Abstract
Caveolin-1 (Cav-1), a major structural protein of caveolae, is an integral membrane protein which plays an important role in the progression of carcinoma. However, whether Cav-1 acts as a tumor promoter or a tumor suppressor still remains controversial. For example, the tumor-promoting function of Cav-1 has been found in renal cancer, prostate cancer, tongue squamous cell carcinoma (SCC), lung SCC and bladder SCC. In contrast, Cav-1 also plays an inhibitory role in esophagus adenocarcinoma, lung adenocarcinoma and cutaneous SCC. The role of Cav-1 is still controversial in thyroid cancer, hepatocellular carcinoma, gastric adenocarcinoma, colon adenocarcinoma, breast cancer, pancreas cancer, oral SCC, laryngeal SCC, head and neck SCC, esophageal SCC and cervical SCC. Besides, it has been reported that the loss of stromal Cav-1 might predict poor prognosis in breast cancer, gastric cancer, pancreas cancer, prostate cancer, oral SCC and esophageal SCC. However, the accumulation of stromal Cav-1 has been found to be promoted by the progression of tongue SCC. Taken together, Cav-1 seems playing a different role in different cancer subtypes even of the same organ, as well as acting differently in the same cancer subtype of different organs. Thus, we hereby explore the functions of Cav-1 in human adenocarcinoma and SCC from the perspective of clinical significances and pathogenesis. We envision that novel targets may come with the further investigation of Cav-1 in carcinogenesis.
Keywords: caveolin-1, squamous cell carcinoma, adenocarcinoma, oncogene, tumor suppressor gene
Introduction
Caveolins, the major structural proteins of caveolae, consist of caveolin-1 (Cav-1), Cav-2 and Cav-3. Interestingly, the expression patterns of Cav-1 and Cav-2 are largely distinct from that of Cav-3. Cav-1 and Cav-2 have similar distribution; they are highly expressed in adipocytes, endothelial cells, pneumocytes, fibroblasts and these terminal differentiation cells, whereas Cav-3 expressed limited to muscle cell types.1 Besides, it has been found that Cav-1 and Cav-2 collectively promote the malignant progress of carcinoma. Recently, the oncogenic role of Cav-1 and Cav-2 has been identified in breast cancer,2 prostate cancer3 and esophageal squamous cell carcinoma (SCC).4 Furthermore, Cav-1 and Cav-2 may be act as novel therapeutic targets in prostate cancer3 and breast cancer2 as well as potential marker in esophageal SCC.4 However, the function of Cav-3 in tumor is under exploration, whereas Cav-1 is involved in multiple cancer-associated processes, including cellular transformation, tumor growth, cell migration and metastasis, cell death and survival, multidrug resistance (MDR) and angiogenesis.5
At present, whether Cav-1 functions as an oncogene or a tumor suppressor in cancer progression is still controversial. Certain studies reported that Cav-1 is downregulated in pancreatic cancer,6 ovarian cancer,7 breast cancer,8 laryngeal SCC,9 lung adenocarcinoma10 and esophageal adenocarcinoma (EAC).11 Consistent with these observations, the human Cav-1 gene locates at chromosome 7q31.1, which has a high incidence of tumor suppressor gene loss in a broad range of tumor types.12 These evidences indicate that Cav-1 may regard as a tumor suppressor. In contrast, the expression of Cav-1 was reported to increase in prostate cancer,13 bladder cancer,14 renal cancer15 and esophageal SCC,4,16 head and neck SCC (HNSCC),17 cervical SCC18 and tongue SCC.19 Interestingly, these upregulations have also been associated with advanced tumor stage, lymph node metastasis and poor prognosis of cancer patients, which may imply that Cav-1 can function as a tumor promoter. In the initial stages of tumor progression, tissue cells can undergo oncogenic transformation through various mechanisms. The decreased expression of Cav-1 can promote the rapid expansion of these abnormal cells. In the later stages, with the larger tumor and malignant progression, cancer cells have to adapt a complex microenvironment. Cav-1 overexpression can suppress apoptosis and acquire MDR. As a consequence, the elevated expression of Cav-1 can enhance the survival ability of cancer cells.20
There is a growing recognition that cancer cells are not independent existence, but surrounded by stromal components in the tumor microenvironment, which are composed of the extracellular matrix (ECM), fibroblasts and myofibroblasts/cancer-associated fibroblasts (CAFs), immune cells, blood and lymphoid vessels.21,22 Recent studies have shown that the expression of stromal Cav-1 has an influence on the progression of carcinoma.23,24 The absence of stromal Cav-1 in CAFs and the loss of Cav-1 may affect the survival of cancer, autophagic tumor stroma model of cancer metabolism. It is well known that cancer cells or hypoxia induces oxidative stress and activate two proautophagic drivers, namely, HIF-1α and nuclear factor kappa-B (NF-κB), in adjacent fibroblasts. Thus, oxidative stress can lead to autophagic/lysosomal degradation of Cav-1. Simultaneously, mitophagy in CAFs would secrete the high-energy nutrients (such as lactate and pyruvate) by aerobic glycolysis that can directly transfer to epithelial cancer cells via a monocarboxylate transporter (MCT). The epithelial cancer cells would apply nutrients to the mitochondrial tricarboxylic acid cycle, thereby promoting ATP generation via oxidative phosphorylation. Moreover, CAFs can upregulate the expression of antiapoptotic protein TP53-induced glycolysis regulatory phosphatase in adjacent epithelial cancer cells, so protecting cancer cells from apoptosis and autophagy.21,25–27 Antioxidants (such as N-acetyl cysteine, metformin or quercetin) or lysosomal inhibitors (eg, chloroquine) can effectively inhibit the degradation of Cav-1, directly implicating autophagy in this process.28 In conclusion, a loss of stromal Cav-1 can promote cancer cell survival and resist to apoptosis. Thus, the absence of stromal Cav-1 is one of the powerful predictor about oxidative stress, autophagic CAFs and reverse Warburg effect.
Downregulation of stromal Cav-1 can promote cancer survival and predict a poor prognosis. Recently, some reporters suggested that the absence of the stromal Cav-1 can cause a “lethal” breast cancer microenvironment and associated with cancer recurrence, metastasis and poor clinical outcome.22,24,29,30 Similar results were obtained from prostate cancer patients; specifically, a loss of stromal Cav-1 is also correlated with reduced relapse-free survival and is functionally relevant to cancer progression.31 Furthermore, the loss of stromal Cav-1 expression in colorectal cancer (CRC) is associated with poor prognosis and could be a prognostic factor for CRC patients.32 The above studies have shown that stromal Cav-1 may play an inhibited role. In contrast, the accumulation of stromal Cav-1 in tongue SCC is significantly associated with poor prognosis.33
Taken together, there is a huge difference about Cav-1 expression in different cancers or different stages of the same cancer. Whether Cav-1 plays a tumor-inhibiting role or a tumor-promoting role may quite depend on the subtypes and stages of cancers. Meanwhile, the stromal Cav-1 may play a complex role in the progression of cancer; therefore, further studies are needed to clarify these contradictory phenomena. In this review, we try to explore the function of Cav-1 in the human adenocarcinomas and SCCs.
The structure of Cav-1
Cav-1 is a 21–24 kDa integral membrane protein, consisting of two isoforms, α-isoform with a slower migration (containing residues 1–178) and β-isoform with a faster migration (containing residues 32–178).34,35 Previous study also demonstrated that both isoforms contain the oligomerization residues 61–101.36 It was reported that Cav-1 has a central hydrophobic domain (residues 102–134), which are considered to form an unusual hairpin loop structure in the membrane, thus leading to both the amino-terminal domain (residues 1–101) and the carboxyl-terminal domain (residues 135–178) of Cav-1 face with the cytoplasm.37
The residues between 80 and 101 have termed the caveolin scaffolding domain (CSD).38 Two caveolin-binding motifs (φχφχχφ and φχχχχφχχ, where φ represents aromatic residues, such as Trp, Phe or Tyr and χ represents non-aromatic amino residues) exist in most caveolae-associated proteins.39 Numerous signal molecules can interact with Cav-1 via the CSD, including Src family tyrosine kinases, Rho-GTPases, growth factor receptors, endothelial nitric oxide synthase (eNOS), G-proteins and G-protein-coupled receptors. However, Cav-1 can negatively or positively regulate these signaling molecules, thus playing a vital role in cancer progression.
The role of Cav-1 in invasion, migration and metastasis
Cav-1 and Rho-GTPases
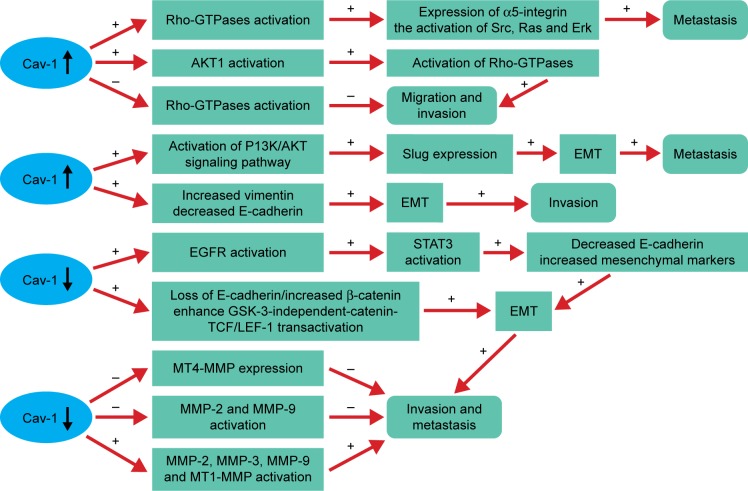
There is increasing evidence that Rho-GTPases are likely to play a role in tumor metastasis and invasion.40,41 Previous studies also have demonstrated the pivotal role of Cav-1 in regulating the activity of Rho-GTPases in various cancers. The cooperation between Cav-1 and Rho-GTPases promotes tumor metastasis, which mainly depend on the elevated expression of α5-integrin and the enhanced activation of Src, Ras and Erk.42 Moreover, an increased expression of Cav-1 can promote the activation of AKT1, leading to the increased phosphorylation of RhoC GTPase (Figure 1). As a consequence, the invasion capacity of inflammatory breast cancer cells is significantly elevated.43 These phenomena are not in accordance with the study reported by Lin et al who indicated that Cav-1 expression inhibits RhoC GTPase activation and subsequently activates the p38 mitogen-activated protein kinase (MAPK) pathway, thus restricting migration and invasion of primary pancreatic cancer cells.44
Figure 1.
Cav-1 plays an important role in tumor migration, invasion and metastasis by regulating the activity of Rho-GTPases, EMT and MMPs.
Notes: Rho-GTPases: the cooperation between Cav-1 and Rho-GTPase can promote metastasis by the elevated expression of α5-integrin and the enhanced activation of Src, Ras and Erk.42 Moreover, Cav-1 overexpression can increase the phosphorylation of RhoC GTPase by stimulating the activation of AKT1,43 whereas the opposite result exists in pancreatic cancer.44 EMT: Cav-1 overexpression promotes bladder cancer metastasis via inducing EMT by activating the PI3K/AKT signaling pathway, which upregulates Slug expression.14 The decreased expression of Cav-1 can also enhance cancer cell invasion and metastasis by the downregulation of E-cadherin, upregulation of β-catenin and enhancing GSK-3-independent-catenin-TCF/LEF-1 transactivation47 or by stimulating EGFR, then promoting the activation of STAT3, resulting in contributing to the EMT.46 MMPs: Cav-1 mediates tumor invasion and metastasis by negatively regulating the activity of MMP-2, MMP-9 and the expression of MT4-MMP54,55 or by positively regulating the activity of MMP-3 and the expression of MMP2, MMP-9 and MT1-MMP.45,50 The “+” represents the promotion and “−” represents the inhibition.
