Abstract
Lipids disorder is the principal cause of atherosclerosis and may present with several forms, according to blood lipoprotein prevalence. One of the most common forms is combined dyslipidemia, characterized by high levels of triglycerides and low level of high-density lipoprotein. Single lipid-lowering drugs may have very selective effect on lipoproteins; hence, the need to use multiple therapy against dyslipidemia. However, the risk of toxicity is a concerning issue. In this review, the effect and safety of an approved combination therapy with simvastatin plus fenofibrate are described, with an analysis of pros and cons resulting from randomized multicenter trials, meta-analyses, animal models, and case reports as well.
Keywords: fibrates, simvastatin, fixed-dose combination, combined dyslipidemia, review
Introduction
Cardiovascular disease (CVD) represents the first cause of death and morbidity of the western countries, despite the progress occurred in the treatment and prevention during the last decades; its prevalence is foreseen to remain stable until 2020, as recent projections illustrate,1 and is continuously increasing also in new developing countries, probably on the basis of the bad influence of western lifestyle and poor awareness of cardiovascular risk among people.1–4
The principal cause of CVD is atherosclerosis, whose development is largely conditioned also by modifiable risk factors, such as smoke, impaired metabolism of lipids and carbohydrates, hypertension, sedentary life, and obesity.2–8
The role of high levels of cholesterol in atherosclerosis pathogenesis is unquestioned5–8 and, while some of the aforementioned risk factors can be directly prevented and treated, dyslipidemia (impaired metabolism of lipids) is hardly manageable for two main reasons: 1) it could have a genetic/familiar component in its genesis and 2) many other extra-cardiac diseases at relatively high incidence and prevalence can engender impairment in lipid control aside from alimentary intake (diabetes mellitus [DM], hypothyroidism, HIV/AIDS, chronic kidney disease, Cushing syndrome, drugs, and alcohol abuse).9,10
Even if the use of statins has been well established in clinical practice for primary and secondary prevention,11,12 CVD risk still remains high in some populations. Fibrates may therefore represent a further pharmacological tool against dyslipidemia. Current international guidelines suggest lowering low-density lipoprotein (LDL) as the principal goal of the therapy for primary and secondary prevention13,14 and non-high-density lipoprotein (HDL) cholesterol, mainly triglyceride (TG) levels, is set as a secondary target of prevention. Since statins contribute to TG decrease is limited, the co-administration of fibrates can further reduce the CVD risk. Experts, however, prudently recommend combined therapy in statin-resistant patients (level of recommendation IIb; class C) because data in favor of double-drug therapy efficacy compared to monotherapy are not conclusive. Among the many possible and existing combinations, the most widely used is simvastatin plus fenofibrate. Nevertheless, the real and net benefit of this specific pharmacological association is still debated.
Other biomarkers (C-reactive protein, fibrinogen, and homocysteine) strongly correlate with dyslipidemia and CV risk, and the effect of these lipid-lowering drugs on such biomarkers is also described in this review.
Methods
Until April 2016, an online search was carried out on PubMed using the following keywords in combination: “adverse effects, cardiovascular disease, combined dyslipidemia, diabetes mellitus, dyslipidemia, fenofibrate, fenofibric acid, fibrate, mixed dyslipidemia, lipid-lowering medications, simvastatin, simvastatin and fenofibrate, statin, statin and fibrate.” After a thorough search, the most relevant randomized clinical trials and comments with regard to reviews, original papers, and case reports were included.
Definition of combined dyslipidemia (CD)
Mixed dyslipidemia or CD is qualitatively defined as an impairment of lipid metabolism characterized by high levels of TG carried within very-low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL), low levels of HDL and higher amount of small and dense LDL (sd-LDL) than normal.15 TG-rich lipoproteins (included Lp-a) are also named Apo-B fraction, since it is the prevalent apo-protein expressed on its surface and constitutes a more accurate measurement of TG-rich lipoprotein because of stoichiometric relation compared to whole LDL concentration.16,17 Furthermore, high values of Apo-B correlate with atherosclerosis and coronary heart disease (CHD).18
A quantitative definition of CD is missing because different cutoff levels were used in studies that recruited people of different age and ethnicity. This disorder is related to a high atherogenic risk profile (hence “atherogenic dyslipidemia”) and is usually associated with other dysmetabolic patterns such as insulin resistance or overt diabetes and obesity, whose essential pathological features are endothelial dysfunction and high risk of thrombosis.15,19 Non-HDL cholesterol represents the sum of IDL, LDL, and VLDL, and sd-LDL is a particular type of LDL characteristically linked to mixed dyslipidemia: the reduction in cholesterol esters and the increase in TG reduce LDL diameter; atherogenic potential is elevated since they are less affine to LDL receptor and the small size increases the accessibility to subintimal space.20,21 Additionally, in type 2 DM (DM2) patients (a condition frequently associated with this phenotype),22 they are more prone to glycation, which further increases its atherogenic effect.23 On the other hand, HDL in the same context of DM2 can be modified by oxidative stress, becoming less protective.24
Epidemiologically, CD prevalence has been estimated to be present in one out of ten subjects in general population and in 15% of statin-treated patients.17 The overlap with DM2 increases the incidence of CD to >50% and the CVD risk is three to four times compared to nondiabetic people and, moreover, many interracial difference exist.17,25
The reduction in LDL only halves the CHD risk; therefore, other lipoproteins are involved in the ongoing atherosclerosis.15 Consequently, although LDL-C has been controlled with optimal statin therapy and/or diet, patients with dysfunctional HDL and elevated TG and sd-LDL maintain a hidden CV risk, namely, the “residual CVD risk.”24
Simvastatin
Statins are the most efficacious lipid-lowering agents. Competitive inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, enzyme catalyzing the conversion of HMG-coA to mevalonic acid, blocks a necessary step in the biosynthesis of cholesterol thanks to a mevalonic-acid-like terminal. Low intra-hepatic levels of cholesterol increase LDL-C receptor expression and therefore removal of this lipoprotein from blood. Some studies indicate that hepatic VLDL production is also decreased due to cholesterol synthesis reduction, cholesterol being a basic component of VLDL; accordingly statins lower TG contained in VLDL, perhaps through direct activation of peroxisome proliferator-activated receptor (PPAR)-α, the same molecular target of fibrates.26,27
Simvastatin was discovered in 1979 as semisynthetic fermentation product of Aspergillus terreus. It is administered as a prodrug, which is rapidly converted in the liver from inactive lactone to its acid form (Table 1). LDL reduction ranges by 20%–48%, from the lowest (10 mg) to the highest dose (80 mg).28–31
Table 1.
Pharmacokinetic profile
| Simvastatin | |
|---|---|
| Absorption | 61%–85% (not altered by food) |
| First pass extraction | >95% of administered dose |
| Bioavailability | <5% |
| Metabolism | CYP4503A4 |
| Tmax | 2.5–4 h |
| Half-life | 2 h |
| Elimination | Feces (58%); urine (13%) |
| Protein binding | 94%–98% |
| Drug–drug interaction | Gemfibrozil, Cyclosporine, Warfarin, Digoxin |
|
| |
| Fenofibrate | |
|
| |
| Absorption | Better in fed state for micronized particles (160 mg) Independent for nanoparticles (145 mg) |
| Bioavailability | 60% |
| Metabolism | Hepatic (glucuronidation) |
| Tmax | 3.5 h |
| Half-life | 19–27 h |
| Elimination | Feces (25%); urine (60%) |
| Protein binding | 99% |
| Drug–drug interaction | Cyclosporine, Pravastatin, Simvastatin (?),Warfarin, Erythromycin |
Simvastatin reduces CV risk as confirmed in large trials. Single study observation reports that simvastatin is able to increase HDL more than atorvastatin.32 Statins are able to stabilize atherosclerotic plaque, and simvastatin has provided evidence of its important contribute.33,34 Furthermore it can, independently from dosages, decrease hs-CRP levels in CHD patients35 and also prevent organ damage (cardiac remodeling after ischemic and nonischemic injury).36,37
The 4S trial,38 a multicenter, randomized, double-blind, placebo-controlled study, showed that cardiovascular risk mortality was reduced by 42% because of the daily dosage of 20–40 mg of simvastatin over a 5-year long period and myocardial revascularization risk decreased by 37%. Moreover, cerebrovascular events and acute myocardial infarction risk were lowered to about a third.
