Abstract
Aortic stenosis is the most common valvular heart disease in the developed world. About 7% of the population over age 65 years suffers from degenerative aortic stenosis. The prognosis of patients with symptomatic severe aortic stenosis is dismal without valve replacement. Even though the American College of Cardiology recommends aortic valve replacement to treat this condition as a class I recommendation, approximately one third of these patients over the age of 75 years are not referred for surgery. Typically, this is from concern about prohibitive surgical risk associated with patient frailty, comorbidities, age, and severe left ventricular dysfunction.
The advent in France of transcatheter aortic valve replacement has raised the hope in the United States for an alternative, less invasive treatment for aortic stenosis. Two recent trials—the Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve (Partner) and the CoreValve US Pivotal—have established transcatheter aortic valve replacement as the preferred approach in patients who are at high or prohibitive surgical risk. The more recently published Partner 2 trial has shown the feasibility of transcatheter aortic valve replacement in intermediate-surgical-risk patients as well. With a profile that promises easier use and better valve performance and delivery, newer-generation valves have shown their potential for further improvement in safety profile and overall outcomes. We review the history and status of this topic.
Keywords: Age factors, aortic valve insufficiency/therapy, aortic valve stenosis/mortality/surgery, evaluation studies as topic, heart valve diseases/therapy, heart valve prosthesis implantation/adverse effects/instrumentation, postoperative complications/prevention & control, prosthesis design/trends, transcatheter aortic valve replacement/mortality/trends, treatment outcome
Severe, symptomatic aortic stenosis (AS) is fatal, if left untreated: the prognosis is worse than those of most other malignancies, for the mortality rate is 50% at 2 years (Fig. 1).1,2 Aortic valve replacement (AVR) is the only treatment that has proved helpful in strengthening the survival prospects of these patients, and no medical therapy has shown its efficacy in improving outcomes. Despite the 2014 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for AVR as a class I indication for severe symptomatic AS,3 nearly one third of patients with severe symptomatic AS are not referred for surgical AVR.2 This is often because multiple comorbidities, advanced age, left ventricular (LV) dysfunction, and frailty all result in poor candidacy for surgical AVR (SAVR).4,5 The operative death risk for SAVR among patients with LV dysfunction is as high as 10%. A similar increase in risk is noted among SAVR patients with chronic renal disease and advanced age.6,7
Fig. 1.
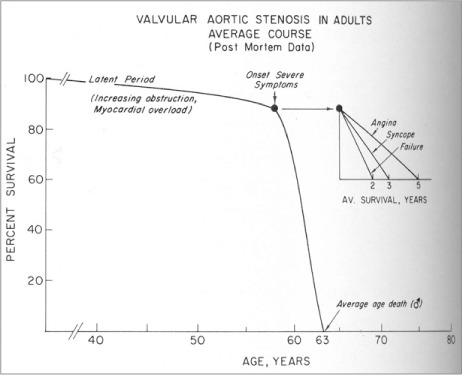
In a postmortem study, this was the prognosis of patients with aortic stenosis who did not undergo valve replacement or valve implantation.
From: Ross J Jr, Braunwald E. Aortic stenosis. Circulation 1968;38(1 Suppl):61–7.1
Aortic stenosis is an age-related degenerative process with an increase in incidence noted among advanced-age groups. Because advanced age and debility remain significant limiting factors in providing these patients access to SAVR, the emergence of transcatheter aortic valve replacement/transcatheter aortic valve implantation (TAVR/TAVI) provides a feasible and lesser-risk option for the frail and the elderly who are deemed poor candidates for surgery.
History of Transaortic Valve Replacement
The first classic description of successful surgical management of aortic insufficiency was published in 1954.8 The first successful replacement, however, was performed in 1960.9 Duran and Gunning described performing AVR in a patient in the early 1960s, using a xenograft porcine aortic valve.10,11 Since then, the art of open-heart valve surgery has made rapid strides.
