Abstract
Adults who underwent complex atrial baffling as children via Mustard or Senning procedures are at heightened risk for atrial arrhythmias. Antiarrhythmic therapies are typically ineffective in this population. Accordingly, our team of pediatric and adult electrophysiologists investigated the effectiveness of early invasive transbaffle-access techniques to perform early radiofrequency ablation at the source of these clinically significant arrhythmias.
For this retrospective study, we selected 11 adult survivors of atrial baffling (mean age, 34 ± 9 yr) who underwent clinically indicated electrophysiologic study after no more than one trial of antiarrhythmic therapy. Using transbaffle-access techniques and 3-dimensional mapping of the venous atria, we found 12 inducible arrhythmias in 10 patients: intra-atrial reentrant tachycardia (n=6), atrioventricular nodal reentrant tachycardia (n=3), focal atrial tachycardia (n=2), and repetitive double firing of the atrioventricular node (n=1). Defining success as short- and midterm freedom from arrhythmia, we analyzed outcomes of radiofrequency ablation at 1 and 6 months.
At 1 month, ablation was 100% successful. At 6 months, after 11 ablations in 9 patients, 5 patients had no clinical recurrence, 2 had improved arrhythmia control from minimal medical therapy, and 2 were to undergo repeat study for recurrent tachycardia. In the recurrence-free patients, arrhythmias during electrophysiology study matched the types found clinically before the study.
To our knowledge, this is the largest one-year cohort of adult survivors of atrial baffling to have undergone study by a combined pediatric–adult electrophysiology team. We conclude that early invasive transbaffle access for ablating diverse atrial tachyarrhythmias was effective in these patients.
Keywords: Arrhythmias, cardiac/epidemiology/etiology; body surface potential mapping/methods; catheter ablation/methods; electrophysiologic techniques, cardiac; postoperative complications; recurrence; retrospective studies; tachycardia/etiology; transposition of great vessels/complications/physiopathology/surgery; treatment outcome
Interatrial baffle procedures, such as Mustard or Senning operations, were the standard of care in the mid- to late 20th century for patients born with dextro-transposition of the great arteries (D-TGA). The surgical process of redirecting systemic and pulmonary venous circulation with complex baffling in the atria is a known risk factor for late atrial tachyarrhythmias.1 Newer surgical techniques, such as the atrial switch, have supplanted interatrial baffle operations.
The substrate for late atrial tachyarrhythmia has historically been reported as atrial flutter or intra-atrial reentrant tachycardia (IART).1 Investigators have since shown that atrioventricular (AV) nodal reentrant tachycardia (AVNRT) and nonautomatic focal atrial tachycardia (NAFAT) are also prevalent.3,4 (Nonautomatic FAT is defined as an arrhythmia inducible with pacing maneuvers and presumably caused by microreentry or by triggered mechanisms.) Original attempts at radiofrequency ablation (RFA) in these patients were reasonably successful.5 The increasing frequency of RFA has engendered newer transbaffle access (TBA) techniques that enable access to the pulmonary venous atrium (PVA).6,7 The goal is to offer more lasting control of atrial arrhythmias.
Our institution has experienced a notable increase in adult Mustard and Senning survivors who present with clinically significant, recurrent atrial arrhythmias. Antiarrhythmic agents are relatively ineffective and can have undesirable side effects. Using a strategy of early RFA intervention (after ≤1 trial of antiarrhythmic therapy) with atrial mapping, our team of adult and pediatric electrophysiologists studied short- and midterm RFA outcomes in adults with a prior Mustard or Senning procedure who presented at our institution over a period of one year for clinically significant arrhythmias.
Patients and Methods
In this retrospective review approved by the local institutional review board, we included sequential adult patients with a childhood diagnosis of D-TGA and prior atrial baffling (Mustard or Senning procedure) who had undergone a clinically indicated electrophysiologic study (EPS) at our institution from April 2013 through March 2014. Data on age, sex, arrhythmia history, prescribed medications, and functional class were collected, and descriptive statistics were generated (Table I). Information specific to the EPS, including inducible arrhythmia, RFA treatment, and complications, were also collected. For all patients, we compiled descriptive and applicable procedural data at baseline, at the time of EPS, and at 1 and 6 months after EPS. If a patient underwent repeat EPS during the review period or up to 6 months after the first EPS, we documented the subsequent study. We established postprocedural short-term outcomes at one month and midterm outcomes at 6 months.
TABLE I.
