Abstract
Crucial to the management of staphylococcal bacteremia is an accurate evaluation of associated endocarditis, which has both therapeutic and prognostic implications. Because the clinical presentation of endocarditis can be nonspecific, the judicious use of echocardiography is important in distinguishing patients at high risk of developing endocarditis. In the presence of high-risk clinical features, an early transesophageal echocardiogram is warranted without prior transthoracic echocardiography.
The purpose of this study was to investigate the clinical risk factors for staphylococcal infective endocarditis that might warrant earlier transesophageal echocardiography and to describe the incidence of endocarditis in cases of methicillin-resistant and methicillin-sensitive Staphylococcus aureus bacteremia.
A retrospective case-control study was conducted by means of chart review of 91 patients consecutively admitted to a community hospital from January 2009 through January 2013. Clinical risk factors of patients with staphylococcal bacteremia were compared with risk factors of patients who had definite diagnoses of infective endocarditis. There were 69 patients with bacteremia alone (76%) and 22 patients with endocarditis (24%), as verified by echocardiography. Univariate analysis showed that diabetes mellitus (P=0.024), the presence of an automatic implantable cardioverter-defibrillator/pacemaker (P=0.006) or a prosthetic heart valve (P=0.003), and recent hospitalization (P=0.048) were significantly associated with developing infective endocarditis in patients with S. aureus bacteremia. The incidence of methicillin-resistant and methicillin-sensitive S. aureus bacteremia was similar in the bacteremia and infective-endocarditis groups (P=0.437).
In conclusion, identified high-risk clinical factors in the presence of bacteremia can suggest infective endocarditis. Early evaluation with transesophageal echocardiography might well be warranted.
Keywords: Echocardiography, transesophageal/utilization; endocarditis, bacterial; retrospective studies; risk factors; Staphylococcus aureus; staphylococcal infections
Infective endocarditis (IE) can be life-threatening in the absence of timely and appropriate management. Staphylococcus aureus has emerged as the most frequent cause of IE, and its prognosis is worse than those associated with other microorganisms.1–5 Staphylococcus aureus IE (SAIE) is a common community-acquired infection; however, cases have also been occurring in the healthcare setting in recent years.6
Nearly every patient with S. aureus bacteremia (SAB) faces the possibility of associated endocarditis, yet only a minority of SAB patients experience cardiac involvement. Often it is difficult to distinguish patients with SAIE from those with uncomplicated SAB.7 The prevalence of SAIE among patients with SAB varies from 13% to 25%.8 Investigators have shown that, in patients with SAB of unknown origin, a valvular prosthesis, persistent fever, and persistent bacteremia are independently associated with SAIE.9 However, the clinical risk factors that predispose SAB patients to IE need further elucidation.
Current guidelines from the Infectious Diseases Society of America10 suggest that echocardiography, preferably TEE, be applied in all cases of SAB. However, one study11 suggested that echocardiography might be “dispensable” in cases of uncomplicated community-associated and nosocomial SAB. We also compared the incidence of IE among patients with methicillin-sensitive (MSSA) versus patients with methicillin-resistant (MRSA) S. aureus bacteremia. In a previous study,12 community MSSA and nosocomial MRSA were the most frequent causes of the community and MRSA endocarditis, respectively. We ourselves investigated the clinical risk factors associated with a higher incidence of SAIE in SAB patients who had either MSSA or MRSA bacteremia, with the intent of stratifying those patient populations to determine who will benefit from early transesophageal echocardiography (TEE).
Patients and Methods
Our data were gathered from inpatient electronic medical records of the Queens Hospital Center (QHC) in Jamaica, NY. The QHC is a member of New York City Health and Hospital Corporation and Queens Health Network and is an affiliate of the Icahn School of Medicine at Mt. Sinai. It is a major acute-care community hospital in the southeast and central Queens area with 293 beds, which averaged 14,000 admissions, 99,000 emergency visits, and 330,000 clinic visits in 2013. It serves a culturally and economically diverse population in New York City.