The Heart Protection Study,39 a larger trial, demonstrated the evident benefit of simvastatin 40 mg in high-risk population (previous CV events, DM1 and DM2, hypertension): as primary endpoint mortality was reduced by 13%. Secondary endpoints were nonfatal myocardial infarction and stroke: both resulted significantly improved (risk reduction was 35% and 28%, respectively). Another relevant study aimed at showing the reduction of coronary plaques through quantitative coronary angiography, the Multicenter Anti-Atheroma Study, reported a significant decrease in number of new lesions and an increase of mean lumen area compared to previous angiograms, and, in addition, a slowed progression of CHD in the group treated with simvastatin 20 mg/day compared to placebo recipients.40
As described, simvastatin plays a chief role as lipid-lowering medication in terms of primary and secondary prevention of the main clinical outcomes using mean dosages of 20–40 mg. These dosages are the same in combination therapy with fibrates.
Even though high dosages of the most potent statins, including simvastatin, are related to an increased risk of new onset diabetes, a meta-analysis concluded that the potential risk is outright balanced from the real clinical benefit of statins.41 Moreover, simvastatin, as typical of its class, is characterized by several pleiotropic effects on different organs and systems: according to some reports, simvastatin might exhibit a superior antidepressant effect in post-CABG-treated patients compared to atorvastatin and be a promising coadjuvant agent in oncology.42,43
Fenofibrate
Fenofibrate is a pro-drug transformed into its active form – fenofibric acid – in the liver. As agonists binding to PPAR-α, fibrates regulate genetic expression of numerous enzymes involved in lipids metabolism.44 Fenofibrate pharmacokinetic profile is summarized in Table 1.
The increased synthesis of lipoprotein lipase (LPL) enhances free fatty acids (FFA) oxidation from TG in adipose tissue.44 Liver production of ApoC-III, a component of VLDL and a LPL inhibitor, is reduced; therefore, first, less-circulating VLDLs are released from hepatocellular compartment and, second, peripheral oxidation is facilitated.44–46 Additionally, de novo FFA and VLDL production is reduced due to acetyl-CoA-carboxylase, fatty acids synthase, and ApoB under-expression. Furthermore, fibrates, particularly fenofibrate, reduce LDL and increase size and density of sd-LDL; also, Lp-PLA1 (lipoprotein – phospholipase A1) reduction diminishes LDL oxidation.44,45
On the other hand, ApoA-I and ApoA-II overtranscription increases HDL levels, and the same effect is achieved via a decrease of cholesteryl ester transfer protein (CETP) activity, reducing the transfer of cholesterol from HDL to VLDL.44–46 HDL, however, can even decrease for not completely clear reasons, if thiazolidinediones are co-administered.47
Eventually the net effect of fibrate is the reduction of TG-rich lipoprotein and elevation of HDL; hence, its indication in nonalcoholic fatty liver disease treatment.48 LDL trend can show a paradoxical pattern: in fact in case of severe hypertriglyceridemia, catabolism of TG induced by fibrate can convert VLDL to LDL rapidly, leading to initial LDL elevation and shift to less-buoyant LDL particles.44
Weak results were obtained in glucose control: in some studies, fenofibrate improved insulin sensitivity and glucose level in metabolic syndrome, DM2 and CD patients.49–52 The underlying mechanism could be an increase of adiponectin (insulin sensitizer) in adipose tissue.53
Also, other studies showed antithrombotic and fibrinolytic properties of fenofibrate (decrease in PAI and fibrinogen),54 neo-angiogenesis reduction, and, on the other side, increase of flow-mediated dilation consistent with beneficial improvement of nitric oxide production, as proved in vitro.44
Last, markers of inflammation as CRP, interleukins (mostly interleukin-6 [IL-6] and tumor necrosis factor-α [TNF-α]), adhesion molecules (VCAM and ICAM) and uric acid were reduced with the administration of fenofibrate in a high-risk group.52–54
On account of what stated, two main trials have been carried out with regard to real clinical efficacy of lipid-lowering effect of fenofibrate and both investigated the efficacy in DM2 population because of frequent incidence of CD with DM.
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study enrolled patients with well-controlled DM2, among which <40% had diagnosis of dyslipidemia and about a fifth had previous diagnosis of CHD.55 The comparison with placebo did not result in superiority of fenofibrate 200 mg/day in terms of primary composite outcomes (CHD events); however, while failed to present minor rates of CHD mortality significantly, fenofibrate-treated group showed a reduction in nonfatal myocardial infarction (hazard ratio, HR: 0.76). Total mortality, total stroke, and total CVD mortality reduced nonsignificantly, whereas fenofibrate decreased the incidence of coronary revascularization and first minor amputation as well as delayed first treatment for any retinopathy or maculopathy (present only in 8% of baseline sample). Apparently, the lack of efficacy of fenofibrate compared to placebo should be related to higher number of randomized placebo patients who were already on treatment with statins. Considering this bias, a successive analysis revealed an improvement in the relative reduction of total CVD events to 15% and a post hoc analysis reported that subgroups affected by marked hypertriglyceridemia, CD, and metabolic syndrome experienced greater benefit of fenofibrate treatment.56 Unexpectedly, fenofibrate slowered glomerular filtration rate (GFR) decline and reduced albuminuria rate.44
The DAIS trial (Diabetes Atherosclerosis Intervention Study) was designed to demonstrate a slower CHD progression in subjects treated with fenofibrate compared to placebo group.57 Although the ambitious endpoint was not satisfied, due to diffuse disease, minimum lumen diameter of focal atherosclerosis appeared higher in fenofibrate recipients, however, similarly to FIELD, fenofibrate decreased albuminuria rate.
Combination therapy
Efficacy
The ACCORD Lipid, a double-blind, randomized, open-label trial, aimed to verify the superiority of fenofibrate plus simvastatin versus placebo plus simvastatin in 5518 patients with DM2 at high risk of CVD.58 The final results showed no significant variations between the two groups for primary and secondary endpoints (Table 2). Beneficial effect on minor outcomes as renal disease progression, measured as micro and macro-albuminuria, was instead confirmed.
Table 2.
| Endpoint | Simvastatin + fenofibrate (N) | Simvastatin + placebo (N) | Hazard ratio (p-value) | |
|---|---|---|---|---|
| Primary outcomes | Major CV event (nonfatal MI, nonfatal stroke or death from CV cause) | 291 | 310 | 0.92 (0.32) |
| Secondary outcomes | Primary outcome plus revascularization or hospitalization for CHD | 641 | 667 | 0.94 (0.3) |
| Major CHD event | 332 | 353 | 0.92 (0.26) | |
| Nonfatal MI | 173 | 186 | 0.91 (0.39) | |
| Stroke | 98 | 88 | 1.05 (0.8) | |
| All-cause mortality | 302 | 335 | 0.91 (0.33) | |
| Fatal or nonfatal CHD | 120 | 143 | 0.82 (0.1) | |
| Other | Progression of diabetic retinopathy | 52 | 80 | 0.6 (0.006) |
| Moderate vision loss | 227 | 233 | 0.95 (0.57) |
Abbreviations: CV, cardiovascular; MI, myocardial infarction; CHD, coronary heart disease
Lipid values were overall reduced; nevertheless, at subgroup analysis, the primary endpoint was reduced in the top tertile of TG and in the lowest tertile of HDL patients (17% of total cohort), thus showing the major benefit in the highest risk CD population (relative risk reduction =31%).59,60 Slight gender differences for primary endpoint were reported, as women were more protected than men (9.1% vs 11.2% in combination therapy groups of respective gender).59 Part of the subjects enrolled in ACCORD Lipid was recruited for the ACCORD Eye trial, which assessed subjects with diabetic retinopathy.60 As previously demonstrated in the FIELD,55 fenofibrate in combination therapy showed its protective effect with regard to this common complication with a substantial relative risk reduction (40%). Furthermore, an ancillary study,61 based on the observations that post-prandial TGs are predictors of CHD,62,63 investigated the supposed superiority of fenofibrate plus simvastatin in lowering non-fasting TG compared to placebo associated with simvastatin: the hypothesis was rejected, but, however, Apo-B48, marker of postprandial chylomicrons, was reduced in a greater extent in subject with high fasting TG at the baseline.