Abbreviations: Cav-1, caveolin-1; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; GSK, glycogen synthase kinase; LEF, lymphoid enhancer factor; MMPs, matrix metalloproteinase; MT, membrane type; PI3K, phosphatidylinositol 3-kinase; STAT3, signal transducer and activator of transcription 3; TCF, T-cell factor.
Cav-1 and epithelial-to-mesenchymal transition (EMT)
Evidences suggested that Cav-1 can mediate the invasion and metastasis of cancer and often accompanied by the EMT. Some studies from different angles have been referred to clarify the effect of Cav-1 in regulating EMT in cancers. Cav-1 can promote bladder cancer metastasis via inducing EMT by activating the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, thus upregulating Slug expression.14 In addition, overexpression of Cav-1 significantly increases vimentin expression, but decreases E-cadherin expression, caused the change of EMT, which may explain the motility and invasion ability in hepatocellular carcinoma (HCC).45 Whereas the reduced levels of Cav-1 function by hypoxia increases epidermal growth factor receptor (EGFR) activation, leading to the activation of signal transducer and activator of transcription 3 (STAT3), resulting in the downregulation of E-cadherin and upregulation of mesenchymal markers (Slug, a-SMA, N-cadherin and vimentin), suggesting that Cav-1 can mediate the EMT and promote the invasive potency in gastric cancer (GC).46 Furthermore, the decreased expression of Cav-1 by EGF leads to the loss of E-cadherin, disruption of cell–cell contacts and enhances glycogen synthase kinase 3 (GSK-3)-independent-catenin-T-cell factor (TCF)/lymphoid enhancer factor 1 (LEF-1) transactivation and increased transcriptional activity of β-catenin, resulting in enhancing cancer cells invasion and metastasis.47
Cav-1 and matrix metalloproteinase (MMP)
MMPs are a family of zinc-containing proteolytic enzymes that degrade various components of ECM.48 Numerous studies indicated that high expression levels of certain MMPs are related to the cancer invasion and metastasis capacity.45,49–52 At present, many researches have investigated the relationship between Cav-1 and MMP, but came out with different results. It has been demonstrated that membrane type 1 (MT1)-MMP colocalizes with caveolae and Cav-1.53 Cav-1 may function as a negative regulator by inhibiting MT4-MMP expression, which is associated with the metastasis in colon cancer.54 Furthermore, Cav-1 overexpression can reduce the metastasis and invasion capacity of metastatic mammary tumor cells by inhibiting the activity of MMP-2 and MMP-9.55 In contrast, the motility and invasion-promoting effect of Cav-1 overexpression in HCC may be partly through increasing secreted MMP-2 and expression levels of MMP-9 and MT1-MMP, as well as inducing an EMT-like phenotype.45 Consistent with the above results, Cav-1 might promote the invasion and metastasis potential via decreasing of E-cadherin protein expression and activate the enzyme activity of MMP3 in human small cell lung cancer NCI-H446 cell.50
The role of Cav-1 in cell cycle
Cav-1 mediates the development of tumor by inversely regulating the cell cycle progression. The control of the cell cycle involved in two major “checkpoints/transitions”, more specifically, the G1→S transition and the G2→M transition. Besides, cyclins/cyclin-dependent kinases (CDKs) and CDK inhibitors are the key regulatory factors of the two transitions.56 Cav-1 may negatively regulate the transcriptional activity of two major components (cyclinD1 and CDC25A) of the cell cycle regulatory apparatus that governs DNA synthesis and cell transformation.57 Moreover, the decreased expression of Cav-1 by small interfering RNA significantly reduces the expression of phospho-AKT, cyclinD1 and CDK4, downstream transducers phosphorylated ERK and STAT3, thus leading to the inhibition of the metastatic lung cancer cells proliferation.58 In addition, Cav-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent pathway.59
The role of Cav-1 in apoptosis
Apoptosis is an active and physiological process of cell death, and the imbalance is one of the important factors for the formation of malignant tumor. Yet, the role of Cav-1 on apoptosis regulation fails to reach a consensus (Figure 2).
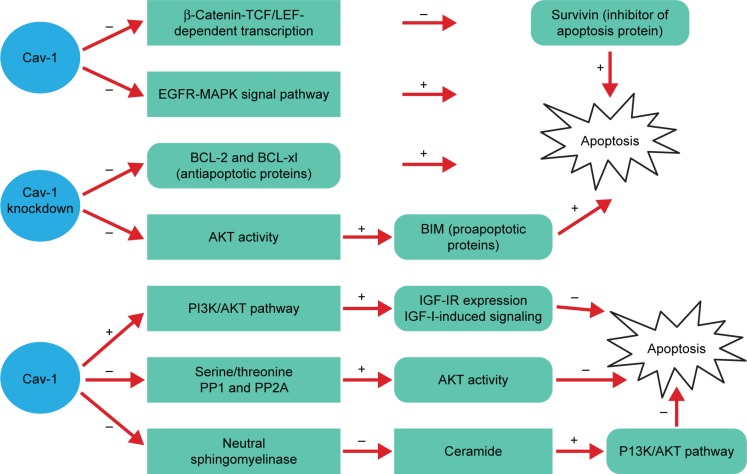
Figure 2.
The role of Cav-1 in apoptosis fails to reach a consensus.
Notes: Increased apoptosis: Cav-1 overexpression can promote cell apoptosis by downregulation of EGFR-MAPK signal pathway6,9 or β-catenin-TCF/LEF-dependent transcription60 and its downstream signal molecules survivin.60 However, Cav-1 knockdown also can inhibit the expression of antiapoptotic proteins BCL-2 and BCL-xl. Simultaneously, Cav-1 can negatively regulate the activity of AKT, leading to the upregulation of BIM.61 Decreased apoptosis: Cav-1 inhibits anoikis by upregulation of IGF-IR expression and IGF-I-induced signaling via the PI3K/AKT pathway.62 Moreover, the elevated expression of Cav-1 can maintain phosphorylated AKT through scaffolding binding site interaction and inhibition of PP1 and PP2A.63 Furthermore, Cav-1 overexpression also significantly reduces staurosporine-induced apoptosis by downregulation of the ceramide via inhibiting the activity of neutral sphingomyelinase, the decreased ceramide inhibits P13K/AKT pathway-induced cell apoptosis.64 The “+” represents the promotion and “−” represents the inhibition.
Abbreviations: BCL, B-cell lymphoma; Cav-1, caveolin-1; EGFR, epidermal growth factor receptor; EMT, epithelial-to-mesenchymal transition; GSK, glycogen synthase kinase; IGF, insulin-like growth factor; IR, insulin receptor; LEF, lymphoid enhancer factor; MAPKs, mitogen-activated protein kinases; PI3K, phosphatidylinositol 3-kinase; PP, protein phosphatase; TCF, T-cell factor.
Many previous studies have demonstrated that Cav-1 is capable of promoting cell apoptosis. The study of HEK293T and ZR75 cells indicated that antiproliferative and proapoptotic properties of Cav-1 may be attributed to reducing survivin (inhibitor of apoptosis protein) expression via a mechanism involving diminished β-catenin-TCF/LEF-dependent transcription.60 What is more, overexpression of Cav-1 exhibits slower growth and promotes cell apoptosis in human cancer by inhibiting the activities of EGFR-MAPK signaling pathway.6,9
However, Cav-1 can inhibit apoptosis through various signal pathways. It has been shown that the absence of Cav-1 significantly reduces the activation of the AKT pathway mediated by TGF-β, thus contributing to the increased expression of proapoptotic proteins BIM. Besides, a significant decrease of antiapoptotic protein B-cell lymphoma (BCL)-2 and BCL-xl expression is observed, suggesting the elevation of the hepatocyte apoptosis.61 Furthermore, the expression of Cav-1 in human breast cancer cells is able to inhibit anoikis and enhances matrix-independent survival by a mechanism, which involves upregulation of insulin-like growth factor (IGF)-insulin receptor (IR) expression and IGF-I-induced PI3K/AKT signaling pathway.62 From another point of view, Cav-1 could maintain phosphorylated AKT through scaffolding binding site interaction and inhibition of serine/threonine protein phosphatases (PP1 and PP2A), and that elevated AKT activities are largely responsible for Cav-1-mediated survival in prostate carcinoma cells.63 Based on the research of the breast cancer cell line Hs578T, the results suggested that Cav-1 overexpression significantly reduces staurosporine-induced apoptosis through inhibition of neutral sphingomyelinase, decrease of ceramide, which can inhibit PI3K/AKT pathway mediating cell apoptosis.64 Taken together, the effect of Cav-1 on cell apoptosis is an extremely complex process.
The different functions of Cav-1 in adenocarcinoma
Cav-1 and thyroid carcinoma
Cav-1 plays a different role in different subunits of thyroid carcinoma. A study has demonstrated that Cav-1 is frequently positive in papillary thyroid carcinoma (PTC), significantly decreases in undifferentiated carcinomas (anaplastic carcinomas), but not the follicular-type carcinomas (FTC). In addition, Cav-1 overexpression plays an important role in the early phase and its decreased expression is associated with the aggressive characteristics including dedifferentiation in PTC, whereas it has little influence on follicular tumors.65 Consistent with the results, Cav-1 expression in the cancer cells is more intense in classical PTC than the other histologic subtypes. In contrast, stromal Cav-1 expression is stronger in the follicular, solid and trabecular PTC variants than in classical PTC. Moreover, clinicopathologic parameters showed that upregulation of Cav-1 in thyroid epithelia correlates with lymph node metastasis, whereas downregulation of Cav-1 in the stromal compartment correlates with the degree of neoplastic infiltration. The study first reported that downregulation of Cav-1 in epithelia and stroma is coincided with BRAF mutation of PTC.66 Inconsistent with the findings, Cav-1 is expressed in the cancer cells with similar frequencies in PTC, diffuse sclerosing variant of papillary carcinoma (DSVPC) and AC, whereas the stromal Cav-1 expression is more frequent in AC compared with PTC and DSVPC.67 In addition to those results, one study showed that galectin-3 can override the tumor suppressor activity of Cav-1, the coordinated expression of Cav-1 and galectin-3; represent a highly precise marker for differentiated thyroid cancer (DTC) diagnosis; and synergistically promote focal adhesion signaling, tumor cell migration, progression and aggressivity of DTC.68 In summary, the different expression of Cav-1 in PTC, AC, FTC and DTC reflects that Cav-1 may play a complicate role in the different histologic subtypes of adenocarcinoma from the same organ.
Cav-1 and EAC
Positive staining of Cav-1 was lost in 95% of EAC specimens (n=100) by immunohistochemistry (IHC) on microar-rays, predominantly concentrating on the mechanism that bile acids could mediate downregulation of Cav-1 expression in EAC cells through the sterol-responsive element-binding protein-1 (SREBP1)/SREBP cleavage-activating protein and nuclear receptors farnesoid X receptor (FXR)/FXR target genes small heterodimer partner-mediated posttranscriptional events. Thus, loss of Cav-1 may activate EGFR signaling and destabilize cell–cell and cell–matrix contacts, contributing to the onset of Barrett esophagus metaplasia and progression to Barrett adenocarcinoma of the esophagus.11 Furthermore, Cav-1 normalized methylation value in EAC is significantly higher than that in corresponding normal esophagus. Whereas OE33 cells are subjected to demethylation by 5-AzadC treatment and could increase Cav-1 mRNA expression, which interpreted that DNA hypermethylation could make Cav-1 gene silencing, and hypermethylation of Cav-1 promoter is a common event in human EAC and occurs early during Barrett’s-associated EAC.69 These two studies suggested that Cav-1 may inhibit the progression of EAC.