With the evolution of minimally invasive percutaneous coronary interventions to treat complex, multivessel coronary artery disease, cardiologists aspired to treat stenotic valves percutaneously with minimal risk. This journey began with the development of balloon aortic valvuloplasty.12 However, it failed to substantially change long-term outcomes. The experimental model of stent-mounted porcine valve implants13 was followed by a successful pulmonic valve implantation in a lamb.14 In 2002, Cribier and colleagues described, in a 57-year-old man with severe calcified bicuspid AS, the first human case of TAVR.15 However, their anterograde, transvenous approach was fraught with limitations.16 Webb and associates subsequently reported the feasibility and safety of TAVR via a transfemoral arterial approach.17 Lichtenstein and co-authors initially described the transapical approach,18 and later reported clinical and hemodynamic outcomes for patients who underwent valve-in-valve aortic valve implantation of an Edwards balloon-expandable valve through the transapical approach.19
Indications for Aortic Valve Replacement
The ACC/AHA guidelines for the indications of AVR underwent significant changes in 2014, upon the growing success of TAVR (Fig. 2).3 The ACC/AHA recommends TAVR as the choice for interventions in the following situations (Table I)3:
1) Patients considered to be at a prohibitive risk for surgery and at a predicted post-TAVR survival longer than 12 months (class I indication, level of evidence B).
2) As an alternative to SAVR in patients considered to be at high risk for surgery (class IIa indication, level of evidence B).
Fig. 2.
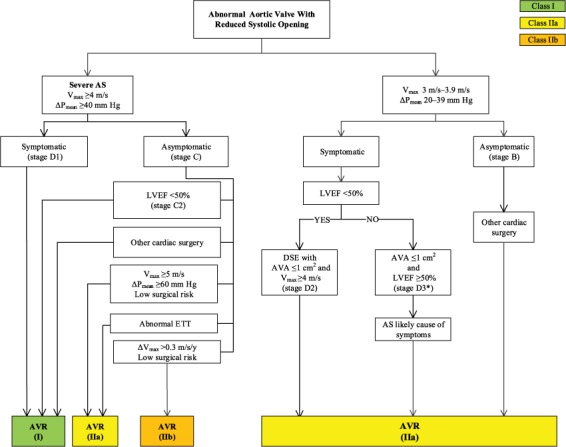
Indications for aortic valve replacement in patients with aortic stenosis.
From: Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published erratum appears in Circulation 2014;129(23):e650]. Circulation 2014;129(23):2440–92.3 Reprinted with permission from Circulation: 2014;129:2440–92. ©2014 American Heart Association, Inc.
AS = aortic stenosis; AVA = aortic valve area; AVR = aortic valve replacement; DSE = dobutamine stress echocardiography; ETT = exercise treadmill test; LVEF = left ventricular ejection fraction; ΔPmean = mean pressure gradient; Vmax = maximum velocity
TABLE I.