According to SAFARI trial (study of simvastatin plus fenofibrate for combined hyper-lipidemia), simvastatin 20 mg plus fenofibrate 160 mg improved the overall lipid profile:64 in particular, while HDL almost doubled, TGs were reduced by 43% in the combination treatment group. Moreover, as previously reported, the addition of fenofibrate caused a shift to larger, buoyant, and less dangerous LDL particles.
In a post hoc analysis, the same investigators assessed a correlation between non-HDL and Apo-B in the co-treated group and suggested a possible role of non-HDL cholesterol as surrogate marker of Apo-B content, thus supporting its use as a secondary target of the lipid-lowering therapy.65
In addition to SAFARI, smaller studies supported existing data with regard to lipid profile: Mohiuddin et al66 used the lowest dose of fenofibrate than the other studies did (135 vs 160 mg in the SAFARI and 200 mg in the ACCORD) associated with simvastatin 20 and obtained favorable results in comparison with statin alone: HDL-C increased (+17.8% vs 7.2%) and TG decreased drastically (−37.4% vs −14.2%). Percentages approximately equivalent were found in fenofibrate 135 mg plus simvastatin 40 mg.
The DIACOR (Diabetes and Combined Lipid Therapy Regimen) study compared the combination therapy with both monotherapies: results showed that the supplemental advantage in HDL rise and TG decrease are derived merely from fenofibrate addition.67 Furthermore, an analysis from DIACOR study confirmed positive modifications found during SAFARI at expense of pro-atherogenic sd-LDL.68
Stefanutti et al reached consistent conclusions: all four groups treated with different double-drug combinations (from simvastatin 10 mg to simvastatin 30 mg plus fenofibrate 200–300 mg) showed improvement in lipid profile, albeit the more statistically significant overall benefit was obtained with simvastatin 20 mg/fenofibrate 200 mg, and no serious adverse reactions were noted in any group.69 If slight differences among various dose combinations might exist, no remarkable disparity was assessed between daily co-administration and alternate day use.70 Outstanding results can be obtained with a new fixed-dose combination with fenofibrate 145 mg in a new nanotechnology formulation compared to single medications.71 As observable from all the studies referred, the higher the baseline dyslipidemia level, the better the effect of combined drugs is.
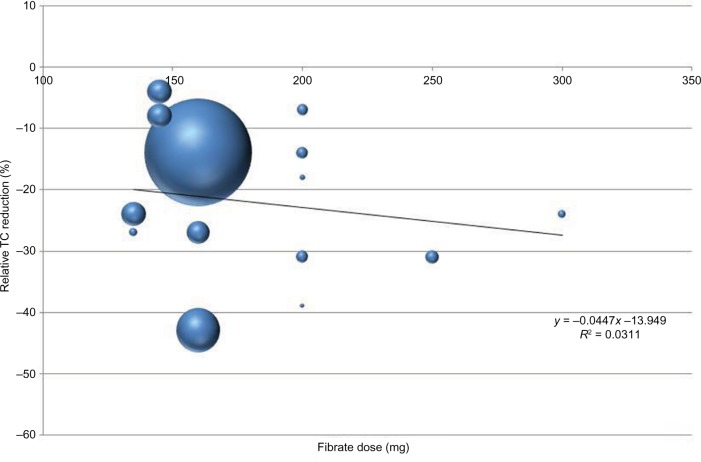
Mean relative total cholesterol level reduction values are given in Table 3; meta-regression analysis of such studies is plotted in Figure 1. Three months is generally required to achieve a substantial cholesterol reduction. A mild, direct, nonsignificant correlation between fenofibrate dosage and total cholesterol reduction is also evident (linear weighted regression analysis).
Table 3.
Mean relative reduction of total cholesterol levels by adding fibrates.
| Study | Patients treated with fibrates | Simvastatin dose (mg) | Fibrate dose (mg) | Follow-up duration (months) | Relative TC reduction (%) | Reference |
|---|---|---|---|---|---|---|
| ACCORD | 2271* | 20** | 160§ | 56 | −14 | 58 |
| Foucher et al | 109 | 20 | 145 | 3 | −4 | 71 |
| 110 | 40 | 145 | 3 | −8 | ||
| DIACOR | 100 | 20 | 160 | 3 | −27 | 67 |
| Stefanutti et al | 5 | 10 | 200 | 12 | −18 | 69 |
| 26 | 20 | 200 | 12 | −31 | ||
| 11 | 20 | 300 | 12 | −24 | ||
| 3 | 30 | 200 | 12 | −39 | ||
| Krysiak et al | 24 | 40 | 200 | 3 | −14 | 75 |
| SAFARI | 374 | 20 | 160 | −43 | 64 | |
| Shah et al | 22 | 20 | 200 | 3 | −7 | 72 |
| Kayikcioglu et al§§ | 32 | 10 | 250 | 6 | −31 | 70 |
| Mohiuddin et al | 112 | 20 | 135 | 3 | −24 | 66 |
| 111 | 40 | 135 | 3 | −27 |
Notes:
Data refer to number of patients followed up to exit visit;
maximal statin daily dose was 40 mg but varied during the follow-up, based on lipid lowering response and according to international guidelines (overall average dose was 22.3 mg in fenofibrate group);
fenofibrate 54 mg was administered in patients with baseline or incidental eGFR <50 mL/min/1.73 m2;
data refer to everyday administration group.
Abbreviation: TC, total cholesterol.
Figure 1.
Mean relative reduction of total cholesterol levels by adding fibrates.
Abbreviation: TC, total cholesterol.
Pleiotropic effects of both drugs act synergistically on novel different biomarkers and risk factors. Combination therapy reduces fibrinogen after 3 months of treatment for an acute coronary syndrome, and this is attributed mainly to fenofibrate addition.72,73 Nevertheless, clinical validations are still expected.
An analysis carried out on animals fed with high fat content diet and treated separately with simvastatin and fenofibrate compared to untreated controls provided preliminary results with regard to metabolomic effects: aside from cholesterol, levels of various small endogenous molecules (aminoacids, fatty acids, carbohydrate, and catabolites) significantly changed, among which important markers involved in CVD genesis (ie, a precursor of vaso-protective prostaglandins, linoleic acid, increased, while creatinine, renal damage marker, decreased).74 However, dosages in animals were different than those generally used in humans, and these data require further confirmations.
Administered together, simvastatin and fenofibrate potentiate their own anti-inflammatory properties in DM2 patients, as proven by a decrease in a greater extent than single monotherapy of monocyte and lymphocyte released atherogenic cytokines (IL-1b, IL-6, IL-2, interferon [IFN]-γ, and TNF-α) and, hence hs-CRP, probably via the same cellular pathways.52,75 Moreover, the effect was exactly more prominent and statistically more significant in patients with overt CD.