Baseline Characteristics of the 11 Patients
Methods. All procedures were performed by a combined team of pediatric and adult electrophysiologists who used TBA techniques. In adults who have atrial baffles, standard catheterization and other techniques to evaluate and treat atrial tachycardia are often unsuccessful. At our center, any adult with D-TGA and a prior atrial switch procedure who will undergo EPS is evaluated by a pediatric (NJK) and adult (SK) electrophysiologist.
According to standard protocol, the pediatric structural team attained access to the PVA by means of echocardiographic guidance and mapped the systemic and pulmonary venous atria. They placed 5 catheters in the adult atrial-switch patient undergoing EPS: in the left atrial appendage (systemic venous atrium), superior vena cava, His bundle, coronary sinus, and right ventricular apex. If present, transbaffle leaks were used for access to the PVA, thereby avoiding a transbaffle puncture to enable TBA. When an intact baffle necessitated TBA, biplane angiography was used to place a standard Brockenbrough curved needle (St. Jude Medical, Inc.; St. Paul, Minn) through a Mullins™ Transseptal Introducer Sheath (Medtronic, Inc.; Minneapolis, Minn). Upon access to the PVA, the sheath was exchanged for an Agilis™ NxT Steerable Sheath (St. Jude Medical) to improve catheter manipulation. After TBA was attained, the systemic venous atrial catheter was advanced to the PVA. Although baffle leaks were often closed when these patients became significantly desaturated, we monitored for active atrial tachyarrhythmias and the necessity of EPS.
Electroanatomic 3-dimensional mapping of the venous atria and baffles was performed with use of the Carto® 3 system (Biosense Webster, a Johnson & Johnson company; Diamond Bar, Calif) and EnSite™ NavX™ (St. Jude Medical). To evaluate electrogram timing and define the anatomy, the catheter was positioned in all major atrial sections. Areas where early and late activation met were used to define the location of the potential macroreentrant circuit. Post-pacing interval mapping was also performed, to confirm the circuit location. A strategy was then devised to create a linear ablation line across a critical isthmus within the circuit. The site of earliest activation enabled focused ablation of NAFAT.
Atrial arrhythmias generated during EPS were defined as macroreentrant or non-macroreentrant atrial tachycardia. Macroreentrant atrial tachycardia (such as IART) was defined by 3 criteria: activation mapping accounting for >90% of the tachycardia cycle length; an early-meets-late pattern along the mitral isthmus or around the left- or right-sided pulmonary veins (in the case of roof-dependent atrial tachycardia); and entrainment mapping of at least 2 opposite segments of the circuit showing a post-pacing interval within 20 ms of the tachycardia cycle length.8 Non-macroreentrant tachycardia was present when most of the cycle length could be accounted for by activation mapping and the reentrant circuit was confined to either the anterior or the posterior wall of the atria and baffle (but not to both of those walls simultaneously), and when entrainment mapping revealed an appropriate post-pacing interval from anywhere along the circuit. The presence of NAFAT was confirmed if mapping showed centrifugal activation from a point source.9
Results
We identified 11 patients with prior atrial baffling (10 who had D-TGA with Mustard correction and one who had levo-TGA with dextroversion and modified Senning correction) and clinical indications for EPS (Table I). The patients' mean age was 34 ± 9 years; 4 were women. All 11 had reported palpitations before EPS; other symptoms were dyspnea in 3 patients, chest pain in 2, and dizziness in 2. The New York Heart Association functional class was 1.6 ± 0.6, and the mean right (systemic) ventricular ejection fraction was 0.37 ± 0.08. Nineteen prior arrhythmias had been documented by means of electrocardiography or 24-hour Holter monitoring; 5 patients had experienced more than one arrhythmia. Eight of those 19 arrhythmias were recorded as IART, 4 as atrial fibrillation, 3 each as supraventricular and ventricular tachycardia, and one as AVNRT. Three patients had been prescribed a conventional class I or III antiarrhythmic medication; the others had been prescribed a β-blocker or a β-blocker and digoxin.
Number and Type of Arrhythmias. We identified 12 clinically significant, inducible arrhythmias in 10 patients (Fig. 1). Patients 2 and 3 underwent 2 studies each; Patient 7's study was canceled because of thyroid storm (Table II). The prevalent inducible arrhythmia was IART (6 instances), followed by AVNRT in 3 and NAFAT in 2; Patient 6 had repetitive double firing of the AV node because of dual AV nodal physiology (Fig. 2).
Fig. 1.