ACC/AHA Recommendations for Aortic Stenosis: Choice of Surgical or Transcatheter Intervention3
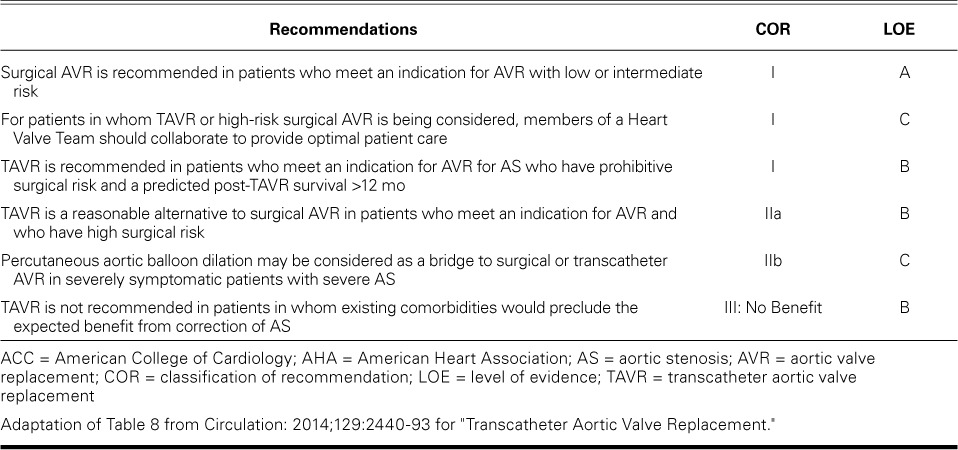
An approach by a heart valve team—consisting of experts in valvular heart disease, interventional cardiology, cardiac imaging, cardiac anesthesia, and cardiac surgery—is considered essential in patients who are being considered for TAVR or high-risk SAVR (class I indication, level of evidence C). Substantial credit for the improvement in TAVR outcomes has been attributed to the improvement in patient selection by the heart valve team.20
Types of Transcatheter Valves
Two types of valves are currently approved by the U.S. Food and Drug Administration (FDA) for TAVR: the CoreValve® Revalving system (Medtronic, Inc.; Minneapolis, Minn) and the Edwards Sapien system (Edwards Lifesciences Corporation; Irvine, Calif). Both valves have proved their effectiveness in the recent outcomes of the CoreValve US Pivotal and Partner trials, respectively.
Edwards Sapien TAVR Valves
The Edwards Sapien aortic valve is a balloon-expandable, trileaflet, equine pericardial valve attached to a stainless-steel framework. The Edwards Sapien XT and the Edwards Sapien 3 valves, available in 20-mm, 23-mm, 26-mm, and 29-mm sizes, are the 2 types of Edwards Sapien TAVR valves available in the U.S. Either can be deployed through transfemoral, transapical, transaxillary, and transaortic approaches. The Edwards Sapien XT system has been approved by the FDA for aortic valve-in-valve procedures. The Sapien 3 system—with a lower device profile, improved delivery catheter, and the additional feature of a polyethylene terephthalate outer skirt—has shown reduced rates of vascular sequelae and paravalvular regurgitation (Fig. 3).
Fig. 3.
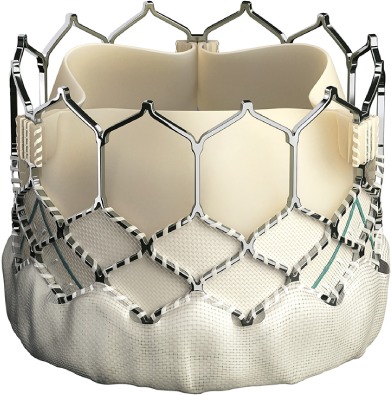
Image shows the unique features of the Edwards Sapien 3 Valve. (Reprinted with permission from Edwards Lifesciences Corporation.)
Partner Trial (NCT00530894)
The Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve (Partner) trial was a randomized prospective trial that divided patients into 2 cohorts: Partner A and Partner B. The Partner A cohort compared TAVR against SAVR in high-surgical-risk patients, defined as predicted risk of operative death ≥15%.21 Partner B included patients who were not considered suitable candidates for surgery because of combined risk of death or irreversible comorbidity of more than 50%, as agreed upon by 2 cardiac surgeons.22
The Partner A trial included 351 patients undergoing SAVR and 348 undergoing TAVR. The 30-day mortality rate for TAVR was 3.4% and for SAVR was 6.5%. The 1-year mortality rate was 24.2% and 26.8% for TAVR and SAVR, respectively (P=0.001 for noninferiority). Reported 5-year results were comparable as well, with mortality rates reported at 67.8% in the TAVR group versus 62.4% in the SAVR group (P=0.76).23
The Partner B trial compared TAVR versus medical therapy, with the results significantly favoring the TAVR group. The 1-year mortality rate for TAVR was 30.7%, versus 50.7% for conventional medical therapy (P <0.001). The benefits of TAVR were sustained at 5 years with a significantly lower mortality rate than medical treatment (71.8% vs 93.6%; P <0.0001).24
CoreValve Revalving System
The CoreValve Revalving system, which has a self-expanding nitinol frame, contains a trileaflet porcine pericardial tissue prosthesis and is delivered by advancing a catheter over a guidewire in retrograde fashion from the femoral, axillary, or subclavian artery to the aortic annulus. The valve is manufactured in 4 diameters (23, 26, 29, and 31 mm) and was the first transcatheter valve approved by the FDA for valve-in-valve replacement. Evolut™ R, the latest-generation CoreValve, is configured with a low delivery profile and is the only repositionable and recapturable device available in the U.S. (Fig. 4).