Our study population was identified by retrospective chart review. Patients' medical records were systematically screened for study eligibility. Included subjects were adult patients older than 18 years of age with at least one positive blood culture for MSSA or MRSA, whether community- or hospital-acquired; whether or not diagnosed with IE as defined by the revised Duke criteria13; and whether supported by transthoracic echocardiography (TTE), TEE, or both. The institution's standard of practice was to obtain at least 2 sets of blood cultures when IE was clinically suspected. Only those patients who met the inclusion criteria from 1 January 2009 through 1 January 2013 were included in the retrospective cohort study, which comprised 2 subgroups: patients with SAB and definite IE, and patients with SAB only. Patients with polymicrobial bacteremia were included, provided that S. aureus had been isolated in the qualifying blood-culture specimen. Patients with equivocal diagnoses of IE were excluded.
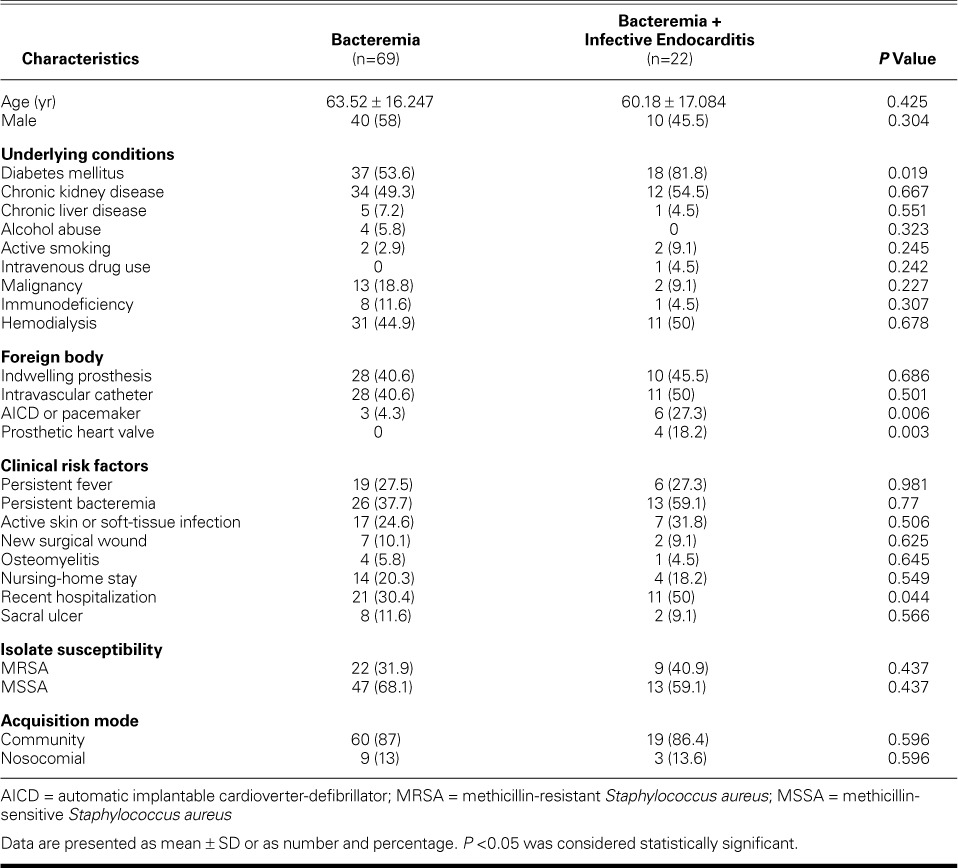
Data collected consisted of age and sex, underlying chronic medical comorbidities, the presence of foreign bodies, clinical risk factors, isolate susceptibility (MRSA or MSSA), and acquisition of community- or hospital-acquired bacteremia (Table I). Chronic liver disease was defined as chronic hepatitis, liver cirrhosis, or hepatocellular carcinoma. Chronic renal insufficiency implied an elevated serum creatinine level of ≥2 mg/dL as a baseline for at least 3 months, with structural or functional evidence of renal injury. Immunodeficiency referred to human immunodeficiency virus, steroid therapy and chemotherapy for malignancy, and autoimmune diseases or immunosuppressive therapy for organ transplantation or autoimmune diseases. Persistent fever or bacteremia was defined as fever (temperature, >38 °C/100.4 °F) or positive blood cultures at longer than 48 hours after initiation of adequate antimicrobial therapy. The mode of acquisition of SAB was considered nosocomial bacteremia, provided that the bacteremia in hospitalized patients had occurred 48 hours or longer after admission.