Cav-1 and lung adenocarcinoma
It was reported that the positive staining of Cav-1 is downregulated in lung adenocarcinomas and loss of Cav-1 may be a critical step for tumor extension and dedifferentiation.10 Whereas our previous study verified that no correlation is detected between Cav-1 expression and clinicopathologic parameters of lung adenocarcinomas.70 Even upregulation of Cav-1 mRNA expression is found in lung adenocarcinomas (29.7%), which is higher than lung SCC (15.8%) but it is significantly lower when compared with matched tumor-free tissues or noncancerous lung tissue. Furthermore, its overexpression is correlated with the advanced pathologic stage and shorter survival rates in lung adenocarcinoma patients.71 Moreover, Cav-1 overexpression is necessary for mediating filopodia formation in human lung adenocarcinoma cell lines, thus promoting the ability of cell invasion and migration.72 The effect and mechanism of Cav-1 in cancer MDR is still controversial. Cav-1 is upregulated in several MDR cancer cells, including taxol-resistant lung adenocarcinoma A549 cell line,73 bleomycin treatment of A549 cell line74 and taxol-resistant A2780 ovarian carcinoma cell line.75 More importantly, A549 cell lines transduced with the Cav-1 K176R mutant would enhance drug resistance of doxorubicin and cisplatin through the influence of interaction between Cav-1 and P-glycoprotein.76 However, low expression of Cav-1 in A549/Taxol cells by transfecting with a Cav-1 shRNA lentiviral vector, inhibited cell proliferation, cell invasion ability and induced G0/G1 arrest and cell apoptosis.77 Taken together, these results may suggest that the Cav-1 serves as a suppressor in the early stage, while shifting its function in the later stage of lung adenocarcinoma patients.
Cav-1 expression in CAFs is closely related with pathologic T stage, lymphatic permeation, vascular invasion and pleural invasion and predicts a poor outcome of lung adenocarcinoma patients, but it cannot serve as an independent prognostic factor partly due to its strong associations with pathologic vascular and/or pleural invasion. Taken together, Cav-1-positive CAFs play a tumor-promoting role in lung adenocarcinoma.78
Cav-1 and breast cancer
There is considerable controversy about the role of Cav-1 in breast cancer. Some people put forward evidence to prove that Cav-1 serves as a tumor suppressor in breast cancer. The decreased Cav-1 expression level is observed in non-metastatic primary tumors, but much lower level is found in highly metastatic tumor.8 Besides, Cav-1 overexpression can suppress the primary breast cancer growth and brain metastases via STAT3 inhibition.79 In addition, Cav-1 can limit the activation of large conductance Ca2+-activated potassium (BKCa) channel, leading to the suppression of the proliferation and invasiveness in breast cancer cells.80 Cav-1 also can regulate breast cancer cell metabolism, the elevated Cav-1 expression is inversely correlated with NF-E2-related factor 2 (Nrf2) and Mn superoxide dismutase (MnSOD) expression, thus preventing MnSOD-driven glycolysis in breast cancer MCF7 cell line.81 These may be critical for understanding the tumor inhibitory mechanisms of Cav-1.
In contrast, Cav-1 overexpression is associated with tumor malignant progression. The increased expression of Cav-1 can elevate the invasion capacity of inflammatory breast cancer cell by activating AKT1.43 Moreover, the coexpression of Cav-1 and MT1-MMP is observed in human breast cancer cell lines, both are required for invadopodia formation, which regulates the invadopodia-mediated invasion by degrading ECM.82 Furthermore, one study also indicated that Cav-1 can inhibit apoptosis and enhance matrix-independent survival in human breast cancer cells.62
Cav-1 can mediate MDR by positively regulating the activity of BCRP in breast cancer.83 Cav-1 knockdown could significantly reduce the tumorigenicity and chemoresistance of breast cancer stem cells by downregulating the β-catenin/ABCG2 pathway.84 Moreover, breast cancer aggressiveness is associated with Cav-1 CGI shore methylation levels.85 An interesting study revealed that 19% estrogen receptor-positive breast cancers involve Cav-1 P132L mutation.86 Further study demonstrated that Cav-1 (P132L) mutation dramatically accentuates cell migration, invasion and metastasis capacity via activating EGF, HGF and TGF signaling pathways.87 However, another study reported that Cav-1 P132L mutation has not found in breast cancer.88 Therefore, further studies are needed to explore the possible mechanism of the Cav-1 P132L mutation on tumorigenicity of breast cancer.
According to the previous reports, epithelial Cav-1 expression could not be an independent prognostic factor for clinical outcome of breast cancer patients.22,89–91 However, Cav-1 expression with tumor (++)/stromal (−) is closely associated with unfavorable prognosis.92 Whereas an absence of stromal Cav-1 has been regarded as an independent poor prognostic indicator of breast cancer and specifically associated with early disease recurrence, advanced tumor stage, lymph node metastasis, a shorter disease-free survival and an overall survival.22,24,29,30
Cav-1 and GC
Cav-1 is frequently lost or reduced in primary GC tissue by comparison with nonneoplastic mucosa, but it is increased in distant metastases cell lines by comparison with primary tumor cell lines.93 In addition, via the activation of the EGFR, TGF-β, Wnt signaling and their downstream signal pathways, the low expression of Cav-1 plays a vital role in enhancing cell proliferation, cell survival and upregulating EMT, which results in promoting GC progression under hypoxic condition.46 Conversely, one study revealed that positive staining of Cav-1 is shown in 22 (5.4%) of 405 cases in GC, significantly correlated with advanced pTNM stage, lymph node metastasis and poor prognosis, while Cav-1 is not expressed in nonneoplastic gastric mucosa.94 However, another study reported that Cav-1 immunoexpression is found in 46 (94%) of 49 cases from surgically resected GC tissues, but it appear that Cav-1 is neither stage-specific nor related to prognosis.95 Furthermore, a meta-analysis showed that Cav-1 expression is only correlated with Lauren classification and its overexpression could predict a better overall survival. Therefore, Cav-1 is a novel prognostic biomarker of GCs.96
In the stromal of gastric adenocarcinoma, our group previously showed that low stromal Cav-1 levels and high autophagy levels may cooperatively promote GC development, and downregulation of stromal Cav-1 is a novel predictor of poor GC prognosis.97 Consistent with our results, loss of stromal Cav-1 can significantly activate fibroblasts in GC microenvironment, and it may function as a potential biomarker for GC progression.98
The role of Cav-1 in GC is so complicated that the above studies have yet to reach a consensus. There are many possible reasons that account for the differences, such as the small sample size, difference in pathological subtypes, using the different antibodies or the different proportion of tumor histologic stage and histologic grade.
Cav-1 and pancreatic carcinoma
In pancreatic carcinoma, the negative regulation of the cell growth and cell invasion capacity, as well as the promotion of apoptosis may be critical for understanding the mechanism of Cav-1 in tumor suppression through downregulating EGFR–MAPK signaling pathway.6 Some reports showed that Cav-1 plays an important role in regulating the migration and invasion in pancreatic carcinoma. Cav-1 expression can restrict migration and invasion of primary pancreatic carcinoma, via inhibiting RhoC GTPase activation.44 This is in contrast to which RhoC-mediated migration and invasion in inflammatory breast cancer.43 Together with Cav-1, expression in pancreatic carcinoma induces an epithelial phenotype and promotes cell–cell contact, with increased expression of plasma membrane bound E-cadherin and β-catenin, resulting in reducing cell migration and invasion. Consistent with Cav-1 as a negative regulator of some signaling pathway, one report indicated that Cav-1 gene could inhibit pancreatic carcinoma cell invasion, at least in part, probably through downregulating ERK-MMP signaling pathways.99 Then, Cav-1 also attenuates doxorubicin-chemoresistance of pancreatic cancer cells.100 Disagreeing with the above results, a previous study suggested that Cav-1 overexpression is positively associated with tumor progression, indicating a poor prognosis for certain patients undergoing surgical resection for pancreatic carcinoma.101 What is more, Cav-1 can make an independent poor prognostic factor and its elevated level is correlated with histologic stage and tumor aggressiveness.102–104 Furthermore, the elevated Cav-1 expression is resistant to therapies.103 Beyond that, Cav-1 is indispensable for the tumor-promoting effect of Cav-1 and promoting its prognostic potency.104 The above results demonstrated that Cav-1 function is very complicated in the pancreatic carcinoma cells.