Fig. 4.
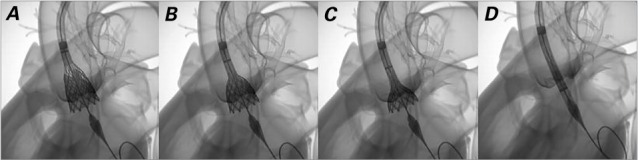
Images show the recapturability feature of the CoreValve Evolut R valve: A) valve too deep; B) recapture begins; C) partially recaptured; D) valve fully recaptured. (Reprinted with permission from Medtronic, Inc.)
CoreValve US Pivotal Trial (NCT01240902)
The CoreValve US Pivotal Trial was a multicenter noninferiority trial performed at 45 clinical sites in the U.S., which compared TAVR using a CoreValve with SAVR, in patients who had severe AS and increased risk of death from surgery.25 The inclusion criteria were New York Heart Association (NYHA) functional class II or greater, severe AS with aortic valve area ≤0.8 cm2 or aortic valve area index ≤0.5 cm2/m2 and a mean gradient >40 mmHg or a peak velocity >4 m/s whether at rest or during dobutamine echocardiography, and at increased surgical risk. The primary endpoint was all-cause death at one year, and 795 patients underwent randomization in the U.S. All-cause death at one year in the TAVR group was 14.2% versus 19.1% in the SAVR group (P <0.001 for noninferiority and P=0.04 for superiority). The 2-year mortality results were congruent with the 1-year results: an all-cause mortality rate at 22.2% in the TAVR group and 28.6% in the SAVR group. Hemodynamic performance was superior in the TAVR group at all times.
There have been promising results from the CoreValve Australia-New Zealand Study, the Italian CoreValve registry, and the UK TAVI registry.26–28 The results from the Italian registry showed durability with the CoreValve at the 5-year follow-up, with only 5 patients (1.4%) noted to have prosthesis failure.28 The Spanish experience yielded a 30-day mortality rate of 7.4% in 108 patients who underwent TAVI.29
CoreValve versus Edwards Sapien Valve
The Randomized Comparison of Transcatheter Heart Valves in High Risk Patients with Severe Aortic Stenosis: Medtronic CoreValve Versus Edwards Sapien XT (Choice) trial (NCT01645202) was the first head-to-head trial of a balloon-expandable TAVR valve versus a self-expandable valve.30 At 30 days, device success occurred in 116 of 121 patients (95.9%) in the balloon-expandable group and 93 of 120 patients (77.5%) in the self-expandable group (P <0.001). This was attributed to a significantly lower frequency of aortic regurgitation and the rare need for implanting more than one valve in the balloon-expandable group. Despite the greater device success in the balloon-expandable group, the mortality rates in these groups were comparable both at the 30-day and at the 1-year follow-up. A recent meta-analysis of these 2 groups showed a similar risk of death and stroke at both stages.31 However, the incidence of new pacemaker implantation, aortic regurgitation, valve embolization, and the need for more than one valve was found to be higher with self-expandable valve implantation than with balloon-expandable valve implantation.