Safety
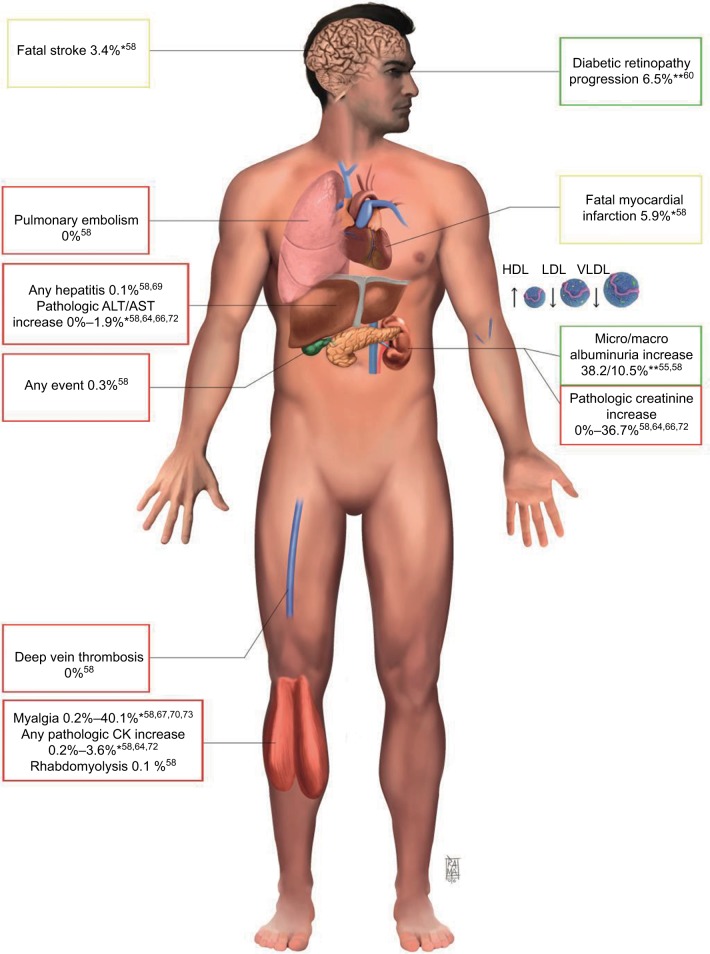
The most common adverse reactions characteristic of statins group are hepato- and myotoxicity, either clinically silent or evident (Figure 2). The association with fibrate can increase the risk of serious adverse reaction for both pharmacodynamic and pharmacokinetic interaction.76
Figure 2.
Principal side effects and clinical benefit of combination therapy.
Notes: Red squares report range of side effect percent resulted in clinical trials. Green squares contain data about clinical benefits. Yellow squares refer to major clinical endpoint that resulted not statistically significant compared to controls (placebo or monotherapy). References are in the brackets. *No overall difference versus placebo or statin monotherapy (depending on the study); **overall lower than in placebo groups.
Abbreviations: ALT, alanine amino transferase; AST, aspartate amino transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
Statins can affect muscles through different mechanisms (membrane integrity perturbation, apoptosis induction, mythocondrial dysfunction, impairment in calcium homeostasis, or rarely autoimmune reaction); however, the incidence according to a meta-analysis of 26 statins trials is 1: 10,000 compared to placebo.77,78
Liver injury is usually asymptomatic and apparently due to elevated membrane permeability, secondary to low cholesterol production, which permits leakage of hepatocellular content, while autoimmune mechanism is quite uncommon.79
Fibrate-related skeletal muscle damage is probably ascribed to reactive oxygen species derived from β-oxidation and mitochondrial dysfunction induced by fibrate, as shown in animal models which were treated with both potent and weak (fenofibrate) compounds;80,81 however, experimental doses are extremely higher than those used in humans. Similar origin for hepatotoxicity has been speculated, even if recent evidences suggest that PPAR-α agonist can directly increase alanine amino transferase (ALT) and aspartate amino transferase (AST) gene expression and can also shift hepatic metabolism into a more ALT/AST-dependent one.82 On the contrary, PPAR activators likely protect against acetaminophen oxidative stress.83 As was observed in FIELD study, fibrates increase cholelithiasis risk, altering cholesterol biliary efflux, and accordingly pancreatitis is significantly more probable.84 Indeed, gallbladder disease is a contraindication to the use of fibrates.
Pharmacokinetic interaction is univocal: fibrates can generally increase statin half-life therefore augmenting the risk of statin side effects. Gemfibrozil, but not fenofibrate, inhibits OATP1B1 (organic anion-transporting polypeptide), a cellular transporter from blood stream to hepatic cells, increasing simvastatin concentration and its adverse effects; thus this association should be avoided.85
When combined, both in fixed dose and in staggered dose, fenofibrate decreases simvastatin AUC (area under the plasma concentration curve) by ~30%, as reported in two isolated studies, while simvastatin acid was not affected.86 Many pharmacological mechanisms have been postulated; however, since the intentional reduction of cholesterol is unaltered with both dosing schedules and no life-threatening reactions occurred, the authors concluded that this unaccountable drug–drug interaction is clinically irrelevant, and risk of myopathy can be considered theoretic. Clinicians and primary care physicians should also mind about other drugs administration (ie, cyclosporine, an OATP1B1 inhibitor) and choose case by case which drug discontinuation provides the best benefit/risk ratio.
Also, in all the aforementioned trials, serious adverse reactions were isolated cases: for instance in the SAFARI trial (618 patients over 12 weeks), there was no significant difference of frequency of muscle symptoms between combined therapy versus monotherapy (1.4% vs 1.5%) and no case of rhabdomyolysis was recorded.64 In the ACCORD Lipid study (with the longest follow-up and the highest number of patients enrolled), the incidence of myotoxicity (myopathy, myositis, and rhabdomyolysis) in the treatment group was equally balanced to placebo plus simvastatin recipients (0.1% for both).58 Although nephroprotective effect resulted in long term, fibrate co-administration caused transient creatinine elevation (>1.3 mg/dL for women; >1.5 mg/dL for men), but no cases of acute renal failure were reported. Despite collateral homocysteine increase, any deep venous thrombosis or pulmonary embolism occurred, in contrast to data recorded in the FIELD study. Additionally, absolute number of deaths was definitely comparable between the two groups (203 in the fenofibrate plus simvastatin group vs 221 in placebo). Overall risk of myotoxicity was very low in combination therapy, including asymptomatic and occasional creatinine phosphokinase (CPK) increment >5 or >10 times the upper limit of normal (ULN, usually between 0.3% and 2.2%).58
Alanine amino transferase (ALT) is more sensitive in case of liver injury compared to AST (present also in muscle tissues), and an increase of its value per three times the ULN should induce to analyze fractionated bilirubin level before drug discontinuations, because bilirubin is a more reliable indicator of damage, as National Lipid Association’s (NLA) Liver Expert Panel recommends.87 In the ACCORD study, ALT elevation occurred because of drug discontinuation in only four patients in combination therapy group and no difference in gallbladder-related events occurred between the two groups.58 However, gallbladder ultrasound should be performed before initiating the therapy.
Minor drug adverse reactions (DAEs), such as allergy, cataract, and interstitial lung disease, have been described in sporadic cases. Obviously, new onset diabetes related to simvastatin could not be evaluated in these statistics because diabetes was a chief inclusion criterion.
Finally, despite overall rarity of DAEs (mostly the serious ones) and the net beneficial effect of lipid-lowering medications in terms of primary and secondary CVD prevention as confirmed by a recent meta-analysis, physicians should be aware of safety aspects and frequently monitor patients on high dosage of statins and other toxic drugs whose interferences may be harmful (almost exclusively for simvastatin) or with preexisting risk factors (chronic kidney disease, hepatic insufficiency, muscular disease, age, and hypothyroidism).88 It is generally recommended to measure ALT and CPK at the baseline and after some weeks of treatment or at the first suspected symptoms in healthy subjects. Food and Drug Administration suggests to test renal function periodically in elderly patients or those with renal insufficiency.89 Transaminases and CPK should be monitored periodically in any case.
