) | |
| Smokers | 78 (60.4) | 44 (60.3) | 34 (60.8) | |
| Nodal classification | <0.01 | |||
| N0 | 87 (67.4) | 57 (78.1) | 30 (53.6) | |
| N+ | 42 (32.6) | 16 (21.9) | 26 (46.4) | |
| Disease stage | <0.05 | |||
| I/II | 80 (62.0) | 51 (69.9) | 29 (51.8) | |
| III/IV | 49 (38.0) | 22 (30.1) | 27 (48.2) | |
| Recurrence | <0.05 | |||
| Yes | 46 (35.6) | 20 (27.4) | 27 (48.2) | |
| No | 83 (64.4) | 53 (72.6) | 29 (51.8) | |
Abbreviation: CRP/ALB, C-reactive protein/albumin.
Survival analysis
During a median of 77 (interquartile range [66–94]) months’ follow-up, a total of 40 (31%) and 44 (34.1%) experienced death or recurrence of LSCC, respectively. In patients with CRP/ALB ratio <0.047, the median OS was 88 months and the interquartile (P25, P75) was 68 and 97 months, respectively. In patients with CRP/ALB ratio ≥0.047, the median OS was 73 months and the interquartile (P25, P75) was 61 and 89 months, respectively. For the entire cohort, the 5-year OS and DFS were 69% and 50.4%, respectively.
In Table 2, univariate analysis reveals that the nodal metastasis (P<0.01), late disease stage (P<0.01), and higher CRP/ALB ratio (P=0.01) had a significant association with OS. The 5-year OS rate was significantly better in patients with CRP/ALB ratio <0.047 (Figure 2). For DFS also, it revealed the same outcome (nodal metastasis [P<0.01]; late disease stage [P<0.01]; higher CRP/ALB ratio [P<0.01]).
Table 2.
Univariate Cox regression analysis for OS and DFS in patients with laryngeal squamous cell carcinoma
| Variables | No of patients | OS
|
DFS
|
||||
|---|---|---|---|---|---|---|---|
| No of events | HR (95% CI) | P-value | No of events | HR (95% CI) | P-value | ||
| Sex | 0.11 | 0.08 | |||||
| Male | 119 | 39 | 43 | ||||
| Female | 10 | 1 | 0.20 (0.03–1.47) | 1 | 0.18 (0.02–1.28) | ||
| Age, years | 0.26 | 0.97 | |||||
| ≤60 | 64 | 23 | 22 | ||||
| >60 | 65 | 17 | 0.70 (0.37–1.31) | 22 | 0.97 (0.54–1.75) | ||
| Smoking | 0.78 | 0.67 | |||||
| Nonsmoker or ex-smoker | 51 | 19 | 20 | ||||
| Smoker | 78 | 21 | 1.23 (0.67–1.99) | 24 | 1.06 (0.56–1.76) | ||
| Nodal classification | <0.01 | <0.01 | |||||
| N0 | 87 | 14 | 22 | ||||
| N+ | 42 | 26 | 4.58 (2.42–8.61) | 20 | 2.43 (1.33–4.45) | ||
| Disease stage | ,0.01 | <0.01 | |||||
| I/II | 80 | 12 | 19 | 4.68 (2.49–8.81) | |||
| III/IV | 49 | 28 | 5.94 (2.99–11.78) | 25 | |||
| CRP/ALB ratio | <0.05 | <0.01 | |||||
| <0.047 | 73 | 17 | 18 | ||||
| ≥0.047 | 56 | 23 | 2.13 (1.15–3.93) | 26 | 2.36 (1.28–4.36) | ||
Abbreviations: CRP/ALB, C-reactive protein/albumin; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
Figure 2.
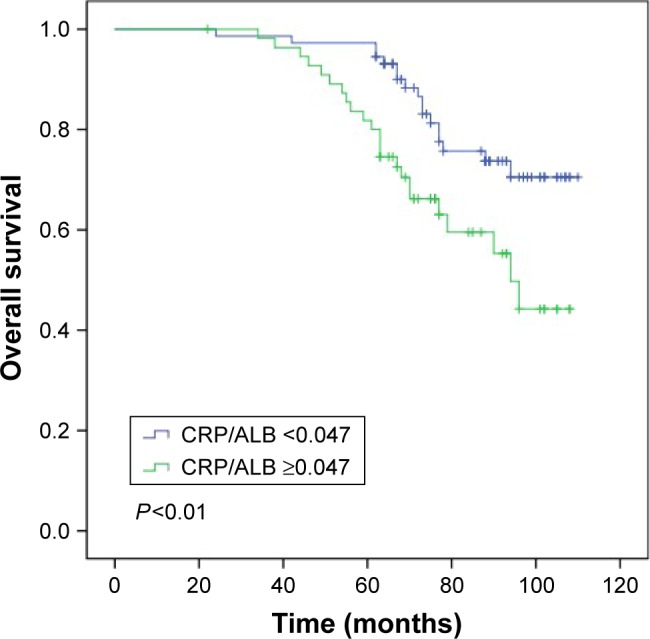
Overall survival curves for the 129 patients with LSCC stratified by the CRP/ALB ratio (P<0.01).