TABLE I.
Baseline Characteristics of the 91 Patients
The study protocol was reviewed and unanimously approved by both the Institutional Review Board committee of the Icahn School of Medicine at Mt. Sinai and the local research committee of the QHC.
The primary outcomes of interest were identification of clinical risk factors for IE in patients with MSSA or MRSA bacteremia and the estimation of the incidence of IE in MSSA and MRSA bacteremia.
Statistical Analysis
Baseline statistical analysis was performed with use of the χ2 and Fisher exact tests for categorical variables, and the t test for continuous variables. Descriptive statistics were expressed as mean ± SD for continuous variables and as number and percentage for categorical variables. Logistic regression analysis was used to calculate the odds ratio for the risk factors for IE. In the logistic regression analysis, the clinical risk factors were considered independent variables, and the presence of bacteremia or bacteremia with IE was considered a dependent variable. A P value <0.05 was considered statistically significant. We used SPSS version 22.0 (IBM Corporation; Armonk, NY) for the statistical analysis.
Results
Of the 197 patients who were screened, 91 (46%) were eligible for inclusion in the study. The patients were categorized into 2 groups: the bacteremia group composed of 69 patients (76%), and the bacteremia with IE group composed of 22 patients (24%). Except for 2 cases, all patients in the bacteremia group had completed echocardiography. All 22 cases of IE were verified with echocardiography. Patients were excluded because of the absence of SAB; the primary isolation of different species of Staphylococcus, such as S. epidermidis; and insufficient clinical data. Of the 106 excluded patients, 23 patients (22%) were categorized as having possible endocarditis. Twenty-five patients (27%) died in the hospital.
The mean ages for both groups of patients with bacteremia and bacteremia with IE were 63.52 ± 16.25 and 60.18 ± 17.08 years, respectively (P=0.425). Fifty-eight percent of patients with bacteremia were male, and 46% had bacteremia with IE (P=0.304). Diabetes mellitus (DM) was more prevalent in patients with bacteremia and IE than in the bacteremia group (82% vs 54%; P=0.019). Furthermore, the odds of developing IE in diabetic patients were 3.8 times greater than the odds of developing bacteremia alone (P=0.024). The frequency of dialysis did not differ between the 2 subgroups (45% in bacteremia vs 50% in bacteremia + IE; P=0.678). These subgroups of patients had no marked differences in such baseline medical comorbidities as chronic kidney or liver disease, alcohol abuse, active smoking, intravenous-drug use, malignancy, and immunodeficiency.
However, patients with IE were more likely to have an automatic implantable cardioverter-defibrillator (AICD) or pacemaker (P=0.006), or a prosthetic heart valve (P=0.003). In terms of clinical risk factors, persistent fever (P=0.98), persistent bacteremia (P=0.77), soft-tissue infection (P=0.506) or surgical wound (P=0.625), osteomyelitis (P=0.645), nursing-home stay (P=0.549), and sacral ulcer (P=0.566) did not distinguish between SAB and endocarditis. Recent hospitalization (within 8 wk) was significantly associated with acquiring bacteremia and IE (P=0.044). Only one patient was identified as an intravenous-drug user in the bacteremia and IE group. The rates of MRSA and MSSA isolation were similar in both groups, as was the mode of acquisition (community- or nosocomial-acquired). The demographics are shown in Table I.