Comparison of simvastatin with other fibrates
Simvastatin plus gemfibrozil have an evidently high risk/benefit ratio because of clear pharmacological interactions, while, generally, the combination with gemfibrozil is associated with higher rates of DAEs compared to fenofibrate (ie, 15 vs 20.7/100,000 for rhabdomyolysis) as evaluated in a retrospective analysis.90 Safety and efficacy about combination of bezafibrate with simvastatin, even if positive, are based only on two single experiences with poor sample sizes and brief follow-up.91,92
Comparison of fenofibrate with other statins
The addition of fenofibrate has been tested with other statins (fluvastatin, atorvastatin, pravastatin, and rosuvastatin) in comparison with monotherapy, with overall favorable results and comparable safety profile among different associations in regard of lipid levels.93–96 Rosuvastatin has alone been demonstrated to be noninferior to combination.96 However, no double-blind, randomized, placebo-controlled, and multicentre clinical trial, as large as ACCORD and SAFARI studies, with hundreds of patients enrolled, has been done, and, thus, any head-to-head comparison is presently unreliable.
Special populations and conditions
DM and metabolic syndrome
DM patients represent the target population of the ACCORD Lipid trial; therefore, such results are the basis for treatment indications. Although not superior to placebo in reducing major CV events, the addition of fenofibrate to lipid-lowering therapy in DM2 patients has a series of healthy effects on endothelial function, inflammation, hemostasis, and glycemic control,50,52,97 which could be the reason of the improvement of microangiopathy and diabetic nephropathy. In a small study, carried out by Vega et al, no impairment of glucose levels occurred, and the low and theoretic risk to develop DM2, mainly related to other preexisting conditions, should not refrain for initiating this vascular protective lipid-lowering therapy.98 Nevertheless, combination therapy should neither reciprocally interfere with antidiabetic medications nor increase the risk of adverse effect (as myopathy risk in case of thiazolidinediones and fibrates).99
Chronic kidney disease
Serum creatinine level ULN (>1.5 mg/dL) was an exclusion criteria in all great trials.58,64 However, mild-to-moderate renal insufficiency is more frequent in real practice; thus, precautionary laboratory and clinical monitoring should be performed in such patients. Fenofibrate alone is contraindicated in IV-V KDOQI stage and dose should be the lowest possible in case of creatinine clearance <50 mg/dL, according to the manufacturer.
Hypothyroidism
Hypothyroidism is associated with mixed dyslipidemia and abnormal CPK is correlated in >90% of cases; furthermore, low thyroid function decreases GFR and renal clearance.100,101 The primary treatment of secondary dyslipidemia is restoring hormones level, although, in case of preexisting or refractory dyslipidemia, the addition of lipid-lowering medications should be considered. Association therapy is not reported; nevertheless, simvastatin and fenofibrate alone have shown to be useful.102,103 Since the risk of muscular toxicity is increased and numerous isolated cases of rhabdomyolysis requiring hemodialysis have been reported,104 great caution should be paid not only in case of known hypothyroidism but also in case of occult hypothyroidism.102,103 Therefore, a screening of thyroid function before initiating double therapy is recommended.
HIV
HIV infection is associated with atherogenic dyslipidemia:105 endogenous IFN-α inhibits hepatic and endothelial lipase, thus increasing TG concentrations.106 Moreover, CETP activity and HDL level are decreased.107 HAART therapy also causes dyslipidemia stimulating lipogenesis.108,109 Double therapy is a reliable option for dyslipidemia: indeed, fenofibrate is very effective also in HIV patients;110 however, simvastatin is contraindicated in case of protease inhibitors treatment, which could elevate simvastatin concentration by 10 times and increase the risk of overexposure via CYP3A4 inhibition.111,112 Therefore, other combinations should be preferred.
Childhood with CD
Acquired CD is a more prevalent form of dyslipidemia in childhood.113 Diagnosis of familial combined hyperlipidemia (FH) requires the presence of increased Apo-B and sd-LDL particles in the patient and in at least two family members and one first-degree relative with the history of CHD. The family history is usually unknown and CHD could be silent, thus few cases of CD can be classified as purely genetic and familial.113 Approximately 40% of obese adolescents have an atherogenic pattern typical of individuals with DM and metabolic syndrome. Intervention of lifestyle change is primary; however, if after 6 months lipid profile has not improved or baseline LDL and TG are severely high, medication should be considered, preferentially in children aged >10 years.113 Low dose of simvastatin alone has demonstrated to improve vascular reactivity in children with FH and to be effective and safe in primary and secondary dyslipidemia.114 Fenofibrate alone was shown to be effective and generally safe in a small randomized trial, but clinical efficacy on prevention of CVD is still unknown.115 Similarly, no data about the use of drugs in combination are currently available.
Conclusion
Beside LDL reduction as a primary goal of lipid-lowering therapy, reduction of non-HDL cholesterol, decreasing TG and small dense LDL may be considered in primary and secondary prevention of CVD and may be pursued, if required, by implementing pharmacological therapy. In case of refractory CD, doubling statin dosage would not be convenient in terms of safety and efficacy; therefore, dual lipid-lowering therapy constitutes a viable solution. Combination therapy with simvastatin and fenofibrate, in various dosages, is an efficient and reliable mean of pharmacological prevention as large, multicenter, randomized trials reported. Moderate but significant clinical benefits are achievable in selected populations and, in absence of predisposing factors, toxicity is a marginal issue. However, cautions should be paid and patients should be clearly informed about risk–benefit ratio and possible adverse events. Moreover, fixed dose is equivalent to staggered dose and increases patient’s compliance.