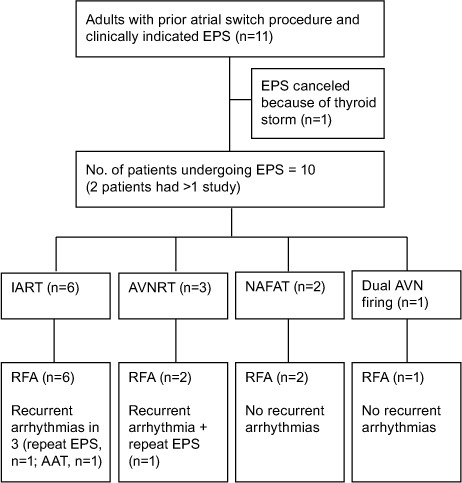
Diagram gives an overview of the study and outcomes of the EPS.
AAT = antiarrhythmic therapy; AVN = atrioventricular node; AVNRT = atrioventricular nodal reentrant tachycardia; AVRT = atrioventricular reciprocating tachycardia; EPS = electrophysiologic study; IART = intra-atrial reentrant tachycardia; NAFAT = nonautomatic focal atrial tachycardia; RFA = radiofrequency ablation
TABLE II.
Electrophysiologic Studies and Outcomes in the 11 Patients
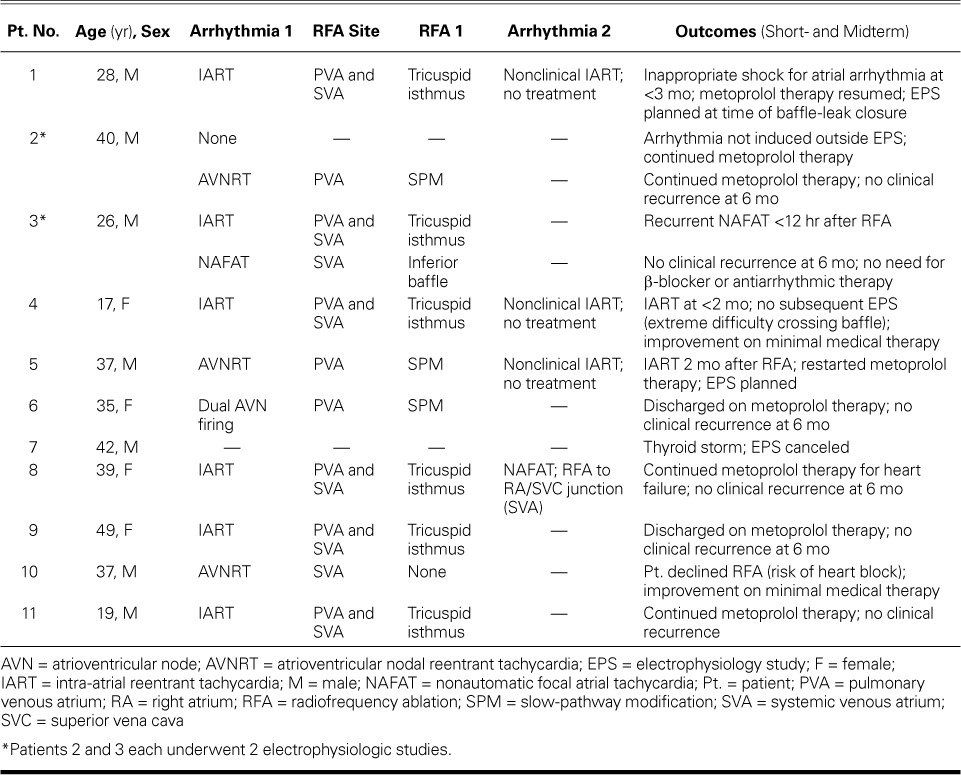
Fig. 2.