Stromal Cav-1 expression in CAFs is lower than that in paracancerous associated and normal fibroblasts, and its absence is closely related to TNM stage, lymph node metastasis, distant metastasis and HER-2/neu amplification, they also indicated that the loss of stromal Cav-1 is an independent prognostic indicator in pancreatic carcinoma.105
Cav-1 and HCC
Comparing with normal liver cell line and nontumorous liver tissues, increased expression of Cav-1 is found in metastatic HCC cell lines and tumor tissues.45,106,107 In addition, Cav-1 overexpression is correlated with invasion and poor prognosis of HCC.49,108 Some reports suggested that Cav-1 contributes to HCC progression and metastasis through the inhibition of autophagy107 or inducing EMT via Wnt/β-catenin pathway.109 Furthermore, Cav-1 overexpression induced by GLI1 also plays a vital role in GLI1-driven EMT.110 In addition, Cav-1 may function as an initiator of the HCC via triggering c-Met signal transduction and the interaction between them will be beneficial to the invasive phenotype.111 Even Cav-1 also can negatively regulate TRAIL-induced apoptosis in hepatoma HepG2 cells.112 These evidences suggest the oncogenic role of Cav-1 in HCC. However, Cav-1 has been reported to function as a tumor suppressor role through its modulation of eNOS in HCC and Cav-1 overexpression means a better overall survival.113
Cav-1 and colon adenocarcinoma
Compared to normal colonic epithelium and adenomas, the expression of Cav-1 is elevated in colon adenocarcinoma.114 In addition, its overexpression is directly associated with the growth rate, contributing to tumorigenesis.115 Moreover, Cav-1 can stimulate the activation of a small GTPase Rab5, thus enhancing the activity of ras-related C3 botulinum toxin substrate 1 (Rac1) and cell migration, invasion in metastatic human colon adenocarcinoma HT-29 (US) cells.116 Whereas Cav-1 may play an inhibitory role during early stages of colon adenocarcinoma, and the decreased expression of Cav-1 is correlated with the loss of wild-type adenomatous polyposis coli via downregulation of Forkhead box protein O1a and upregulation of c-myc transcription factors.117
Cav-1 and renal cancer
Previous studies have reached an agreement about the role of Cav-1 in renal cell carcinoma (RCC). Using IHC, increased Cav-1 expression correlates with tumor progression and predicts a poor prognosis in RCC.118 Furthermore, Cav-1 mRNA expression is remarkably increased in RCC by comparison with normal renal tissue, and the elevated Cav-1 mRNA levels are associated with tumor stage and predicted malignant progression.119 In addition, the coexpression of Cav-1 and activated AKT/mTOR pathway can predict a poor disease-free survival, contributing to disease progression and metastasis in RCC.120 Moreover, Cav-1 in coordination with pERK can predict metastasis risk in RCC.15 Furthermore, Cav-1 may boost the angiogenesis, associating with microvessel density (MVD), and the coexpression of Cav-1 and MVD is significantly correlated with metastasis and a worse prognosis in clear cell RCC. Taken together, Cav-1 plays an important role in the progression of RCC.121
Cav-1 and prostate cancer
It is widely accepted that Cav-1 is elevated in prostate cancer cells and its overexpression is associated with disease progression. High expression of Cav-1 has a significant positive association with higher stage and grade tumor.31 Cav-1 also might help to identify patients at high risk of developing aggressive prostate cancer recurrence.122 Recently, the role of Cav-1 in prostate cancer is explored in some researches from different angles. Cav-1 can cause phosphorylated AKT, and elevated AKT activities are benefit to Cav-1-mediated survival activities.63 Moreover, the positive combination of c-myc and Cav-1 likely reflects the aggressiveness of prostate carcinoma and may offer prognostic value for this malignancy.123 Furthermore, Cav-1 mediates angiogenesis via cooperating with VEGFR2 during prostate cancer progression.124 Besides, the physical interaction between Id-1 and Cav-1 plays a key role in the EMT and increases cell migration rate as well as resistance to taxol-induced apoptosis in prostate cancer cells.125 This phenomenon is in accordance with other researches that have shown a link between Cav-1 expression and EMT in bladder14 and pancreatic cancers.126
Compared to stromal Cav-1 expression in patients with benign prostatic hypertrophy, primary prostate cancers and prostate cancer metastases, the results indicated that the loss of stromal Cav-1 is associated with advanced prostate cancer and metastatic disease, and it could be a powerful prognostic marker for patients with prostate cancer.127 In concordance with their findings, the low expression of stromal Cav-1 is correlated with clinical stage, increased Gleason score and reduced relapse-free survival. What is more, an absence of stromal Cav-1 confers the metastatic capacity of tumor cells by upregulation of TGF-β1 and γ-synuclein via AKT activation.128
The different functions of Cav-1 in SCCs
Cav-1 and cutaneous SCC
Cav-1 can be observed in the cytoplasm of cutaneous SCC, and its expression level is significantly lower than that of normal skin cells.129 Furthermore, Cav-1 overexpression has the ability to suppress cutaneous SCC progression by decreasing cell proliferation, migration and invasion capabilities. Whereas Cav-1 knockdown occurs the opposite results, simultaneously, increases the invasive ability and incidence of spontaneous lymph node metastasis. The possible molecular mechanism is that Cav-1 can negatively regulate the MAPK/activator protein-1 pathway activation.130
Cav-1 and oral SCC (OSCC)
The role of Cav-1 in OSCC still remains complex and controversial. Cav-1 overexpression is observed in the cytoplasm of OSCC by comparison with normal oral mucosa and serves as a prognostic marker for poor prognosis in OSCC.131,132 Moreover, Cav-1 upregulation plays an important role in the tumor progression and correlates with cisplatin sensitivity in OSCC.133 The opposite results are found in other studies, and genetic evidence showed the inactivation of Cav-1 by a mutation or reduced expression may take effect in the pathogenesis of OSCC.134 Whereas an increased Cav-1 expression is seen in the stepwise carcinogenesis from normal oral mucosa (8%), noncancerous-matched tissues (18%), oral precancerous lesions (53%) to primary OSCC (79%), a drastic decrease in expression from primary OSCC to metastatic OSCC (37%), which indicates the value to explore its biphasic functions in oral carcinogenesis.135
In the tumor stroma, a reverse Warburg metabolism in OSCC is not dependent upon myofibroblasts,136 and no negative correlation is detected between the loss of stromal Cav-1 expression and the elevated expression of MCT4, which has been shown in breast cancer.137 Furthermore, the reduced Cav-1 can lead to an increase in oxidative stress in OSCC microenvironment.138
Cav-1 and tongue SCC
Regarding the correlation between the expression of Cav-1 and tongue SCC, little research has been reported. Our previous reports showed that an increased expression of Cav-1 in the carcinogenesis from normal tongue mucosa, hyperplastic tongue mucosa, tongue precancerous lesions to tongue SCC by quantum dots immunofluorescence histochemistry, moreover, Cav-1 expression level is correlated positively with clinical stage and histologic grade in tongue SCC, which suggested that Cav-1 might be an oncogene in the development of tongue SCC.19 Whereas Cav-1 expression in the tumor microenvironment components of human tongue SCC is higher than those in the tumor cells, and it had a negative prognostic value. One of the ways can enable the accumulation of Cav-1 in the tumor microenvironment, which is secreted by exosomes. As such, exosomal Cav-1 can be present in the ECM or it may be ingested by cells that undergo EMT, by CAFs or by other types of cells. The secretion of exosomes is a way to enhance the accumulation of Cav-1 in the tumor microenvironment.33 In agreement with the idea, increased stromal Cav-1 is found in the human renal carcinoma, colon carcinoma and melanoma and it would promote local invasiveness and metastasis via remodeling of ECM, and also linked to poor survival.139 However, these clinical findings appear to conflict with those reports from prostate cancer,127,128 breast cancer,24 pancreatic cancer105 and esophageal SCC23 patients, where the loss of stromal Cav-1 expression is associated with recurrent disease, advanced stage, metastatic spread and poor survival.
Cav-1 and laryngeal SCC
Cav-1 is capable of inhibiting the growth of human laryngeal SCC HEp-2 cell line both in vitro and in vivo, reducing the capacity of anchorage-independent growth, inducing G0/G1 arrest in a cell cycle and increasing the apoptotic cell fraction. The mechanism of its tumor suppressing action may be interpretation via the negative regulation of the EGFR–MAPK signaling pathway.9 Other study demonstrated that Cav-1 overexpression induced by low-intensity ultrasound will be involved in low-intensity ultrasound-induced apoptosis via downregulation of STAT3 in HEp-2 cells.140 In contrast, the univariate analysis indicates that Cav-1 overexpression in membranous and cytoplasmic means a worse disease-specific survival (DSS). However, Cav-1 cannot act as an independent prognostic factor for DSS due to its strong associations with other recognized clinical prognostic factors.141
Cav-1 and HNSCC
Cav-1 overexpression in HNSCC is associated with the simultaneous abnormal expression of at least one member of the E-cadherin/α-β catenin complex and multiple ErbB receptors as well as with lymph node metastases.142 The elevated expression of Cav-1 may promote tumor cell migration and invasion in HNSCC due to the loss of miR-133a.17 Furthermore, increased expression of Cav-1 may play a pivotal role in the metastasis of HNSCC, possibly through the induction of EMT and the formation of cancer stem cells.143 However, Cav-1 may have the potential to suppress carcinogenesis and lung metastasis via disrupting integrin β1/Src-mediated tumor cell growth, invasion and survival.144 Similarly, low levels of Cav-1 can enable HNSCC cells to undergo EMT and enhanced prometastatic properties by inducing the expression of α5β1 integrins.145
Cav-1 and esophageal SCC
In esophageal SCC, overexpression of Cav-1 is associated with lymph node metastasis and poor prognosis for long-term survival.16 In another research, positive Cav-1 immunostaining is correlated with T factor, lymphatic invasion, vein invasion, differentiation and overall survival of esophageal SCC patients.4 In vitro study demonstrated that Cav-1 is upregulated in esophageal SCC cell lines using human cancer cDNA arrays.146 Furthermore, Cav-1 can mediate MDR by positively regulating the expressions of P-glycoprotein and MDR1 in esophageal SCC cell line Ec9706.147 Downregulation of miR-138 promotes lipid raft formation via upregulating multiple components of lipid rafts, including flotillin-1 (FLOT1) and FLOT2, and then Cav-1 facilitates the recruitment of the tumor necrosis factor receptor and inhibitor of NF-κB kinase signalosome into lipid rafts and activates the NF-κB signaling pathway, consequently leading to the progression of aggressiveness and poorer clinical outcomes in human esophageal SCCs.148 Moreover, there is evidence that Cav-1 downregulation induced by β-carotene enhances apoptosis via inhibiting the AKT/NF-κB pathway in esophageal SCC cells.149 Inconsistent with the previous reports, hypermethylation of the Cav-1 promoter in esophageal SCC is significantly higher than that in corresponding normal esophagus, which can lead to gene silencing. These inconsistent results may be due to the different analytic methods used, ethnic groups studied and smaller sample sizes in the previous studies.69 However, downregulation of stromal Cav-1 expression in esophageal SCC may promote malignant progression and heralds worse outcome, which is significantly associated with lymph node metastases, early tumor recurrence and poor prognosis.23
Cav-1 and lung SCC
Many researchers have reported that increased expression of Cav-1 has been observed in lung SCC.10,70,150 Overexpression of Cav-1 may be correlated with tumor extension,10 advanced pathologic stage, pT and poor prognosis in lung SCC.151 Whereas our previous study reported that its overexpression is only correlated with lymph node metastasis in lung SCC, suggesting that Cav-1 plays a critical role in the metastasis of lung SCC.70 Even there is no significant correlation between Cav-1 expression and clinicopathologic parameters of lung SCC.71 Taken together, Cav-1 plays a tumor-promoting role in the progression of lung SCC, whereas the relationship between Cav-1 and clinicopathologic parameters is still controversial and further studies are needed.
Cav-1 and bladder SCC
Hypermethylation of the Cav-1 promoter is found in bladder SCC (25.9%) by comparison with adenocarcinomas (0%), nonneoplastic urothelium (0%) via methylation-specific PCR. However, a strong diffuse immunostaining of Cav-1 protein is detected in all the specimens of bladder SCC, suggesting that aberrant methylation and protein expression of the Cav-1 are related to bladder SCC. Whereas any expression of Cav-1 is not detected in the normal transitional cell epithelium and adenocarcinomas, supporting epigenetic control of Cav-1 gene is not involved in the histogenesis of adenocarcinomas.152,153 These results may be due to the small sample size, 5 normal sample and 10 adenocarcinomas sample are involved in this study.153
Cav-1 and cervical SCC
Cav-1 expression is dramatically reduced in a human cervical SCC SiHa cell. The possible molecular mechanisms is that the human papillomavirus (HPV) oncoprotein E6 can downregulate Cav-1 via the inactivation of p53, and restore Cav-1 expression that can partially revert HPV-mediated cell transformation, which supports an emerging role for Cav-1 as a tumor suppressor in cervical SCC.154 This is not in accordance with our previous study, which demonstrated that the positive rates of Cav-1 protein by quantum dot-based immunofluorescence staining from normal cervical mucosa, CIN, cervical adenocarcinoma and SCC were 0%, 33%, 19% and 55%, respectively. Furthermore, Cav-1 has a positive correlation with the PCNA protein and high-risk of HPV infection; however, there is no significant association between Cav-1 and any other clinicopathologic characteristics in cervical SCC. Furthermore, a study revealed that Cav-1 is critical for cervical SCC invasion and metastasis.155 These results suggested that Cav-1 might be an oncogene in the progress of cervical SCC.18
The absence of stromal Cav-1 is detected in 39 patients (67%) of cervical SCC. However, stromal Cav-1 expression is not correlated with cell differentiation degree and invasive range. At present, the role of Cav-1 in the stromal microenvironment remains unknown.18 Therefore, further studies are needed to discover the functions of stromal Cav-1 in cervical SCC.
Conclusion
Expression of Cav-1 may affect the malignant progression of carcinoma. From Tables 1 and 2, we make a conclusion that Cav-1 plays a different role in different histologic types, such as adenocarcinoma and SCC. Even in the same histologic type, its expression is significantly different. In fact, the expression level of Cav-1 has differences even in the different histologic types of the same organ as shown in Table 3. Cav-1 plays a complex role in different subunits of the same histologic type in the same organ. Stromal Cav-1 may also play a vital role in influencing the progression of carcinoma. Taken together, the multifunction of Cav-1 can regulate cell proliferation, migration, apoptosis, autophagy and cell cycle in cancer by various pathways. Therefore, we need to further explore the mechanism of Cav-1, which may shed light on the discovery of new targets for cancer treatment.