Acknowledgments
We sincerely thank our trusted illustrator Raffaele Matteucci, professionally known as RaMat, for his precious graphic contribution in this and in other works (Figure 2). The paper is not under consideration elsewhere. None of the paper’s contents have been previously published.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Comparative Risk Assessment Collaborating Group. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 3.Sharma M, Ganguly NK. Premature coronary artery disease in Indians and its associated risk factors. Vasc Health Risk Manag. 2005;1:217–225. [PMC free article] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211. doi: 10.1016/j.pop.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 7.Hossain P, Kawar B, El NM. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 8.Member S, Bruger M. Experimental atherosclerosis; the effect of feeding olive oil on the absorption and deposition of cholesterol. Arch Pathol. 1945;40:373–375. [PubMed] [Google Scholar]
- 9.Qi Q, Liang L, Doria A, Hu FB, Qi L. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes. 2012;61:745–752. doi: 10.2337/db11-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodnala D, Rubenfire M, Brook RD. Secondary causes of dyslipidemia. Am J Cardiol. 2012;110:823–825. doi: 10.1016/j.amjcard.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 13.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217:3–46. doi: 10.1016/j.atherosclerosis.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP. Combination therapy in mixed dyslipidemia. J Intern Med. 2008;263:35365. doi: 10.1111/j.1365-2796.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 16.Lamarche B, Lemieux I, Després JP. The small, dense LDL phenotype and the risk of coronary heart disease: epidemiology, patho-physiology and therapeutic aspects. Diabetes Metab. 1999;25:199–211. [PubMed] [Google Scholar]
- 17.Halcox J, Misra A. Type 2 diabetes mellitus, metabolic syndrome, and mixed dyslipidemia: how similar, how different, and how to treat? Metab Syndr Relat Disord. 2015;13:1–21. doi: 10.1089/met.2014.0049. [DOI] [PubMed] [Google Scholar]
- 18.Brunzell JD. Increased ApoB in Small Dense LDL Particles Predicts Premature Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2005;25:474–475. doi: 10.1161/01.ATV.0000156537.78366.1d. [DOI] [PubMed] [Google Scholar]
- 19.Shimabukuro M, Higa N, Masuzaki H, Sata M, Ueda S. Impact of individual metabolic risk components or its clustering on endothelial and smooth muscle cell function in men. Cardiovasc Diabetol. 2016;15:77. doi: 10.1186/s12933-016-0394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23:97–104. [PubMed] [Google Scholar]
- 21.Lamarche B, Tchernof A, Cantin B, Dagenais GR, Lupien PJ, Després JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs MJ, Kleisli T, Pio JR, Malik S, L’Italien GJ, Chen RS, Wong ND. Prevalence and control of dyslipidemia among persons with diabetes in the United States. Diabetes Res Clin Pract. 2005;70:263–269. doi: 10.1016/j.diabres.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Younis NN, Soran H, Pemberton P, Charlton-Menys V, Elseweidy MM, Durrington PN. Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clin Sci. 2013;124:343–349. doi: 10.1042/CS20120304. [DOI] [PubMed] [Google Scholar]
- 24.Agouridis AP, Rizos CV, Elisaf MS, Filippatos TD. Does combination therapy with statins and fibrates prevent cardiovascular disease in diabetic patients with atherogenic mixed dyslipidemia? Rev Diabet Stud. 2013;10:171–190. doi: 10.1900/RDS.2013.10.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gitt AK, Junger C, Smolka W, Bestehorn K. Prevalence and overlap of different lipid abnormalities in statin-treated patients at high cardiovascular risk in clinical practice in Germany. Clin Res Cardiol. 2010;99:723–733. doi: 10.1007/s00392-010-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet. 1993;24:195–202. doi: 10.2165/00003088-199324030-00002. [DOI] [PubMed] [Google Scholar]
- 27.Myerson M, Ngai C, Jones J, Holleran S, Ramakrishnan R, Berglund L, Ginsberg HN. Treatment with high-dose simvastatin reduces secretion of apolipoprotein B-lipoproteins in patients with diabetic dyslipidemia. J Lipid Res. 2005;46:2735–2744. doi: 10.1194/jlr.M500335-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Nawrocki JW, Weiss SR, Davidson MH, et al. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler Thromb Vasc Biol. 1995;15(5):678–682. doi: 10.1161/01.atv.15.5.678. [DOI] [PubMed] [Google Scholar]
- 29.Farnier M, Portal JJ, Maigret P. Efficacy of atorvastatin compared with simvastatin in patients with hypercholesterolemia. J Cardiovasc Pharmacol Ther. 2000;5(1):27–32. doi: 10.1177/107424840000500104. [DOI] [PubMed] [Google Scholar]
- 30.Kapur NK, Musunuru K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vasc Health Risk Manag. 2008;4(2):341–353. doi: 10.2147/vhrm.s1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ose L, Davidson MH, Stein EA, et al. Lipid-altering efficacy and safety of simvastatin 80 mg/day: long-term experience in a large group of patients with hypercholesterolemia. World Wide Expanded Dose Simvastatin Study Group. Clin Cardiol. 2000;23(1):39–46. doi: 10.1002/clc.4960230108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunninghake DB, Ballantyne CM, Maccubbin DL, Shah AK, Gumbiner B, Mitchel YB. Comparative effect of simvastatin and atorvastatin on high-density lipoprotein cholesterol and apolipoprotein A1. Clin Ther. 2003;25:1670–1686. doi: 10.1016/s0149-2918(03)80162-5. [DOI] [PubMed] [Google Scholar]
- 33.Serra N, Rosales R, Masana L, Vallvé JC. Simvastatin increases fibulin-2 expression in human coronary artery smooth muscle cells via rhoA/rho-kinase signaling pathway inhibition. PLoS One. 2015;10:e0133875. doi: 10.1371/journal.pone.0133875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellosta S, Via D, Canavesi M, Pfister P, Fumagalli R, Paoletti R, Bernini F. HMG-CoA reductase inhibitors reduce MMP-9 secretion by macrophages. Arterioscler Thromb Vasc Biol. 1998;18:1671–1678. doi: 10.1161/01.atv.18.11.1671. [DOI] [PubMed] [Google Scholar]
- 35.Meredith KG, Horne BD, Pearson RR, Maycock CA, Lappe DL, Anderson JL, Muhlestein JB. Comparison of effects of high (80 mg) versus low (20 mg) dose of simvastatin on C-reactive protein and lipoproteins in patients with angiographic evidence of coronary arterial narrowing. Am J Cardiol. 2007;99:149–153. doi: 10.1016/j.amjcard.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 36.Zou C, Qi H, Liu ZH, Han L, Zhao C, Yang X. Simvastatin activates the PPARγ-dependent pathway to prevent left ventricular hypertrophy associated with inhibition of RhoA signaling. Tex Heart Inst J. 2013;40:140–147. [PMC free article] [PubMed] [Google Scholar]
- 37.Almansob MA, Xu B, Zhou L, et al. Simvastatin reduces myocardial injury undergoing noncoronary artery cardiac surgery: a randomized controlled trial. Arterioscler Thromb Vasc Biol. 2012;32:2304–2313. doi: 10.1161/ATVBAHA.112.252098. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 39.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 40.MAAS investigators Effect of simvastatin on coronary atheroma: the multicentre anti-atheroma study (MAAS) Lancet. 1994;344:633–638. [PubMed] [Google Scholar]
- 41.Sattar N, Preiss D, Murray H, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 42.Abbasi SH, Mohammadinejad P, Shahmansouri N, et al. Simvastatin versus atorvastatin for improving mild to moderate depression in post-coronary artery bypass graft patients: a double-blind, placebo-controlled, randomized trial. J Affect Disord. 2015;183:149–155. doi: 10.1016/j.jad.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 43.Kretzer IF, Maria DA, Guido MC, Contente TC, Maranhão RC. Simvastatin increases the antineoplastic actions of paclitaxel carried in lipid nanoemulsions in melanoma-bearing mice. Int J Nanomedicine. 2016;11:885–904. doi: 10.2147/IJN.S88546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs. 2011;71:1917–1946. doi: 10.2165/11208090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPAR alpha and PPAR gamma activators direct a distinct tissue-specific transcriptional response via PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 46.Farnier M. Update on the clinical utility of fenofibrate in mixed dyslipidemias: mechanisms of action and rational prescribing. Vasc Health Risk Manag. 2008;4:991–1000. doi: 10.2147/vhrm.s3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linz PE, Lovato LC, Byington RP, et al. Paradoxical reduction in HDL-C with fenofibrate and thiazolidinedione therapy in type 2 diabetes: the ACCORD Lipid Trial. Diabetes Care. 2014;37:686–693. doi: 10.2337/dc13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musso G, Cassader M, Gambino R. Cholesterol-lowering therapy for the treatment of nonalcoholic fatty liver disease: an update. Curr Opin Lipidol. 2011;22:489–496. doi: 10.1097/MOL.0b013e32834c37ee. [DOI] [PubMed] [Google Scholar]