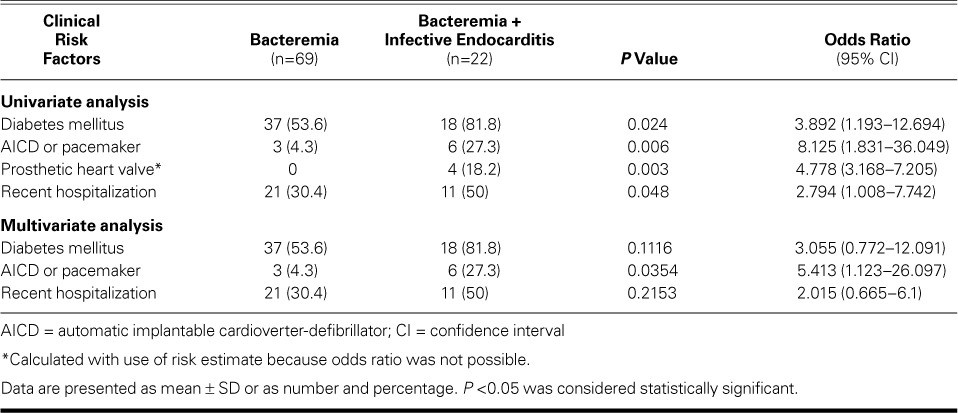
Univariate analysis identified the clinical risk factors significantly associated with IE: the presence of DM (P=0.024), an AICD or pacemaker (P=0.006), prosthetic heart valve (P=0.003); and recent hospitalization (P=0.048). The risk of prosthetic heart valves was considered relative because no patient in the bacteremia group had one. Table II shows the odds ratios for the clinical risk factors. Upon multivariate analysis, an AICD or pacemaker appears to be significantly associated with IE, after adjusting for the presence of DM and recent hospitalization.
TABLE II.
Univariate and Multivariate Analysis of Clinical Risk Factors for Infective Endocarditis
Discussion
Distinguishing the presence of endocarditis from mere SAB has both therapeutic and prognostic implications.14 In this retrospective case-control study in an acute-care community hospital, 24% (22/91) of patients consecutively identified from the medical records within a 4-year period were found to have staphylococcal endocarditis. In contrast to the results in our study, IE was diagnosed in 4.3% and 9.3% of patients with SAB in the “Invasive Staphylococcus aureus Infection Cohort” (INSTINCT) and the “Staphylococcus aureus Bacteremia Group” (SABG), respectively.15 The rising incidence of SAB could be attributed to the extensive use of vascular-access devices, invasive diagnostic and therapeutic procedures, prosthetic cardiovascular devices, and an aging population with multiple complicated medical comorbidities.16 As a consequence of the increasing incidence of SAB and its complications in the United States and in some European countries, there has been a substantial economic burden on healthcare systems.17
In the present study, univariate analysis of risk factors has identified DM, a prosthetic heart valve, an AICD or pacemaker, and recent hospitalization with the development of IE among patients with SAB. It has been reported that the presence of valvular prostheses9 and permanent intracardiac devices (such as a prosthetic valve, pacemaker, or AICD)15 were independently associated with the development of SAIE in patients with SAB. It is of note that DM and a history of recent hospitalization (within 8 wk) proved to be significantly associated with the development of endocarditis among patients with SAB. This finding is in stark contrast with the prospective observational study by Chang and colleagues,14 the results of which showed that DM was not a significant risk factor for endocarditis. Whereas it has been shown that persistent fever and bacteremia are closely linked to increased risk of IE,9,18 the findings of the present study do not suggest such a significant association, which can be explained by the relatively small sample size and by the composition of the study population. The varying epidemiology of SAB reported in the previous literature is highly influenced by the population served by the hospital, especially by the proportion of dialysis patients, intravenous-drug users, and immunocompromised patients.14
On multivariate analysis of the patients in our study, the presence of an AICD or pacemaker seems to suggest a higher risk of associated IE. However, the limited sample size in our study precludes definitive conclusion. A more robust sample size could elucidate the association between clinical risk factors and risk of IE.
In our sample, staphylococcal endocarditis developed in 50% of patients on hemodialysis (11/22), which is comparatively higher than was reported in a multicenter observational study showing endocarditis to be associated with 12% (11/95) of SAB cases in hemodialysis patients.14 The use of a central venous catheter has been implicated as one of the most significant risk factors for both nosocomial SAB and SAIE (whether nosocomial or community-acquired).19,20 It was suggested that hemodialysis patients are at increased risk of IE because of frequent use of percutaneous access for dialysis, increased calcium deposition in the cardiac valves (predisposing to valvular defect), and a high incidence of patients who are nasal carriers of S. aureus.21,22
Although not statistically significant, MSSA was isolated more often in both of our groups of patients than was MRSA. Previous investigators did not observe that sensitivity to methicillin was a risk factor for IE.23 Yet one group24 did report that MSSA is a risk factor for IE, when compared with MRSA.