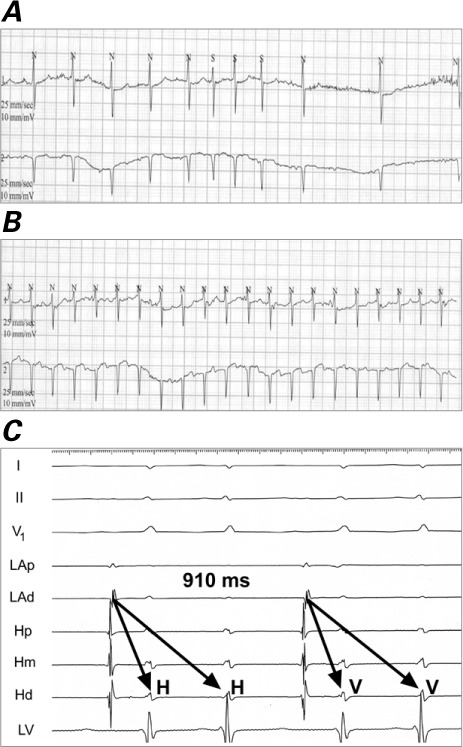
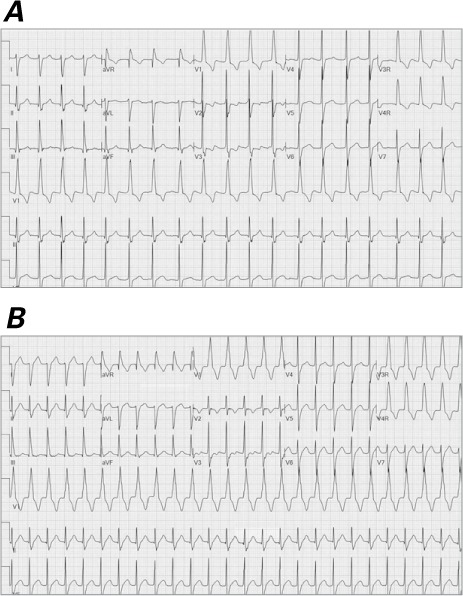
Patient 6. Electrocardiograms show repetitive double firing of the atrioventricular node A) at baseline and B) with ambulation. C) Intracardiac tracing shows 2 His-bundle (H) and ventricular responses (V) for one atrial impulse displaying simultaneous conduction down the fast and slow pathway.
Hd = His distal; Hm = His mid; Hp = His proximal; LAd = left atrium distal; LAp = left atrium proximal; LV = left ventricle; N = normal; S = supraventricular
Atrial Access. In most cases, intact baffles mandated baffle puncture for EPS. In Patients 2 and 5, access to the PVA was attained through a baffle leak. Patient 3 did not need TBA for RFA of an inducible NAFAT. Patient 10, who had AVNRT, was concerned about heart block and declined our recommendation of TBA.
Ablation Sites. The patients with AVNRT needed TBA for ablation of the slow pathway at the PVA. Two instances of NAFAT were ablated upon access to the systemic venous atrium: one at the superior vena cava and right atrial junction (Patient 8), and the other along the inferior baffle (Patient 3). Inducible IART was ablated at the tricuspid isthmus at both the pulmonary (necessary TBA) and systemic venous baffle sites. Figure 3 shows representative Carto images of the venous atria in IART.
Fig. 3.
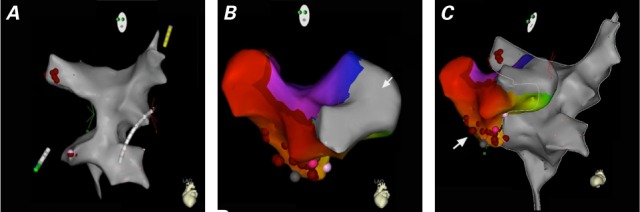
Representative 3-dimensional anatomic mapping of intra-atrial reentrant tachycardia reveals A) the pulmonary venous atrium; B) the tricuspid valve region (arrow) and systemic venous atrium; and C) a complete map of both venous atria, and the red dots (arrow) denoting the line of radiofrequency ablation overlying scar tissue.
Outcomes. In the short term, ablation was successful in all patients (Table II). However, Patient 3, whose IART was ablated, had a recurrent NAFAT—different from the ablated arrhythmia—within 12 hours (Fig. 4). The midterm results of 11 RFAs in 9 patients were as follows: Patients 2, 3, 6, 8, and 9 had no clinical recurrence; Patients 4 and 10 had improved arrhythmia control from minimal medical therapy, and Patients 1 and 5 were to undergo repeat EPS for recurrent tachycardia. In the 5 patients free from clinical recurrence, the arrhythmia during EPS matched the clinical arrhythmia identified before EPS. No adverse procedural sequelae occurred during follow-up.
Fig. 4.
Patient 3. Surface electrocardiograms show separate sources of different atrial tachyarrhythmias: A) intra-atrial reentrant tachycardia and B) nonautomatic focal atrial tachycardia. Note the low-voltage P waves.
Discussion
Ours appears to be the largest single-center cohort of adult atrial-baffle survivors to have undergone clinically indicated EPS by a combined pediatric–adult electrophysiology team in one year.5,6,10
As did earlier investigators,3,4 we found that IART, AVNRT, and NAFAT can all occur with clinical significance in this group of patients. Of note in our series, short-term success did not necessarily predict complete midterm success. The investigators in another retrospective series11 found that RFA yielded less short-term procedural success in adult atrial-switch patients than in other adults with congenital heart disease, and that, if success was nevertheless documented, involvement of the cavotricuspid isthmus was more likely in those cases. Conversely, in our cohort, the type of arrhythmia treated with RFA had no effect on freedom from arrhythmia. In addition, post-RFA arrhythmic recurrences in our patients were easily controlled with β-blocker therapy—unlike their pre-RFA arrhythmias, which often provoked symptoms despite medical therapy.