- 49.Krysiak R, Stachura-Kulach A, Okopien B. Metabolic and monocyte-suppressing actions of fenofibrate in patients with mixed dyslipidemia and early glucose metabolism disturbances. Pharmacol Rep. 2010;62:120–130. doi: 10.1016/s1734-1140(10)70249-8. [DOI] [PubMed] [Google Scholar]
- 50.Pruski M, Krysiak R, Okopien B. Pleiotropic action of short-term metformin and fenofibrate treatment, combined with lifestyle intervention, in type 2 diabetic patients with mixed dyslipidemia. Diabetes Care. 2009;32:1421–1424. doi: 10.2337/dc08-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Idzior-Walus B, Sieradzki J, Rostworowski W, et al. Effects of comicronised fenofibrate on lipid and insulin sensitivity in patients with polymetabolic syndrome X. Eur J Clin Invest. 2000;30:871–878. doi: 10.1046/j.1365-2362.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- 52.Krysiak R, Gdula-Dymek A, Okopien B. The effect of fenofibrate on lymphocyte release of proinflammatory cytokines and systemic inflammation in simvastatin-treated patients with atherosclerosis and early glucose metabolism disturbances. Basic Clin Pharmacol Toxicol. 2013;112:198–202. doi: 10.1111/bcpt.12003. [DOI] [PubMed] [Google Scholar]
- 53.Filippatos TD, Elisaf MS. Safety considerations with fenofibrate/simvastatin combination. Expert Opin Drug Saf. 2015;14:1481–1493. doi: 10.1517/14740338.2015.1056778. [DOI] [PubMed] [Google Scholar]
- 54.Ye J, Kiage JN, Arnett DK, Bartolucci AA, Kabagambe EK. Short-term effect of fenofibrate on C-reactive protein: a meta-analysis of randomized controlled trials. Diabetol Metab Syndr. 2011;3:24. doi: 10.1186/1758-5996-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 56.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steiner G. The Diabetes Atherosclerosis Intervention Study (DAIS): a study conducted in cooperation with the World Health Organization. The DAIS Project Group. Diabetologia. 1996;39:1655–1661. doi: 10.1007/s001250050630. [DOI] [PubMed] [Google Scholar]
- 58.Ginsberg HN, Elam MB, Lovato LC, et al. The ACCORD Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elam M, Lovato LC, Ginsberg H. Role of fibrates in cardiovascular disease prevention, the ACCORD-Lipid perspective. Curr Opin Lipidol. 2011;22:55–61. doi: 10.1097/MOL.0b013e328341a5a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The ACCORD Study Group and ACCORD Eye Study Group Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reyes-Soffer G, Ngai CI, Lovato L, Karmally W, Ramakrishnan R, Holleran S, Ginsberg HN. Effect of combination therapy with fenofibrate and simvastatin on postprandial lipemia in the ACCORD lipid trial. Diabetes Care. 2013;36:422–428. doi: 10.2337/dc11-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 63.Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 64.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am J Cardiol. 2005;95:462–468. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Grundy SM, Vega GL, Tomassini JE, Tershakovec AM. Correlation of non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol with apolipoprotein B during simvastatin + fenofibrate therapy in patients with combined hyperlipidemia (a subanalysis of SAFARI trial) Am J Cardiol. 2009;104:548–553. doi: 10.1016/j.amjcard.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Mohiuddin SM, Pepine CJ, Kelly MT, Buttler SM, Setze CM, Sleep DJ, Stolzenbach JC. Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study. Am Heart J. 2009;157:195–203. doi: 10.1016/j.ahj.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 67.Muhlestein JB, May HT, Jensen JR, et al. The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia: the DIACOR (Diabetes and Combined Lipid Therapy Regimen) study. J Am Coll Cardiol. 2006;48:396–401. doi: 10.1016/j.jacc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 68.May HT, Anderson JL, Pearson RR, et al. Comparison of effects of simvastatin alone versus fenofibrate alone versus simvastatin plus fenofibrate on lipoprotein subparticle profiles in diabetic patients with mixed dyslipidemia (from the Diabetes and Combined Lipid Therapy Regimen study) Am J Cardiol. 2008;101:486–489. doi: 10.1016/j.amjcard.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 69.Stefanutti C, Bucci A, Di Giacomo S, et al. Efficacy, safety and tolerability of combined low-dose simvastatin-fenofibrate treatment in primary mixed hyperlipidaemia. Clin Drug Investig. 2004;24:465–477. doi: 10.2165/00044011-200424080-00005. [DOI] [PubMed] [Google Scholar]
- 70.Kayikçioğlu M, Ozerkan F, Soydan I. Effectiveness and safety of alternate-day simvastatin and fenofibrate on mixed hyperlipidemia. Am J Cardiol. 1999;83:1135–1137. A9. doi: 10.1016/s0002-9149(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 71.Foucher C, Aubonnet P, Reichert P, et al. New fixed-dose combinations of fenofibrate/simvastatin therapy significantly improve the lipid profile of high-risk patients with mixed dyslipidemia versus monotherapies. Cardiovasc Ther. 2015;33:329–337. doi: 10.1111/1755-5922.12148. [DOI] [PubMed] [Google Scholar]
- 72.Shah HD, Parikh KH, Chag MC, et al. Beneficial effects of the addition of fenofibrate to statin therapy in patients with acute coronary syndrome after percutaneous coronary interventions. Exp Clin Cardiol. 2007;12:91–96. [PMC free article] [PubMed] [Google Scholar]
- 73.Sahebkar A, Serban MC, Mikhailidis DP, et al. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: a systematic review and meta-analysis. Pharmacol Res. 2016;103:236–252. doi: 10.1016/j.phrs.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Xu QY, Liu YH, Zhang Q, et al. Metabolomic analysis of simvastatin and fenofibrate intervention in high-lipid diet-induced hyperlipidemia rats. Acta Pharmacol Sin. 2014;35:1265–1273. doi: 10.1038/aps.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krysiak R, Gdula-Dymek A, Okopien B. Effect of simvastatin and fenofibrate on cytokine release and systemic inflammation in type 2 diabetes mellitus with mixed dyslipidemia. Am J Cardiol. 2011;107:1010–1018. doi: 10.1016/j.amjcard.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 76.Bannwarth B. Drug-induced myopathies. Expert Opin Drug Saf. 2002;1:65–70. doi: 10.1517/14740338.1.1.65. [DOI] [PubMed] [Google Scholar]
- 77.Tomaszewski M, Stępień KM, Tomaszewska J, Czuczwar SJ. Statin-induced myopathies. Pharmacol Rep. 2011;63:859–866. doi: 10.1016/s1734-1140(11)70601-6. [DOI] [PubMed] [Google Scholar]
- 78.Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374:664–669. doi: 10.1056/NEJMra1515161. [DOI] [PubMed] [Google Scholar]
- 79.Calderon RM, Cubeddu LX, Goldberg RB, Schiff ER. Statins in the treatment of dyslipidemia in the presence of elevated liver aminotransferase levels: a therapeutic dilemma. Mayo Clin Proc. 2010;85:349–356. doi: 10.4065/mcp.2009.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pettersen JC, Pruimboom-Brees I, Francone OL, Amacher DE, Boldt SE, Kerlin RL, Ballinger WE. The PPARα agonists fenofibrate and CP-778875 cause increased β-oxidation, leading to oxidative injury in skeletal and cardiac muscle in the rat. Toxicol Pathol. 2012;40:435–447. doi: 10.1177/0192623311431945. [DOI] [PubMed] [Google Scholar]
- 81.Balfour JA, McTavish D, Heel RC. Fenofibrate. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in dyslipidaemia. Drugs. 1990;40:260–290. doi: 10.2165/00003495-199040020-00007. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi A, Suzuki Y, Kuno H, Sugai S, Sakakibara H, Shimoi K. Effects of fenofibrate on plasma and hepatic transaminase activities and hepatic transaminase gene expression in rats. J Toxicol Sci. 2009;34:377–387. doi: 10.2131/jts.34.377. [DOI] [PubMed] [Google Scholar]
- 83.Patterson AD, Shah YM, Matsubara T, Krausz KW, Gonzalez FJ. Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology. 2012;56:281–290. doi: 10.1002/hep.25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noé J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35:1308–1314. doi: 10.1124/dmd.106.012930. [DOI] [PubMed] [Google Scholar]
- 86.Winsemius A, Ansquer JC, Olbrich M, et al. Pharmacokinetic interaction between simvastatin and fenofibrate with staggered and simultaneous dosing: does it matter? J Clin Pharmacol. 2014;54:1038–1047. doi: 10.1002/jcph.291. [DOI] [PubMed] [Google Scholar]
- 87.Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. Am J Cardiol. 2006;97:77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Guo J, Meng F, Ma N, et al. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyper-lipidemia. Am J Cardiol. 2012;110:1296–1301. doi: 10.1016/j.amjcard.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 89.Food and Drug Administration FDA drug safety communication: review update of Trilipix (fenofibric acid) and the ACCORD Lipid trial. Nov 9, 2011. [Accessed June 22, 2016]. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm278837.html.