The present study has provided insights into the clinical risk factors that one should consider in performing echocardiography in patients with SAB; however, a definitive conclusion cannot be reached because of the small sample size. Nevertheless, the findings of the study appear to suggest a possibly higher risk of IE in patients with staphylococcal bacteremia in the presence of intracardiac devices, such as a prosthetic valve. We have also shown that DM and a history of recent hospitalization might be predictive of endocarditis. In consideration of these high-risk clinical features, one must consider TEE without the need of prior TTE.
Limitations of the Study
This study has several limitations that might affect broad application of its findings.
First, our sample size is relatively small and is confined to one acute-care community hospital. Cognizant of that fact, we included in the analysis all patients who met the inclusion criteria. Nevertheless, our study contributes to the growing body of literature that attempts to identify subgroups of patients with staphylococcal bacteremia and associated IE, who should need earlier echocardiographic evaluation. It must be acknowledged that selection bias might have influenced the results of the study. In retrospective studies, the sample size is dependent upon the available data. The breadth of the gathered data is also subject to the accuracy of the secondary information documented in the charts.
Second, TEE was not always performed in the SAB patients: endocarditis cases could have been missed, and we might have underestimated the true incidence of staphylococcal endocarditis. It has been suggested by Incani and colleagues25 that a considerable percentage of IE in SAB cases is not clinically evident, which necessitates examination with TEE to improve sensitivity in the detection of endocarditis.
Third, the use of TTE or TEE was not based on the common algorithms followed by clinicians. This could lead to the unintended exclusion of some potential IE cases.
Fourth, our study varied the timing of the echocardiography, which (if performed very early) might cause a missed diagnosis.
Finally, the lack of long-term follow-up data to evaluate the possible recurrence of IE and the eventual outcome is pertinent in understanding whether the clinical risk factors have influenced the morbidity and mortality rates of the SAIE patients.
Summary
Echocardiographic screening is recommended in patients with SAB. The presence of DM, intracardiac devices, prosthetic valves, and recent hospitalization is significantly associated with developing endocarditis in patients with SAB. In the presence of these risk factors in SAB patients, we recommend the performance of screening echocardiography—preferably TEE, because it has higher diagnostic accuracy than does TTE. However, TTE might be used for first-line screening in the absence of high-risk clinical features and other clinical evidence of IE in patients with SAB. Future studies are warranted to elucidate the optimal time for screening echocardiography and to analyze the cost versus the benefit of performing TTE before TEE in diagnosing endocarditis.
Acknowledgment
The authors thank Ms Media Oliver of QHC for assistance in gathering the data.
References
- 1. Mouly S, Ruimy R, Launay O, Arnoult F, Brochet E, Trouillet JL, . et al. The changing clinical aspects of infective endocarditis: descriptive review of 90 episodes in a French teaching hospital and risk factors for death. J Infect 2002; 45( 4): 246– 56. [DOI] [PubMed] [Google Scholar]
- 2. Loupa C, Mavroidi N, Boutsikakis I, Paniara O, Deligarou O, Manoli H, Saroglou G. . Infective endocarditis in Greece: a changing profile. Epidemiological, microbiological and therapeutic data. Clin Microbiol Infect 2004; 10( 6): 556– 61. [DOI] [PubMed] [Google Scholar]
- 3. Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, . et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293( 24): 3012– 21. [DOI] [PubMed] [Google Scholar]
- 4. Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. . Infective endocarditis: changing epidemiology and predictors of 6 month mortality: a prospective cohort study. Eur Heart J 2007; 28( 2): 196– 203. [DOI] [PubMed] [Google Scholar]