It is important to emphasize that arrhythmias in this population can be difficult to characterize on surface electrocardiograms because of low-voltage P waves, as in our Patient 3 (Fig. 4). More than one arrhythmia might exist, so one must strongly suspect a separate arrhythmia and not prematurely conclude that prior EPS has failed. It has been thought that AVNRT cannot occur in this adult group if there is evidence of ventriculoatrial (VA) block at baseline. However, we have found that provocation with isoproterenol can reestablish VA conduction, like the nodal activity seen in pediatric populations; thus, AVNRT should not be ruled out in these adults on the basis of baseline VA conduction.
Conclusions
Adult survivors of atrial baffling from Mustard and Senning procedures can be burdened with atrial arrhythmias that are refractory to medical therapy. Our study—with a high one-year volume of electrophysiologic procedures and RFA for atrial arrhythmias performed by a combined pediatric–adult team—revealed good short- and midterm results, including freedom from arrhythmia. As this patient population continues to age, we anticipate an even greater need for repeat and early invasive therapy. Skillful TBA will be necessary, and detailed pulmonary and systemic atrial and baffle mapping will be crucial toward identifying the varied substrates of atrial tachyarrhythmias in these patients.
References
- 1. Gelatt M, Hamilton RM, McCrindle BW, Connelly M, Davis A, Harris L, . et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol 1997; 29( 1): 194– 201. [DOI] [PubMed] [Google Scholar]
- 2. Jatene AD, Fontes VF, Paulista PP, Souza LC, Neger F, Galantier M, Sousa JE. . Anatomic correction of transposition of the great vessels. J Thorac Cardiovasc Surg 1976; 72( 3): 364– 70. [PubMed] [Google Scholar]
- 3. Greene AE, Skinner JR, Dubin AM, Collins KK, Van Hare GF. . The electrophysiology of atrioventricular nodal reentry tachycardia following the Mustard or Senning procedure and its radiofrequency ablation. Cardiol Young 2005; 15( 6): 611– 6. [DOI] [PubMed] [Google Scholar]
- 4. Khairy P, Van Hare GF. . Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm 2009; 6( 2): 283– 9. [DOI] [PubMed] [Google Scholar]
- 5. Kanter RJ, Papagiannis J, Carboni MP, Ungerleider RM, Sanders WE, Wharton JM. . Radiofrequency catheter ablation of supraventricular tachycardia substrates after Mustard and Senning operations for d-transposition of the great arteries. J Am Coll Cardiol 2000; 35( 2): 428– 41. [DOI] [PubMed] [Google Scholar]
- 6. Correa R, Walsh EP, Alexander ME, Mah DY, Cecchin F, Abrams DJ, Triedman JK. . Transbaffle mapping and ablation for atrial tachycardias after Mustard, Senning, or Fontan operations. J Am Heart Assoc 2013; 2( 5): e000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esch JJ, Triedman JK, Cecchin F, Alexander ME, Walsh EP. . Radiofrequency-assisted transseptal perforation for electrophysiology procedures in children and adults with repaired congenital heart disease. Pacing Clin Electrophysiol 2013; 36( 5): 607– 11. [DOI] [PubMed] [Google Scholar]
- 8. Ouyang F, Ernst S, Vogtmann T, Goya M, Volkmer M, Schaumann A, . et al. Characterization of reentrant circuits in left atrial macroreentrant tachycardia: critical isthmus block can prevent atrial tachycardia recurrence. Circulation 2002; 105( 16): 1934– 42. [DOI] [PubMed] [Google Scholar]
- 9. Yokokawa M, Latchamsetty R, Ghanbari H, Belardi D, Makkar A, Roberts B, . et al. Characteristics of atrial tachycardia due to small vs large reentrant circuits after ablation of persistent atrial fibrillation. Heart Rhythm 2013; 10( 4): 469– 76. [DOI] [PubMed] [Google Scholar]
- 10. Wu J, Deisenhofer I, Ammar S, Fichtner S, Reents T, Zhu P, . et al. Acute and long-term outcome after catheter ablation of supraventricular tachycardia in patients after the Mustard or Senning operation for D-transposition of the great arteries. Europace 2013; 15( 6): 886– 91. [DOI] [PubMed] [Google Scholar]
- 11. Yap SC, Harris L, Silversides CK, Downar E, Chauhan VS. . Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol 2010; 56( 19): 1589– 96. [DOI] [PubMed] [Google Scholar]