Table 1.
The different functions of Cav-1 in human adenocarcinoma
| Organ | Tumor
|
Stromal
|
||
|---|---|---|---|---|
| Function | Prognosis | Function | Prognosis | |
| Thyroid | The level of Cav-1 depends on the different subunits in thyroid cancer, whereas the results fail to reach an agreement.65–68 | |||
| Esophagus | Cav-1 plays a negative role in the pathogenesis of BAC.11,69 | The elevated expression of Cav-1 in a small subgroup of BAC patients was correlated with poor survival prognosis.11 | ||
| Lung | Cav-1 serves as a tumor inhibitor in lung ADs,10,70,150 whereas the tumor- promoting function also has been observed.72 | The higher expression Cav-1 correlated with poorer survival in lung ADs patients.71 | Cav-1 expression in CAFs is closely related with T stage, lymphatic permeation, vascular invasion and pleural invasion.78 | Cav-1 overexpression in CAFs predicts a poor outcome, but it cannot serve as an independent prognostic factor.78 |
| Breast | The tumor-promoting function of Cav-1 in breast cancer has been found by many reports.43,82,156,157 However, some studies insist that Cav-1 functions as a tumor inhibitor.79,80 | Cav-1 expression has no prognostic value in breast cancer.22,89–91 | Cav-1 acts as a tumor suppressor in the stromal microenvironment of breast cancer.22,24,29,30 | The absence of stromal Cav-1 may serve as a poor independent prognostic maker in breast cancer.22,24,29,30 |
| Liver | The elevated Cav-1 expression contributes to HCC progression and metastasis via inhibiting autophagy,107 inducing EMT109 or triggering c-Met signal transduction.111 | Cav-1 overexpression predicts a poor prognosis in HCC.49,108 | ||
| Stomach | Cav-1 can suppress gastric cancer tumorigenesis.46,93 The opposite results also have been observed.94,95 | Cav-1 is associated with poor prognosis,94 good prognosis96 or no prognosis95 remains controversial. | Loss of stromal Cav-1 can promote GC development.97,98 | Loss of stromal Cav-1 can predict the poor prognosis in GC.97 |
| Pancreas | Cav-1 acts as tumor inhibitor6,99,100 or tumor promotor,101,103,104 even with the both in pancreatic AD still remains controversial.44 | Cav-1 has been regarded as an independent unfavorable prognostic factor in pancreatic ductal AD.101,102 | The stromal Cav-1 serves as a tumor suppressor in pancreatic cancer.105 | Loss of stromal Cav-1 in pancreatic cancer can be a strong poor prognosis biomarker.105 |
| Colon | The oncogenic function of Cav-1 was found in colon AD,114–116 whereas Cav-1 may play a tumor-inhibiting role during the early stages.117 | |||
| Kidney | Cav-1 can promote the progression and metastasis of renal cell carcinoma.118,120,121 | Cav-1 may be capable of predicting a worse prognosis.118,120 | ||
| Prostate | The tumorigenic function of Cav-1 has been found in prostate cancer.63,122,124,125 | The increased expression of Cav-1 is associated with poor prognosis.31,122 | The stromal Cav-1 plays a tumor-inhibiting role in the progression of prostate cancer.127 | Loss of stromal Cav-1 in prostate cancer heralds a worse prognosis.127,128 |
Abbreviations: ADs, adenocarcinomas; CAFs, cancer-associated fibroblasts; Cav-1, caveolin-1; EMT, epithelial-to-mesenchymal transition; GC, gastric cancer; HCC, hepatocellular carcinoma.
Table 2.
The different functions of Cav-1 in human SCC
| Organ | Tumor
|
Stromal
|
||
|---|---|---|---|---|
| Function | Prognosis | Function | Prognosis | |
| Skin | Cav-1 serves as a tumor inhibitor in the progression of cutaneous SCC.129,130 | |||
| Oral | The tumor-promoting function131,132 or tumor-inhibiting function,134 even the biphasic functions135 of Cav-1 in OSCC still remains controversial. | Cav-1 serves as a prognostic marker for poor prognosis in OSCC.131,132 | The stromal Cav-1 serves as a tumor suppressor in OSCC.136,138 | |
| Tongue | Cav-1 may serve as an oncogene in the development of tongue SCC.19 | Whether Cav-1 can predict the prognosis still need the extension of clinical studies.19 | The stromal Cav-1 plays a tumor-promoting role in the progression of tongue SCC.33 | The accumulation of Cav-1 in TME is associated with poor prognosis.33 |
| Throat | Cav-1 plays a tumor-inhibiting role in larynges SCC via the negative regulation of the EGFR–MAPK signaling pathway,9 whereas the increased expression of Cav-1 means a worse DSS.141 | Cav-1 cannot serve as an independent prognostic factor for DSS.141 | ||
| Head and neck | Increased expression Cav-1 promotes tumor cell migration and metastasis, indicating the tumor-promoting function in the progression of HNSCC.17,142,143 However, Cav-1 may have the potential to suppress tumorigenesis.144,145 | |||
| Esophagus | Cav-1 expression is upregulated during ESCC carcinogenesis,4,16,146,148 whereas Cav-1 may function as a tumor inhibitor in ESCC.69 | The overexpression of Cav-1 correlates with poor prognosis in ESCC.4,16 | The decreased expression of Cav-1 may promote malignant progression and heralds worse outcome.23 | The absence of stromal Cav-1 is closely related to the poor prognosis in ESCC.23 |
| Lung | The tumor-promoting function has been observed in lung SCC.10,70,71 | Cav-1 can predict a poor prognosis in lung SCC.70,151 | ||
| Bladder | Cav-1 can drive tumorigenesis in bladder SCC.152,153 | |||
| Uterine | Cav-1 may function as an oncogene in the development of cervical SCC,18 and Cav-1 plays a vital role in cervical SCC invasion and metastasis.155 However, the tumor-inhibiting role of Cav-1 also has been found.154 | Further studies are needed to determine the role of stromal Cav-1, which acts as a suppressor or promoter in cervical SCC.18 | ||
Abbreviations: BAC, Barrett esophageal adenocarcinoma; Cav-1, caveolin-1; DSS, disease-specific survival; ESCC, esophageal squamous cell carcinoma; EGFR-MAPK, epidermal growth factor receptor-mitogen-activated protein kinase; HNSCC, head and neck squamous cell carcinoma; OSCC, oral squamous cell carcinoma; SCC, squamous cell carcinoma; TME, tumor microenvironment.
Table 3.
The role of Cav-1 in different histological types of the same organ
| Organ | Function |
|---|---|
| Esophagus | Cav-1 overexpression is associated with lymph node metastasis, pathologic stage and poor prognosis in ESCC, which have reported by many studies.4,16,23 However, Jin et al showed that both Cav-1 methylation frequency and NMV were significantly higher in EAC and ESCC than in corresponding normal esophagus.69 The loss of Cav-1 may contribute to the progression to Barrett adenocarcinoma of the esophagus.11 Taken together, Cav-1 plays a tumor-inhibiting role in EAC, whereas Cav-1 plays a tumor-promoting role of in ESCC |
| Lung | The significant difference of Cav-1 expression is found between lung SCC and lung ADs. More specifically, the expression of Cav-1 is usually low or lost in lung ADs,10,70,150 even its overexpression is correlated with the advanced pathologic stage and shorter survival rates in lung AD patients.71 Taken together, Cav-1 may serve as a suppressor in the early stage, while it shift its function in the later stage of lung adenocarcinoma patients. However, Cav-1 overexpression was observed in lung SCC.10,70,150 Moreover, the relationship between Cav-1 and clinicopathologic parameters is still controversial in adenocarcinoma and SCC.10,70,71,151 Therefore, the function of Cav-1 in lung cancer is histotype dependent. |
| Bladder | Hypermethylation of the Cav-1 promoter was found in bladder SCC by comparison with ADs, non- neoplastic urothelium. However, IHC demonstrated that all the specimens exhibited a strong diffuse immunostaining in bladder SCC, suggesting that the aberrant methylation of Cav-1 promoter and abnormal protein expression of the Cav-1 are related to bladder SCC, whereas no expression of Cav-1 can be detected in the normal transitional cell epithelium and adenocarcinomas, supporting epigenetic control of Cav-1 gene is not involved in the histogenesis of adenocarcinomas.152,153 |
Abbreviations: ADs, adenocarcinomas; Cav-1, caveolin-1; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; NMV, normalized methylation value; SCC, squamous cell carcinoma.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of Hubei Province (No ZRY2015001716) and Public Welfare Technology Application Research of Zhejiang Province (No 2016C33236).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen D, Che G. Value of caveolin-1 in cancer progression and prognosis: emphasis on cancer-associated fibroblasts, human cancer cells and mechanism of caveolin-1 expression (Review) Oncol Lett. 2014;8(4):1409–1421. doi: 10.3892/ol.2014.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Eynden GG, Van Laere SJ, Van der Auwera I, et al. Overexpres-sion of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancer. Breast Cancer Res Treat. 2006;95(3):219–228. doi: 10.1007/s10549-005-9002-1. [DOI] [PubMed] [Google Scholar]
- 3.Sugie S, Mukai S, Yamasaki K, Kamibeppu T, Tsukino H, Kamoto T. Significant association of caveolin-1 and caveolin-2 with prostate cancer progression. Cancer Genomics Proteomics. 2015;12(6):391–396. [PubMed] [Google Scholar]
- 4.Ando T, Ishiguro H, Kimura M, et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep. 2007;18(3):601–609. [PubMed] [Google Scholar]
- 5.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27(4):715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 6.Han F, Gu D, Chen Q, Zhu H. Caveolin-1 acts as a tumor suppressor by down-regulating epidermal growth factor receptor-mitogen-activated protein kinase signaling pathway in pancreatic carcinoma cell lines. Pancreas. 2009;38(7):766–774. doi: 10.1097/MPA.0b013e3181b2bd11. [DOI] [PubMed] [Google Scholar]
- 7.Wiechen K, Diatchenko L, Agoulnik A, et al. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001;159(5):1635–1643. doi: 10.1016/S0002-9440(10)63010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sloan EK, Stanley KL, Anderson RL. Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene. 2004;23(47):7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- 9.Gu D, Li H, Wang Z, Chen Q, Jiang J, Zhu H. Caveolin-1 inhibits the growth of human laryngeal squamous cell carcinoma and down regulates EGFR-MAPKs signaling pathway. Laryngoscope. 2007;117(10):1782–1789. doi: 10.1097/MLG.0b013e31811edd31. [DOI] [PubMed] [Google Scholar]