Abbreviations: CRP/ALB, C-reactive protein/albumin; LSCC, laryngeal squamous cell carcinoma.
Variables with significance in univariate analysis were involved in multivariate analysis to identify independent prognostic factors for OS and DFS, respectively (Table 3). Multivariate analysis suggested that nodal metastasis (hazard ratio [HR] =2.56; 95% confidence interval [CI] =1.12–5.87; P<0.05), late disease stage (HR [95% CI] =3.20 [1.36–7.48], P<0.01), and higher CRP/ALB ratio (HR [95% CI] =2.56 [1.09–5.86], P<0.05) were the independent indicators affecting OS. However, in DFS, only late disease stage (HR [95% CI] =2.91 [1.40–6.07], P<0.01) and higher CRP/ALB ratio (HR [95% CI] =1.97 [1.12–4.06], P=0.02) were the independent indicators affecting DFS.
Table 3.
Multivariate Cox regression analysis for OS and DFS in patients with laryngeal squamous cell carcinoma
| Variables | No of patients | OS
|
DFS
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Nodal classification | <0.05 | 0.72 | |||
| N0 | 87 | ||||
| N+ | 42 | 2.56 (1.12–5.87) | 1.15 (0.545–2.41) | ||
| Disease stage | <0.01 | <0.01 | |||
| I/II | 80 | ||||
| III/IV | 49 | 3.20 (1.36–7.48) | 2.91 (1.40–6.07) | ||
| CRP/ALB ratio | <0.05 | <0.05 | |||
| <0.047 | 73 | 2.56 (1.09–5.86) | 1.97 (1.12–4.06) | ||
| ≥0.047 | 56 | ||||
Abbreviations: CRP/ALB, C-reactive protein/albumin; CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OS, overall survival.
Discussion
The CRP/ALB ratio in this study was an independent prognostic factor for OS and DFS in the LSCC patients. To the best of our knowledge, this is the first study that evaluates the prognostic value of the CRP/ALB ratio in LSCC patients.
Several studies revealed the significant role of systemic inflammation in the prognosis of patients.10 In the tumor microenvironment, inflammatory cytokines produced by cancer cells can promote inflammation11 and chemokines may promote cancer metastasis.12 Some parameters, including NLR and PLR, have been reported for their significance in assessing the systemic inflammatory response.3,5,13 Tu et al3 and Fu et al14 reported that preoperative NLR was associated with recurrence and poor OS. Also, Wang et al reported PLR to be an independent predictor for poor OS and relapse-free survival.13
Recently, an increasing number of studies exploring the relationship between CRP/ALB ratio and several cancers have been conducted, including liver, lung, gastric, and esophageal cancers.7–9,15,16 Besides, Ranzani et al revealed that the CRP/ALB ratio had a more precise prognosis value than CRP level alone to predict 90-day mortality in sepsis patients.17 In the current study, the CRP/ALB ratio had a significant connection with OS and DFS; the CRP/ALB cutoff value was 0.047 and had an interactive role in systemic inflammatory responses and dystrophia.9 However, the underlying mechanisms by which the CRP/ALB ratio regulates the survival of cancer patients are still uncertain. To start with, an elevated CRP level may have a connection with the existence of a systemic inflammatory response.9 Also, tumor cell necrosis and invasion could positively upregulate an inflammatory response.18 Marsik et al retrospectively analyzed a large cohort of patients and reported that there were nearly 30% in 10 years all-cause mortality exhibited a higher CRP level (>80 mg/L) and that a highly increased CRP level had a connection with higher mortality due to cancer and noncancer all-cause vascular diseases.10 Furthermore, laryngeal carcinoma was found to generate interleukin-6, which could induce liver to synthesize more CRP and reduce the levels of ALB synthesized by hepatic cells,19,20 and play a role as an autocrine growth factor, so as to enhance the proliferation of cancer cells.21
Our current finding may suggest that LSCC patients with an increased CRP/ALB ratio may need a more frequent follow-up. Also, they may benefit from anti-inflammatory and nutritional support therapy.22,23 Indeed, the relationship between nutrition and inflammation had been confirmed by many researches, and systemic inflammatory response was found to lead to a worse nutritional status and, subsequently, a poor prognosis in cancer patients.24–26 Furthermore, several studies had revealed that cancer patients could benefit from ω-3 polyunsaturated fatty acids in fish oil as it could improve their CRP/ALB status, immune function, as well as prevent weight loss during treatment.27,28 It is also significant that CRP/ALB ratio should be applied with a combination of the standard TNM staging system to better individualized treatment and surveillance of LSCC patients.
This study has several limitations. The retrospective nature of this study meant that many patients without their detailed medical records were excluded, which may lead to inevitable bias. Also, there may be other unknown factors that could influence serum CRP, ALB, and other biochemical factors. The area under the curve created by ROC curve was relatively low; a relatively small number of patients enrolled in this study may explain this. We also did not investigate the relationship between nutritional support therapy and CRP/ALB ratio in this study. A well-designed prospective study with a large cohort is required to solve these clinical as well as basic life science problems in order to provide a better answer to patients’ prognosis in the future.