- 90.Curtin PO, Jones WN. Therapeutic rationale of combining therapy with gemfibrozil and simvastatin. J Am Pharm Assoc. 2007;47:140–146. doi: 10.1331/EN88-W778-261P-3134. [DOI] [PubMed] [Google Scholar]
- 91.Horsmans Y, Desager JP, Harvengt C. Effects of combined bezafibrate-simvastatin appraised in healthy subjects. J Clin Pharmacol. 1992;32:422–426. doi: 10.1002/j.1552-4604.1992.tb03857.x. [DOI] [PubMed] [Google Scholar]
- 92.Kehely A, MacMahon M, Barbir M, Wray R, Hunt BJ, Prescott RJ, Thompson GR. Combined bezafibrate and simvastatin treatment for mixed hyperlipidaemia. QJM. 1995;88:749. [PubMed] [Google Scholar]
- 93.Farnier M, Dejager S. Effect of combined fluvastatin-fenofibrate therapy compared with fenofibrate monotherapy in severe primary hypercholesterolemia. French Fluvastatin Study Group. Am J Cardiol. 2000;85:53–57. doi: 10.1016/s0002-9149(99)00606-2. [DOI] [PubMed] [Google Scholar]
- 94.Farnier M, Steinmetz A, Retterstøl K, Császár A. Fixed-dose combination fenofibrate/pravastatin 160/40 mg versus simvastatin 20 mg monotherapy in adults with type 2 diabetes and mixed hyperlipidemia uncontrolled with simvastatin 20 mg: a double-blind, randomized comparative study. Clin Ther. 2011;33:1–12. doi: 10.1016/j.clinthera.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 95.Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25:1198–202. doi: 10.2337/diacare.25.7.1198. [DOI] [PubMed] [Google Scholar]
- 96.Chen YP, Chang KC, Tseng WK, Yin WH, Chen JW, Lee YT, Wu CC. Increased rosuvastatin dose versus concomitant fenofibrate and rosuvastatin therapy to achieve lipid goal in patients with diabetes or atherosclerosis with metabolic syndrome. Zhonghua Minguo Xin Zang Xue Hui Za Zhi. 2013;29:421–428. [PMC free article] [PubMed] [Google Scholar]
- 97.Kei A, Liberopoulos E, Elisaf M. Effect of hypolipidemic treatment on glycemic profile in patients with mixed dyslipidemia. World J Diabetes. 2013;4:365–371. doi: 10.4239/wjd.v4.i6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vega GL, Ma PT, Cater NB, Filipchuk N, Meguro S, Garcia-Garcia AB, Grundy SM. Effects of adding fenofibrate (200 mg/day) to simvastatin (10 mg/day) in patients with combined hyperlipidemia and metabolic syndrome. Am J Cardiol. 2003;91:956–960. doi: 10.1016/s0002-9149(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 99.Ledl M, Hohenecker J, Francesconi C, Roots I, Bauer MF, Roden M. Acute myopathy in a type 2 diabetic patient on combination therapy with metformin, fenofibrate and rosiglitazone. Diabetologia. 2005;48:1996–1998. doi: 10.1007/s00125-005-1919-8. [DOI] [PubMed] [Google Scholar]
- 100.Chertow BS, Motto GS, Shah JH. A biochemical profile of abnormalities in hypothyroidism. Am J Clin Pathol. 1974;61:785–788. doi: 10.1093/ajcp/61.6.785. [DOI] [PubMed] [Google Scholar]
- 101.Sindoni A, Rodolico C, Pappalardo MA, Portaro S, Benvenga S. Hypothyroid myopathy: a peculiar clinical presentation of thyroid failure. Review of the literature. Rev Endocr Metab Disord. 2016 May 7; doi: 10.1007/s11154-016-9357-0. Epub. [DOI] [PubMed] [Google Scholar]
- 102.Kiernan TJ, Rochford M, McDermott JH. Simvastatin induced rhab-domyolysis and an important clinical link with hypothyroidism. Int J Cardiol. 2007;119:374–376. doi: 10.1016/j.ijcard.2006.07.233. [DOI] [PubMed] [Google Scholar]
- 103.Qari FA. Severe rhabdomyolysis and acute renal failure secondary to the use of simvastatin in undiagnosed hypothyroidism. Indian J Nephrol. 2008;18:28–29. doi: 10.4103/0971-4065.41287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Clouâtre Y, Leblanc M, Ouimet D, Pichette V. Fenofibrate-induced rhabdomyolysis in two dialysis patients with hypothyroidism. Nephrol Dial Transplant. 1999;14:1047–1048. doi: 10.1093/ndt/14.4.1047. [DOI] [PubMed] [Google Scholar]
- 105.Feeney ER, Mallon PWG. HIV and HAART-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:49–63. doi: 10.2174/1874192401105010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ehnholm C, Aho K, Huttunen JK, Kostiainen E, Mattila K, Pakkarainen J, Cantell K. Effect of interferon on plasma lipoproteins and on the activity of post heparin plasma lipases. Arteriosclerosis. 1982;2:68–73. doi: 10.1161/01.atv.2.1.68. [DOI] [PubMed] [Google Scholar]
- 107.Vu CN, Ruiz-Esponda R, Yang E, et al. Altered relationship of plasma triglycerides to HDL cholesterol in patients with HIV/HAART-associated dyslipidemia: further evidence for a unique form of metabolic syndrome in HIV patients. Metabolism. 2013;62:1014–1020. doi: 10.1016/j.metabol.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lum YD, He JG, Slatter JG, et al. Gene expression profiling of rat liver reveals a mechanistic basis for ritonavir-induced hyperlipidemia. Genomics. 2007;90:464–473. doi: 10.1016/j.ygeno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Penzak SR, Chuck SK. Management of protease inhibitor-associated hyperlipidemia. Am J Cardiovasc Drugs. 2002;2:91–106. doi: 10.2165/00129784-200202020-00003. [DOI] [PubMed] [Google Scholar]
- 110.Visnegarwala F, Maldonado M, Sajja P, et al. Lipid lowering effects of statins and fibrates in the management of HIV dyslipidemias associated with antiretroviral therapy in HIV clinical practice. J Infect. 2004;49:283–290. doi: 10.1016/j.jinf.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 111.Husain NE, Ahmed MH. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV AIDS. 2014;7:1–10. doi: 10.2147/HIV.S46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol. 2015;115:1760–1766. doi: 10.1016/j.amjcard.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 113.Kavey RE. Combined dyslipidemia in childhood. J Clin Lipidol. 2015;9(5 Suppl):S41–S56. doi: 10.1016/j.jacl.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 114.Stefanutti C, Lucani G, Vivenzio A, Di Giacomo S. Diet only and diet plus simvastatin in the treatment of heterozygous familial hypercholesterolemia in childhood. Drugs Exp Clin Res. 1999;25:23–28. [PubMed] [Google Scholar]
- 115.Steinmetz J, Morin C, Panek E, Siest G, Drouin P. Biological variations in hyperlipidemic children and adolescents treated with fenofibrate. Clin Chim Acta. 1981;112:43–53. doi: 10.1016/0009-8981(81)90267-9. [DOI] [PubMed] [Google Scholar]