- 10.Kato T, Miyamoto M, Kato K, et al. Difference of caveolin-1 expression pattern in human lung neoplastic tissue. Atypical adenomatous hyper-plasia, adenocarcinoma and squamous cell carcinoma. Cancer Lett. 2004;214(1):121–128. doi: 10.1016/j.canlet.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Prade E, Tobiasch M, Hitkova I, et al. Bile acids down-regulate caveolin-1 in esophageal epithelial cells through sterol responsive element-binding protein. Mol Endocrinol. 2012;26(5):819–832. doi: 10.1210/me.2011-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurlstone AF, Reid G, Reeves JR, et al. Analysis of the CAVEOLIN-1 gene at human chromosome 7q31.1 in primary tumours and tumour-derived cell lines. Oncogene. 1999;18(10):1881–1890. doi: 10.1038/sj.onc.1202491. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Goltsov AA, Ren C, et al. Caveolin-1 upregulation contributes to c-Myc-induced high-grade prostatic intraepithelial neoplasia and prostate cancer. Mol Cancer Res. 2012;10(2):218–229. doi: 10.1158/1541-7786.MCR-11-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang W, Hao Z, Han JL, Zhu DJ, Jin ZF, Xie WL. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol Oncol. 2014;32(6):855–863. doi: 10.1016/j.urolonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Campbell L, Al-Jayyoussi G, Gutteridge R, et al. Caveolin-1 in renal cell carcinoma promotes tumour cell invasion, and in co-operation with pERK predicts metastases in patients with clinically confined disease. J Transl Med. 2013;11:255. doi: 10.1186/1479-5876-11-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato K, Hida Y, Miyamoto M, et al. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94(4):929–933. [PubMed] [Google Scholar]
- 17.Nohata N, Hanazawa T, Kikkawa N, et al. Caveolin-1 mediates tumor cell migration and invasion and its regulation by miR-133a in head and neck squamous cell carcinoma. Int J Oncol. 2011;38(1):209–217. [PubMed] [Google Scholar]
- 18.Sun J, Gao J, Hu JB, et al. Expression of Cav-1 in tumour cells, rather than in stromal tissue, may promote cervical squamous cell carcinoma proliferation, and correlates with high-risk HPV infection. Oncol Rep. 2012;27(6):1733–1740. doi: 10.3892/or.2012.1703. [DOI] [PubMed] [Google Scholar]
- 19.Xue J, Chen H, Diao L, Chen X, Xia D. Expression of caveolin-1 in tongue squamous cell carcinoma by quantum dots. Eur J Histochem. 2010;54(2):e20. doi: 10.4081/ejh.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3(8):571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, He Y, Chen H. Autophagic tumor stroma: mechanisms and roles in tumor growth and progression. Int J Cancer. 2013;132(1):1–8. doi: 10.1002/ijc.27664. [DOI] [PubMed] [Google Scholar]
- 22.Witkiewicz AK, Dasgupta A, Sotgia F, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174(6):2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia Y, Wang N, Wang J, et al. Down-regulation of stromal caveolin-1 expression in esophageal squamous cell carcinoma: a potent predictor of lymph node metastases, early tumor recurrence, and poor prognosis. Ann Surg Oncol. 2014;21(1):329–336. doi: 10.1245/s10434-013-3225-x. [DOI] [PubMed] [Google Scholar]
- 24.Koo JS, Park S, Kim SI, Lee S, Park BW. The impact of caveolin protein expression in tumor stroma on prognosis of breast cancer. Tumour Biol. 2011;32(4):787–799. doi: 10.1007/s13277-011-0181-6. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Outschoorn UE, Trimmer C, Lin Z, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microen-vironment. Cell Cycle. 2010;9(17):3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavlides S, Whitaker-Menezes D, Castello-Cros R, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 27.Pavlides S, Tsirigos A, Migneco G, et al. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9(17):3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Outschoorn UE, Pavlides S, Howell A, et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43(7):1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witkiewicz AK, Kline J, Queenan M, et al. Molecular profiling of a lethal tumor microenvironment, as defined by stromal caveolin-1 status in breast cancers. Cell Cycle. 2011;10(11):1794–1809. doi: 10.4161/cc.10.11.15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Gendi SM, Mostafa MF, El-Gendi AM. Stromal caveolin-1 expression in breast carcinoma. Correlation with early tumor recurrence and clinical outcome. Pathol Oncol Res. 2012;18(2):459–469. doi: 10.1007/s12253-011-9469-5. [DOI] [PubMed] [Google Scholar]
- 31.Gumulec J, Sochor J, Hlavna M, et al. Caveolin-1 as a potential high-risk prostate cancer biomarker. Oncol Rep. 2012;27(3):831–841. doi: 10.3892/or.2011.1587. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Z, Han FH, Yang SB, Hua LX, Wu JH, Zhan WH. Loss of stromal caveolin-1 expression in colorectal cancer predicts poor survival. World J Gastroenterol. 2015;21(4):1140–1147. doi: 10.3748/wjg.v21.i4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vered M, Lehtonen M, Hotakainen L, et al. Caveolin-1 accumulation in the tongue cancer tumor microenvironment is significantly associated with poor prognosis: an in-vivo and in-vitro study. BMC Cancer. 2015;15:25. doi: 10.1186/s12885-015-1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherer PE, Tang Z, Chun M, Sargiacomo M, Lodish HF, Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and sub-cellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270(27):16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 35.Podar K, Tai YT, Cole CE, et al. Essential role of caveolae in interleukin- 6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278(8):5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 36.Sargiacomo M, Scherer PE, Tang Z, et al. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci U S A. 1995;92(20):9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273(10):5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271(46):29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272(10):6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 40.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 41.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28(1–2):65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 42.Arpaia E, Blaser H, Quintela-Fandino M, et al. The interaction between caveolin-1 and Rho-GTPases promotes metastasis by controlling the expression of alpha5-integrin and the activation of Src, Ras and Erk. Oncogene. 2012;31(7):884–896. doi: 10.1038/onc.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joglekar M, Elbazanti WO, Weitzman MD, Lehman HL, van Golen KL. Caveolin-1 mediates inflammatory breast cancer cell invasion via the Akt1 pathway and RhoC GTPase. J Cell Biochem. 2015;116(6):923–933. doi: 10.1002/jcb.25025. [DOI] [PubMed] [Google Scholar]
- 44.Lin M, DiVito MM, Merajver SD, Boyanapalli M, van Golen KL. Regulation of pancreatic cancer cell migration and invasion by RhoC GTPase and caveolin-1. Mol Cancer. 2005;4(1):21. doi: 10.1186/1476-4598-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cokakli M, Erdal E, Nart D, et al. Differential expression of Caveolin-1 in hepatocellular carcinoma: correlation with differentiation state, motility and invasion. BMC Cancer. 2009;9:65. doi: 10.1186/1471-2407-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kannan A, Krishnan A, Ali M, Subramaniam S, Halagowder D, Sivasithamparam ND. Caveolin-1 promotes gastric cancer progression by up-regulating epithelial to mesenchymal transition by crosstalk of signalling mechanisms under hypoxic condition. Eur J Cancer. 2014;50(1):204–215. doi: 10.1016/j.ejca.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Lu Z, Ghosh S, Wang Z, Hunter T. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4(6):499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 48.Annabi B, Lachambre M, Bousquet-Gagnon N, Page M, Gingras D, Beliveau R. Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem J. 2001;353(Pt 3):547–553. doi: 10.1042/0264-6021:3530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Y, Zeng X, He F, Liao Y, Qian N, Toi M. Caveolin-1 is related to invasion, survival, and poor prognosis in hepatocellular cancer. Med Oncol. 2012;29(2):977–984. doi: 10.1007/s12032-011-9900-5. [DOI] [PubMed] [Google Scholar]
- 50.Yeh D, Chen C, Sun MZ, et al. Caveolin-1 is an important factor for the metastasis and proliferation of human small cell lung cancer NCI-H446 cell. Anat Rec (Hoboken) 2009;292(10):1584–1592. doi: 10.1002/ar.20974. [DOI] [PubMed] [Google Scholar]
- 51.Weng Y, Cai M, Zhu J, et al. Matrix metalloproteinase activity in early-stage lung cancer. Onkologie. 2013;36(5):256–259. doi: 10.1159/000350304. [DOI] [PubMed] [Google Scholar]
- 52.Makinen LK, Hayry V, Hagstrom J, et al. Matrix metalloproteinase-7 and matrix metalloproteinase-25 in oral tongue squamous cell carcinoma. Head Neck. 2014;36(12):1783–1788. doi: 10.1002/hed.23539. [DOI] [PubMed] [Google Scholar]
- 53.Puyraimond A, Fridman R, Lemesle M, Arbeille B, Menashi S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp Cell Res. 2001;262(1):28–36. doi: 10.1006/excr.2000.5069. [DOI] [PubMed] [Google Scholar]
- 54.Nimri L, Barak H, Graeve L, Schwartz B. Restoration of caveolin-1 expression suppresses growth, membrane-type-4 metalloproteinase expression and metastasis-associated activities in colon cancer cells. Mol Carcinog. 2013;52(11):859–870. doi: 10.1002/mc.21927. [DOI] [PubMed] [Google Scholar]
- 55.Williams TM, Medina F, Badano I, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279(49):51630–51646. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 56.Qin L, Yang YB, Tuo QH, et al. Effects and underlying mechanisms of curcumin on the proliferation of vascular smooth muscle cells induced by Chol:MbetaCD. Biochem Biophys Res Commun. 2009;379(2):277–282. doi: 10.1016/j.bbrc.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulit J, Bash T, Fu M, et al. The cyclin D1 gene is transcriptionally repressed by caveolin-1. J Biol Chem. 2000;275(28):21203–21209. doi: 10.1074/jbc.M000321200. [DOI] [PubMed] [Google Scholar]
- 58.Pancotti F, Roncuzzi L, Maggiolini M, Gasperi-Campani A. Caveolin-1 silencing arrests the proliferation of metastatic lung cancer cells through the inhibition of STAT3 signaling. Cell Signal. 2012;24(7):1390–1397. doi: 10.1016/j.cellsig.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Galbiati F, Volonte D, Liu J, et al. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell. 2001;12(8):2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torres VA, Tapia JC, Rodriguez DA, et al. Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J Cell Sci. 2006;119(Pt 9):1812–1823. doi: 10.1242/jcs.02894. [DOI] [PubMed] [Google Scholar]
- 61.Meyer C, Liu Y, Kaul A, Peipe I, Dooley S. Caveolin-1 abrogates TGF-beta mediated hepatocyte apoptosis. Cell Death Dis. 2013;4:e466. doi: 10.1038/cddis.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits anoikis and promotes survival signaling in cancer cells. Adv Enzyme Regul. 2006;46:163–175. doi: 10.1016/j.advenzreg.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23(24):9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu P, Qi B, Zhu H, Zheng Y, Li F, Chen J. Suppression of staurosporine-mediated apoptosis in Hs578T breast cells through inhibition of neutral-sphingomyelinase by caveolin-1. Cancer Lett. 2007;256(1):64–72. doi: 10.1016/j.canlet.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Ito Y, Yoshida H, Nakano K, et al. Caveolin-1 overexpression is an early event in the progression of papillary carcinoma of the thyroid. Br J Cancer. 2002;86(6):912–916. doi: 10.1038/sj.bjc.6600172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paskas S, Jankovic J, Marecko I, et al. Caveolin-1 expression in papillary thyroid carcinoma: correlation with clinicopathological parameters and BRAF mutation status. Otolaryngol Head Neck Surg. 2014;150(2):201–209. doi: 10.1177/0194599813512781. [DOI] [PubMed] [Google Scholar]