Conclusion
This is the first study showing that the CRP/ALB ratio is an independent prognostic factor for OS and DFS in patients with LSCC. As an easily accessible preoperative biomarker, its role in combination with the TNM staging system could be evaluated to predict a more accurate clinical outcome in LSCC patients in the future.
Acknowledgments
This study was supported by grants from the Sun Yat-sen University Clinical Research 5010 Program (grant 2010008).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sadri M, McMahon J, Parker A. Management of laryngeal dysplasia: a review. Eur Arch Otorhinolaryngol. 2006;263(9):843–852. doi: 10.1007/s00405-006-0078-y. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 3.Tu XP, Qiu QH, Chen LS, et al. Preoperative neutrophil-to-lymphocyte ratio is an independent prognostic marker in patients with laryngeal squamous cell carcinoma. BMC Cancer. 2015;15:743. doi: 10.1186/s12885-015-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kara M, Uysal S, Altinisik U, Cevizci S, Guclu O, Derekoy FS. The pretreatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and red cell distribution width predict prognosis in patients with laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2017;274(1):535–542. doi: 10.1007/s00405-016-4250-8. [DOI] [PubMed] [Google Scholar]
- 5.Rachidi S, Wallace K, Wrangle JM, Day TA, Alberg AJ, Li Z. Neutrophil-to-lymphocyte ratio and overall survival in all sites of head and neck squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E1068–E1074. doi: 10.1002/hed.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong BY, Stafford ND, Green VL, Greenman J. Prognostic value of the neutrophil-to-lymphocyte ratio in patients with laryngeal squamous cell carcinoma. Head Neck. 2016;38(Suppl 1):E190–E1908. doi: 10.1002/hed.24346. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol. 2015;22(3):803–810. doi: 10.1245/s10434-014-4048-0. [DOI] [PubMed] [Google Scholar]
- 8.Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation-based prognostic score, the c-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10(9):e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Zhou GQ, Liu X, et al. Exploration and validation of c-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinoma. J Cancer. 2016;7(11):1406–1412. doi: 10.7150/jca.15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54(2):343–349. doi: 10.1373/clinchem.2007.091959. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wang S, Song X, et al. The prognostic value of systemic and local inflammation in patients with laryngeal squamous cell carcinoma. Onco Targets Ther. 2016;9:7177–7185. doi: 10.2147/OTT.S113307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Liu W, OuYang D, Yang A, Zhang Q. Preoperative neutrophil-to-lymphocyte ratio predicts long-term survival in patients undergoing total laryngectomy with advanced laryngeal squamous cell carcinoma: a single-center retrospective study. Medicine (Baltimore) 2016;95(6):e2689. doi: 10.1097/MD.0000000000002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Sun X, Liu J, et al. Preoperative c-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol. 2015;8(4):339–345. doi: 10.1016/j.tranon.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Zhan J, Hong S, et al. Ratio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep. 2015;5:10481. doi: 10.1038/srep10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8(3):e59321. doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang P, Xi M, Li QQ, et al. The modified glasgow prognostic score is an independent prognostic factor in patients with inoperable thoracic esophageal squamous cell carcinoma undergoing chemoradiotherapy. J Cancer. 2014;5(8):689–695. doi: 10.7150/jca.9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliopoulos AG, Stack M, Dawson CW, et al. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kappaB pathway involving TNF receptor-associated factors. Oncogene. 1997;14(24):2899–2916. doi: 10.1038/sj.onc.1201258. [DOI] [PubMed] [Google Scholar]
- 20.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113(4):750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 22.Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000;320(7250):1642–1646. doi: 10.1136/bmj.320.7250.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzo A, Cengel KA. Anti-inflammatory therapy for pancreatic cancer: a sorely needed advance in therapeutics. Cancer Biol Ther. 2008;7(7):1051–1052. doi: 10.4161/cbt.7.7.6581. [DOI] [PubMed] [Google Scholar]
- 24.Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One. 2014;9(9):e106914. doi: 10.1371/journal.pone.0106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Support Care Cancer. 2012;20(8):1823–1829. doi: 10.1007/s00520-011-1282-x. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Lara K, Turcott JG, Juarez E, et al. Association of nutrition parameters including bioelectrical impedance and systemic inflammatory response with quality of life and prognosis in patients with advanced non-small-cell lung cancer: a prospective study. Nutr Cancer. 2012;64(4):526–534. doi: 10.1080/01635581.2012.668744. [DOI] [PubMed] [Google Scholar]
- 27.Long H, Yang H, Lin Y, Situ D, Liu W. Fish oil-supplemented parenteral nutrition in patients following esophageal cancer surgery: effect on inflammation and immune function. Nutr Cancer. 2013;65(1):71–75. doi: 10.1080/01635581.2013.741761. [DOI] [PubMed] [Google Scholar]
- 28.Mocellin MC, Pastore e Silva Jde A, Camargo Cde Q, et al. Fish oil decreases c-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids. 2013;48(9):879–888. doi: 10.1007/s11745-013-3816-0. [DOI] [PubMed] [Google Scholar]