- 5. Cabell CH, Abrutyn E, Fowler VG Jr, Hoen B, Miro JM, Corey GR, . et al. Use of surgery in patients with native valve infective endocarditis: results from the International Collaboration on Endocarditis Merged Database. Am Heart J 2005; 150( 5): 1092– 8. [DOI] [PubMed] [Google Scholar]
- 6. Finkelstein R, Agmon Y, Braun E, Kassis I, Sprecher H, Raz A, . et al. Incidence and risk factors for endocarditis among patients with health care-associated Staphylococcus aureus bacteraemia. Scand J Infect Dis 2012; 44( 12): 934– 40. [DOI] [PubMed] [Google Scholar]
- 7. Petti CA, Fowler VG Jr. . Staphylococcus aureus bacteremia and endocarditis. Infect Dis Clin North Am 2002; 16( 2): 413– 35, x– xi. [DOI] [PubMed] [Google Scholar]
- 8. Mylonakis E, Calderwood SB. . Infective endocarditis in adults. N Engl J Med 2001; 345( 18): 1318– 30. [DOI] [PubMed] [Google Scholar]
- 9. Hill E, Vanderschueren S, Verhaegen J, Herijgers P, Claus P, Herregods MC, Peetermans WE. . Risk factors for infective endocarditis and outcome of patients with Staphylococcus aureus bacteremia. Mayo Clin Proc 2007; 82( 10): 1165– 9. [DOI] [PubMed] [Google Scholar]
- 10. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, . et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52( 3): 285– 92. [DOI] [PubMed] [Google Scholar]
- 11. Khatib R, Sharma M. . Echocardiography is dispensable in uncomplicated Staphylococcus aureus bacteremia. Medicine (Baltimore) 2013; 92( 3): 182– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. . Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J 2004; 147( 3): 536– 9. [DOI] [PubMed] [Google Scholar]
- 13. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, . et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America [published errata appear in Circulation 2005;112(15):2373; Circulation 2007;115(15):e408; Circulation 2007;116(21):e547; Circulation 2008;118(12):e497]. Circulation 2005; 111( 23): e394– 434. [DOI] [PubMed] [Google Scholar]
- 14. Chang FY, MacDonald BB, Peacock JE Jr, Musher DM, Triplett P, Mylotte JM, . et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82( 5): 322– 32. [DOI] [PubMed] [Google Scholar]
- 15. Kaasch AJ, Fowler VG Jr, Rieg S, Peyerl-Hoffmann G, Birkholz H, Hellmich M, . et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53( 1): 1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palraj BR, Sohail MR. . Appropriate use of echocardiography in managing Staphylococcus aureus bacteremia. Expert Rev Anti Infect Ther 2012; 10( 4): 501– 8. [DOI] [PubMed] [Google Scholar]
- 17. Naber CK. . Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 2009; 48 Suppl 4: S231– 7. [DOI] [PubMed] [Google Scholar]
- 18. Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. . Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. Arch Intern Med 2007; 167( 17): 1861– 7. [DOI] [PubMed] [Google Scholar]
- 19. Jensen AG, Wachman CH, Poulsen KB, Espersen F, Scheibel J, Skinhoj P, Frimodt-Moller N. . Risk factors for hospital-acquired Staphylococcus aureus bacteremia. Arch Intern Med 1999; 159( 13): 1437– 44. [DOI] [PubMed] [Google Scholar]
- 20. Benito N, Miro JM, de Lazzari E, Cabell CH, del Rio A, Altclas J, . et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med 2009; 150( 9): 586– 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanslik T, Flahault A, Vaillant JN, Boulard JC, Moulonguet-Doleris L, Prinseau J, Baglin A. . High risk of severe endocarditis in patients on chronic dialysis. Nephrol Dial Transplant 1997; 12( 6): 1301– 2. [DOI] [PubMed] [Google Scholar]
- 22. Yu VL, Goetz A, Wagener M, Smith PB, Rihs JD, Hanchett J, Zuravleff JJ. . Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med 1986; 315( 2): 91– 6. [DOI] [PubMed] [Google Scholar]
- 23. Harbarth S, Rutschmann O, Sudre P, Pittet D. . Impact of methicillin resistance on the outcome of patients with bacteremia caused by Staphylococcus aureus. Arch Intern Med 1998; 158( 2): 182– 9. [DOI] [PubMed] [Google Scholar]
- 24. Joseph JP, Meddows TR, Webster DP, Newton JD, Myerson SG, Prendergast B, . et al. Prioritizing echocardiography in Staphylococcus aureus bacteremia. J Antimicrob Chemother 2013; 68( 2): 444– 9. [DOI] [PubMed] [Google Scholar]
- 25. Incani A, Hair C, Purnell P, O'Brien DP, Cheng AC, Appelbe A, Athan E. . Staphylococcus aureus bacteraemia: evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis 2013; 32( 8): 1003– 8. [DOI] [PubMed] [Google Scholar]