- 67.Kim D, Kim H, Koo JS. Expression of caveolin-1, caveolin-2 and caveolin-3 in thyroid cancer and stroma. Pathobiology. 2012;79(1):1–10. doi: 10.1159/000329472. [DOI] [PubMed] [Google Scholar]
- 68.Shankar J, Wiseman SM, Meng F, et al. Coordinated expression of galectin-3 and caveolin-1 in thyroid cancer. J Pathol. 2012;228(1):56–66. doi: 10.1002/path.4041. [DOI] [PubMed] [Google Scholar]
- 69.Jin Z, Wang L, Cao Z, et al. Temporal evolution in caveolin 1 methy-lation levels during human esophageal carcinogenesis. BMC Cancer. 2014;14:345. doi: 10.1186/1471-2407-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen HL, Fan LF, Gao J, Ouyang JP, Zhang YX. Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol Rep. 2011;25(2):359–366. doi: 10.3892/or.2010.1095. [DOI] [PubMed] [Google Scholar]
- 71.Zhan P, Shen XK, Qian Q, et al. Expression of caveolin-1 is correlated with disease stage and survival in lung adenocarcinomas. Oncol Rep. 2012;27(4):1072–1078. doi: 10.3892/or.2011.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;161(5):1647–1656. doi: 10.1016/S0002-9440(10)64442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang CP, Galbiati F, Volonte D, Horwitz SB, Lisanti MP. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998;439(3):368–372. doi: 10.1016/s0014-5793(98)01354-4. [DOI] [PubMed] [Google Scholar]
- 74.Linge A, Meleady P, Henry M, Clynes M, Kasper M, Barth K. Bleomycin treatment of A549 human lung cancer cells results in association of MGr1-Ag and caveolin-1 in lipid rafts. Int J Biochem Cell Biol. 2011;43(1):98–105. doi: 10.1016/j.biocel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Wang NN, Zhao LJ, Wu LN, et al. Mechanistic analysis of taxol-induced multidrug resistance in an ovarian cancer cell line. Asian Pac J Cancer Prev. 2013;14(9):4983–4988. doi: 10.7314/apjcp.2013.14.9.4983. [DOI] [PubMed] [Google Scholar]
- 76.Lee CY, Lai TY, Tsai MK, et al. The influence of a caveolin-1 mutant on the function of P-glycoprotein. Sci Rep. 2016;6:20486. doi: 10.1038/srep20486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han F, Zhang L, Zhou Y, Yi X. Caveolin-1 regulates cell apoptosis and invasion ability in paclitaxel-induced multidrug-resistant A549 lung cancer cells. Int J Clin Exp Pathol. 2015;8(8):8937–8947. [PMC free article] [PubMed] [Google Scholar]
- 78.Shimizu K, Kirita K, Aokage K, et al. Clinicopathological significance of caveolin-1 expression by cancer-associated fibroblasts in lung adenocarcinoma. J Cancer Res Clin Oncol. 2016 Oct 22; doi: 10.1007/s00432-016-2285-2. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiu WT, Lee HT, Huang FJ, et al. Caveolin-1 upregulation mediates suppression of primary breast tumor growth and brain metastases by stat3 inhibition. Cancer Res. 2011;71(14):4932–4943. doi: 10.1158/0008-5472.CAN-10-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Du C, Chen L, Zhang H, et al. Caveolin-1 limits the contribution of BKCa channel to MCF-7 breast cancer cell proliferation and invasion. Int J Mol Sci. 2014;15(11):20706–20722. doi: 10.3390/ijms151120706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hart PC, Ratti BA, Mao M, et al. Caveolin-1 regulates cancer cell metabolism via scavenging Nrf2 and suppressing MnSOD-driven glycolysis. Oncotarget. 2016;7(1):308–322. doi: 10.18632/oncotarget.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 2009;69(22):8594–8602. doi: 10.1158/0008-5472.CAN-09-2305. [DOI] [PubMed] [Google Scholar]
- 83.Herzog M, Storch CH, Gut P, et al. Knockdown of caveolin-1 decreases activity of breast cancer resistance protein (BCRP/ABCG2) and increases chemotherapeutic sensitivity. Naunyn Schmiedeberg’s Arch Pharmacol. 2011;383(1):1–11. doi: 10.1007/s00210-010-0568-8. [DOI] [PubMed] [Google Scholar]
- 84.Wang Z, Wang N, Li W, et al. Caveolin-1 mediates chemoresistance in breast cancer stem cells via beta-catenin/ABCG2 signaling pathway. Carcinogenesis. 2014;35(10):2346–2356. doi: 10.1093/carcin/bgu155. [DOI] [PubMed] [Google Scholar]
- 85.Rao X, Evans J, Chae H, et al. CpG island shore methylation regulates caveolin-1 expression in breast cancer. Oncogene. 2013;32(38):4519–4528. doi: 10.1038/onc.2012.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li T, Sotgia F, Vuolo MA, et al. Caveolin-1 mutations in human breast cancer: functional association with estrogen receptor alpha-positive status. Am J Pathol. 2006;168(6):1998–2013. doi: 10.2353/ajpath.2006.051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonuccelli G, Casimiro MC, Sotgia F, et al. Caveolin-1 (P132L), a common breast cancer mutation, confers mammary cell invasiveness and defines a novel stem cell/metastasis-associated gene signature. Am J Pathol. 2009;174(5):1650–1662. doi: 10.2353/ajpath.2009.080648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koike S, Kodera Y, Nakao A, Iwata H, Yatabe Y. Absence of the caveolin-1 P132L mutation in cancers of the breast and other organs. J Mol Diagn. 2010;12(5):712–717. doi: 10.2353/jmoldx.2010.090180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liedtke C, Kersting C, Burger H, Kiesel L, Wulfing P. Caveolin-1 expression in benign and malignant lesions of the breast. World J Surg Oncol. 2007;5:110. doi: 10.1186/1477-7819-5-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elsheikh SE, Green AR, Rakha EA, et al. Caveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99(2):327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma X, Liu L, Nie W, et al. Prognostic role of caveolin in breast cancer: a meta-analysis. Breast. 2013;22(4):462–469. doi: 10.1016/j.breast.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 92.Qian N, Ueno T, Kawaguchi-Sakita N, et al. Prognostic significance of tumor/stromal caveolin-1 expression in breast cancer patients. Cancer Sci. 2011;102(8):1590–1596. doi: 10.1111/j.1349-7006.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 93.Burgermeister E, Xing X, Rocken C, et al. Differential expression and function of caveolin-1 in human gastric cancer progression. Cancer Res. 2007;67(18):8519–8526. doi: 10.1158/0008-5472.CAN-07-1125. [DOI] [PubMed] [Google Scholar]
- 94.Nam KH, Lee BL, Park JH, et al. Caveolin 1 expression correlates with poor prognosis and focal adhesion kinase expression in gastric cancer. Pathobiology. 2013;80(2):87–94. doi: 10.1159/000341685. [DOI] [PubMed] [Google Scholar]
- 95.Barresi V, Giuffre G, Vitarelli E, Todaro P, Tuccari G. Caveolin-1 immuno-expression in human gastric cancer: histopathogenetic hypotheses. Virchows Arch. 2008;453(6):571–578. doi: 10.1007/s00428-008-0681-y. [DOI] [PubMed] [Google Scholar]
- 96.Ye Y, Miao SH, Lu RZ, Zhou JW. Prognostic value of caveolin-1 expression in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(19):8367–8370. doi: 10.7314/apjcp.2014.15.19.8367. [DOI] [PubMed] [Google Scholar]
- 97.He Y, Zhao X, Gao J, et al. Quantum dots-based immunofluorescent imaging of stromal fibroblasts caveolin-1 and light chain 3B expression and identification of their clinical significance in human gastric cancer. Int J Mol Sci. 2012;13(11):13764–13780. doi: 10.3390/ijms131113764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen XJ, Zhang H, Tang GS, et al. Caveolin-1 is a modulator of fibroblast activation and a potential biomarker for gastric cancer. Int J Biol Sci. 2015;11(4):370–379. doi: 10.7150/ijbs.10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han F, Zhu HG. Caveolin-1 regulating the invasion and expression of matrix metalloproteinase (MMPs) in pancreatic carcinoma cells. J Surg Res. 2010;159(1):443–450. doi: 10.1016/j.jss.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 100.Salem AF, Bonuccelli G, Bevilacqua G, et al. Caveolin-1 promotes pancreatic cancer cell differentiation and restores membranous E-cadherin via suppression of the epithelial-mesenchymal transition. Cell Cycle. 2011;10(21):3692–3700. doi: 10.4161/cc.10.21.17895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuoki M, Miyamoto M, Kato K, et al. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87(10):1140–1144. doi: 10.1038/sj.bjc.6600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tanase CP. Caveolin-1: a marker for pancreatic cancer diagnosis. Expert Rev Mol Diagn. 2008;8(4):395–404. doi: 10.1586/14737159.8.4.395. [DOI] [PubMed] [Google Scholar]
- 103.Chatterjee M, Ben-Josef E, Thomas DG, et al. Caveolin-1 is associated with tumor progression and confers a multi-modality resistance phenotype in pancreatic cancer. Sci Rep. 2015;5:10867. doi: 10.1038/srep10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L, Xu HX, Wang WQ, et al. Cavin-1 is essential for the tumorpromoting effect of caveolin-1 and enhances its prognostic potency in pancreatic cancer. Oncogene. 2014;33(21):2728–2736. doi: 10.1038/onc.2013.223. [DOI] [PubMed] [Google Scholar]
- 105.Shan T, Lu H, Ji H, et al. Loss of stromal caveolin-1 expression: a novel tumor microenvironment biomarker that can predict poor clinical outcomes for pancreatic cancer. PLoS One. 2014;9(6):e97239. doi: 10.1371/journal.pone.0097239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tse EY, Ko FC, Tung EK, et al. Caveolin-1 overexpression is associated with hepatocellular carcinoma tumourigenesis and metastasis. J Pathol. 2012;226(4):645–653. doi: 10.1002/path.3957. [DOI] [PubMed] [Google Scholar]
- 107.Liu WR, Jin L, Tian MX, et al. Caveolin-1 promotes tumor growth and metastasis via autophagy inhibition in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2016;40(2):169–178. doi: 10.1016/j.clinre.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 108.Zhang ZB, Cai L, Zheng SG, Xiong Y, Dong JH. Overexpression of caveolin-1 in hepatocellular carcinoma with metastasis and worse prognosis: correlation with vascular endothelial growth factor, microvessel density and unpaired artery. Pathol Oncol Res. 2009;15(3):495–502. doi: 10.1007/s12253-008-9144-7. [DOI] [PubMed] [Google Scholar]
- 109.Yu H, Shen H, Zhang Y, et al. CAV1 promotes HCC cell progression and metastasis through Wnt/beta-catenin pathway. PLoS One. 2014;9(9):e106451. doi: 10.1371/journal.pone.0106451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gai X, Lu Z, Tu K, Liang Z, Zheng X. Caveolin-1 is up-regulated by GLI1 and contributes to GLI1-driven EMT in hepatocellular carcinoma. PLoS One. 2014;9(1):e84551. doi: 10.1371/journal.pone.0084551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korhan P, Erdal E, Kandemis E, et al. Reciprocal activating crosstalk between c-Met and caveolin 1 promotes invasive phenotype in hepa-tocellular carcinoma. PLoS One. 2014;9(8):e105278. doi: 10.1371/journal.pone.0105278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao X, Liu Y, Ma Q, et al. Caveolin-1 negatively regulates TRAIL-induced apoptosis in human hepatocarcinoma cells. Biochem Biophys Res Commun. 2009;378(1):21–26. doi: 10.1016/j.bbrc.2008.10.123. [DOI] [PubMed] [Google Scholar]
- 113.Yang SF, Yang JY, Huang CH, et al. Increased caveolin-1 expression associated with prolonged overall survival rate in hepatocellular carcinoma. Pathology. 2010;42(5):438–445. doi: 10.3109/00313025.2010.494293. [DOI] [PubMed] [Google Scholar]
- 114.Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001;115(5):719–724. doi: 10.1309/YL54-CCU7-4V0P-FDUT. [DOI] [PubMed] [Google Scholar]
- 115.Patlolla JM, Swamy MV, Raju J, Rao CV. Overexpression of caveolin-1 in experimental colon adenocarcinomas and human colon cancer cell lines. Oncol Rep. 2004;11(5):957–963. [PubMed] [Google Scholar]
- 116.Diaz J, Mendoza P, Ortiz R, et al. Rab5 is required in metastatic cancer cells for caveolin-1-enhanced Rac1 activation, migration and invasion. J Cell Sci. 2014;127(Pt 11):2401–2406. doi: 10.1242/jcs.141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roy UK, Henkhaus RS, Ignatenko NA, Mora J, Fultz KE, Gerner EW. Wild-type APC regulates caveolin-1 expression in human colon adenocarcinoma cell lines via FOXO1a and C-myc. Mol Carcinog. 2008;47(12):947–955. doi: 10.1002/mc.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campbell L, Gumbleton M, Griffiths DF. Caveolin-1 overexpression predicts poor disease-free survival of patients with clinically confined renal cell carcinoma. Br J Cancer. 2003;89(10):1909–1913. doi: 10.1038/sj.bjc.6601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waalkes S, Eggers H, Blasig H, et al. Caveolin 1 mRNA is overexpressed in malignant renal tissue and might serve as a novel diagnostic marker for renal cancer. Biomark Med. 2011;5(2):219–225. doi: 10.2217/bmm.11.12. [DOI] [PubMed] [Google Scholar]
- 120.Campbell L, Jasani B, Edwards K, Gumbleton M, Griffiths DF. Combined expression of caveolin-1 and an activated AKT/mTOR pathway predicts reduced disease-free survival in clinically confined renal cell carcinoma. Br J Cancer. 2008;98(5):931–940. doi: 10.1038/sj.bjc.6604243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ. Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int. 2004;93(3):291–296. doi: 10.1111/j.1464-410x.2004.04604.x. [DOI] [PubMed] [Google Scholar]
- 122.Karam JA, Lotan Y, Roehrborn CG, Ashfaq R, Karakiewicz PI, Shariat SF. Caveolin-1 overexpression is associated with aggressive prostate cancer recurrence. Prostate. 2007;67(6):614–622. doi: 10.1002/pros.20557. [DOI] [PubMed] [Google Scholar]
- 123.Yang G, Timme TL, Frolov A, Wheeler TM, Thompson TC. Combined c-Myc and caveolin-1 expression in human prostate carcinoma predicts prostate carcinoma progression. Cancer. 2005;103(6):1186–1194. doi: 10.1002/cncr.20905. [DOI] [PubMed] [Google Scholar]
- 124.Yang G, Addai J, Wheeler TM, et al. Correlative evidence that prostate cancer cell-derived caveolin-1 mediates angiogenesis. Hum Pathol. 2007;38(11):1688–1695. doi: 10.1016/j.humpath.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 125.Zhang X, Ling MT, Wang Q, et al. Identification of a novel inhibitor of differentiation-1 (ID-1) binding partner, caveolin-1, and its role in epithelial-mesenchymal transition and resistance to apoptosis in prostate cancer cells. J Biol Chem. 2007;282(46):33284–33294. doi: 10.1074/jbc.M705089200. [DOI] [PubMed] [Google Scholar]
- 126.Huang C, Qiu Z, Wang L, et al. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012;72(3):655–665. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8(15):2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ayala G, Morello M, Frolov A, et al. Loss of caveolin-1 in prostate cancer stroma correlates with reduced relapse-free survival and is functionally relevant to tumour progression. J Pathol. 2013;231(1):77–87. doi: 10.1002/path.4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res. 2010;70(10):4222–4232. doi: 10.1158/0008-5472.CAN-09-3281. [DOI] [PubMed] [Google Scholar]
- 130.Trimmer C, Bonuccelli G, Katiyar S, et al. Cav1 suppresses tumor growth and metastasis in a murine model of cutaneous SCC through modulation of MAPK/AP-1 activation. Am J Pathol. 2013;182(3):992–1004. doi: 10.1016/j.ajpath.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang CF, Yu GT, Wang WM, Liu B, Sun ZJ. Prognostic and predictive values of SPP1, PAI and caveolin-1 in patients with oral squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7(9):6032–6039. [PMC free article] [PubMed] [Google Scholar]
- 132.Auzair LB, Vincent-Chong VK, Ghani WM, et al. Caveolin 1 (Cav-1) and actin-related protein 2/3 complex, subunit 1B (ARPC1B) expressions as prognostic indicators for oral squamous cell carcinoma (OSCC) Eur Arch Otorhinolaryngol. 2016;273(7):1885–1893. doi: 10.1007/s00405-015-3703-9. [DOI] [PubMed] [Google Scholar]
- 133.Nakatani K, Wada T, Nakamura M, Uzawa K, Tanzawa H, Fujita S. Expression of caveolin-1 and its correlation with cisplatin sensitivity in oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131(7):445–452. doi: 10.1007/s00432-004-0662-8. [DOI] [PubMed] [Google Scholar]
- 134.Han SE, Park KH, Lee G, Huh YJ, Min BM. Mutation and aberrant expression of Caveolin-1 in human oral squamous cell carcinomas and oral cancer cell lines. Int J Oncol. 2004;24(2):435–440. [PubMed] [Google Scholar]
- 135.Hung KF, Lin SC, Liu CJ, Chang CS, Chang KW, Kao SY. The biphasic differential expression of the cellular membrane protein, caveolin-1, in oral carcinogenesis. J Oral Pathol Med. 2003;32(8):461–467. doi: 10.1034/j.1600-0714.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 136.Jensen DH, Therkildsen MH, Dabelsteen E. A reverse Warburg metabolism in oral squamous cell carcinoma is not dependent upon myofibroblasts. J Oral Pathol Med. 2015;44(9):714–721. doi: 10.1111/jop.12297. [DOI] [PubMed] [Google Scholar]
- 137.Martins D, Beca FF, Sousa B, Baltazar F, Paredes J, Schmitt F. Loss of caveolin-1 and gain of MCT4 expression in the tumor stroma: key events in the progression from an in situ to an invasive breast carcinoma. Cell Cycle. 2013;12(16):2684–2690. doi: 10.4161/cc.25794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Routray S. Caveolin-1 in oral squamous cell carcinoma microenvironment: an overview. Tumour Biol. 2014;35(10):9487–9495. doi: 10.1007/s13277-014-2482-z. [DOI] [PubMed] [Google Scholar]
- 139.Goetz JG, Minguet S, Navarro-Lerida I, et al. Biomechanical remod-eling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146(1):148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ye Q, Meng C, Shen Y, et al. Caveolin-1 mediates low-intensity ultrasound-induced apoptosis via downregulation of signal transducer and activator of transcription 3 phosphorylation in laryngeal carcinoma cells. Ultrasound Med Biol. 2016;42(9):2253–2260. doi: 10.1016/j.ultrasmedbio.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 141.Greco A, De Virgilio A, Rizzo MI, Pandolfi F, Rosati D, de Vincentiis M. The prognostic role of E-cadherin and beta-catenin overexpression in laryngeal squamous cell carcinoma. Laryngoscope. 2016;126(4):E148–E155. doi: 10.1002/lary.25736. [DOI] [PubMed] [Google Scholar]
- 142.Masuelli L, Budillon A, Marzocchella L, et al. Caveolin-1 overexpression is associated with simultaneous abnormal expression of the E-cadherin/alpha-beta catenins complex and multiple ErbB receptors and with lymph nodes metastasis in head and neck squamous cell carcinomas. J Cell Physiol. 2012;227(9):3344–3353. doi: 10.1002/jcp.24034. [DOI] [PubMed] [Google Scholar]
- 143.Masood R, Hochstim C, Cervenka B, et al. A novel orthotopic mouse model of head and neck cancer and lymph node metastasis. Oncogenesis. 2013;2:e68. doi: 10.1038/oncsis.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang H, Su L, Muller S, et al. Restoration of caveolin-1 expression suppresses growth and metastasis of head and neck squamous cell carcinoma. Br J Cancer. 2008;99(10):1684–1694. doi: 10.1038/sj.bjc.6604735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jung AC, Ray AM, Ramolu L, et al. Caveolin-1-negative head and neck squamous cell carcinoma primary tumors display increased epithelial to mesenchymal transition and prometastatic properties. Oncotarget. 2015;6(39):41884–41901. doi: 10.18632/oncotarget.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu YC, Lam KY, Law S, Wong J, Srivastava G. Profiling of differentially expressed cancer-related genes in esophageal squamous cell carcinoma (ESCC) using human cancer cDNA arrays: overexpression of oncogene MET correlates with tumor differentiation in ESCC. Clin Cancer Res. 2001;7(11):3519–3525. [PubMed] [Google Scholar]
- 147.Zhang S, Cao W, Yue M, et al. Caveolin-1 affects tumor drug resistance in esophageal squamous cell carcinoma by regulating expressions of P-gp and MRP1. Tumour Biol. 2016;37(7):9189–9196. doi: 10.1007/s13277-015-4778-z. [DOI] [PubMed] [Google Scholar]
- 148.Gong H, Song L, Lin C, et al. Downregulation of miR-138 sustains NF-kappaB activation and promotes lipid raft formation in esophageal squamous cell carcinoma. Clin Cancer Res. 2013;19(5):1083–1093. doi: 10.1158/1078-0432.CCR-12-3169. [DOI] [PubMed] [Google Scholar]
- 149.Zhu X, Zhang Y, Li Q, et al. Beta-carotene induces apoptosis in human esophageal squamous cell carcinoma cell lines via the Cav-1/AKT/NF-kappaB signaling pathway. J Biochem Mol Toxicol. 2016;30(3):148–157. doi: 10.1002/jbt.21773. [DOI] [PubMed] [Google Scholar]
- 150.Cassoni P, Daniele L, Maldi E, et al. Caveolin-1 expression in lung carcinoma varies according to tumour histotype and is acquired de novo in brain metastases. Histopathology. 2009;55(1):20–27. doi: 10.1111/j.1365-2559.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 151.Yoo SH, Park YS, Kim HR, et al. Expression of caveolin-1 is associated with poor prognosis of patients with squamous cell carcinoma of the lung. Lung Cancer. 2003;42(2):195–202. doi: 10.1016/s0169-5002(03)00287-3. [DOI] [PubMed] [Google Scholar]
- 152.Kunze E, Von Bonin F, Werner C, Wendt M, Schlott T. Transitional cell carcinomas and nonurothelial carcinomas of the urinary bladder differ in the promoter methylation status of the caveolin-1, hDAB2IP and p53 genes, but not in the global methylation of Alu elements. Int J Mol Med. 2006;17(1):3–13. [PubMed] [Google Scholar]
- 153.Kunze E, Schlott T. High frequency of promoter methylation of the 14-3-3 sigma and CAGE-1 genes, but lack of hypermethylation of the caveolin-1 gene, in primary adenocarcinomas and signet ring cell carcinomas of the urinary bladder. Int J Mol Med. 2007;20(4):557–563. [PubMed] [Google Scholar]
- 154.Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry. 2000;39(45):13916–13924. doi: 10.1021/bi001489b. [DOI] [PubMed] [Google Scholar]
- 155.Cheng Y, Ma D, Zhang Y, Li Z, Geng L. Cervical squamous cancer mRNA profiles reveal the key genes of metastasis and invasion. Eur J Gynaecol Oncol. 2015;36(3):309–317. [PubMed] [Google Scholar]
- 156.Agelaki S, Spiliotaki M, Markomanolaki H, et al. Caveolin-1 regulates EGFR signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol Ther. 2009;8(15):1470–1477. doi: 10.4161/cbt.8.15.8939. [DOI] [PubMed] [Google Scholar]
- 157.Savage K, Lambros MB, Robertson D, et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13(1):90–101. doi: 10.1158/1078-0432.CCR-06-1371. [DOI] [PubMed] [Google Scholar]