Four registries—the UK TAVI registry, the Pooled-RotterdAm-Milano-Toulouse In Collaboration (Pragmatic Plus Initiative), the Spanish National TAVI registry, and the Belgian TAVI Registry—reported comparable results for the Edwards Sapien and the CoreValve.32–35
Anatomic Evaluation before TAVR
Both transthoracic and transesophageal echocardiography have been found inferior to real-time 3-dimensional imaging for measuring annular size.36 Multidetector computed tomography has proved to be the method of choice for pre-TAVR evaluation of the suitability of iliofemoral access and for detailed anatomic evaluation of the aortic root and valve annulus; it has also shown promise in the detection of coronary disease pre-TAVR.37,38 Left- and right-sided heart catheterization are used to evaluate coexisting coronary artery disease and pulmonary hypertension; pulmonary function tests are also part of the routine pre-TAVR evaluation. Although revascularization of coronary disease typically takes place before TAVR, revascularization after TAVR is feasible as well.39
Procedural Success after TAVR
Although early TAVR experiences yielded a procedural success rate of 70% to 80%,40 the Society of Thoracic Surgeons/ACC Transcatheter Valve Therapy Registry investigators have reported a 92% procedural success rate since the approval of TAVR in the U.S.41
Changes in the Heart after TAVR
Replacement of the stenotic aortic valve alleviates the increase in LV pressure load and improves LV hemodynamic performance.42 Transcatheter AVR has been shown to normalize ventricular-arterial coupling, decrease LV hypertrophy, and restore LV function in patients with systolic dysfunction.43,44 Elevated left-sided filling pressures in severe AS can lead to severe pulmonary hypertension in nearly one third of cases. Patients undergoing TAVR were noted to have a sustained reduction in pulmonary hypertension.45 Although these results were reciprocated by balloon aortic valvuloplasty in the short term, the pulmonary pressures returned to preprocedural levels in the long term. Transthoracic echocardiograms from 95 patients evaluated before and after implantation of Edwards Sapien valves at selected intervals in the TRanscatheter EndoVascular Implantation of VALves (Revival) trial revealed the mean valve area (1.6 cm2) achieved after TAVR to be comparable to that achieved by SAVR, and the clinical improvements to be sustainable at 1 year.46 Despite slight progression of the aortic regurgitation at 1 year, most patients had improvement in LV structure and function.47
Changes in Quality of Life
Quality-of-life indices such as the Short Form 36 score, the Kansas City Cardiomyopathy Questionnaire, the Short Form 12v2 scoring system, and the Heart Failure Questionnaire have all indicated marked improvement in patients' quality of life after TAVR. The transfemoral TAVR cohort in the Partner A trial scored higher in the short term (via the Kansas City Cardiomyopathy Questionnaire summary score for evaluation of quality of life) than did the cohort who underwent surgery.48
Predictors of Death after TAVR
The 2 most frequently used death-prediction models are the Logistic EuroSCORE and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) algorithms. The EuroSCORE, however, has been shown to overestimate the expected mortality rate in high-risk candidates.49 Table II summarizes the independent predictors of death.50 Low flow was identified as an independent predictor of death in a Partner trial analysis.51 The transapical approach has been associated with a higher mortality rate than has the transfemoral route.52,53 Sequelae of TAVR—like cerebral embolism, paravalvular regurgitation, and vascular sequelae—are well-known predictors of death. Elevation of brain-type natriuretic peptide levels at 30 days from baseline, along with moderate-to-severe aortic regurgitation, were independent predictors of death in the transfemoral cohort of the Partner trial.54 Female sex, preoperative NYHA functional class IV, LV ejection fraction <0.30, preoperative intravenous inotropic agents, a higher degree of calcification, and arteriovascular disease were found to be independent predictors of severe sequelae in the German Aortic Valve Registry (GARY).55
TABLE II.
Predictors of Death after Transcatheter Aortic Valve Replacement49
Technical Sequelae of TAVR
The Valve Academic Research Consortium (VARC), the organization that oversees the TAVR trials, has proposed standard endpoints for the TAVR clinical trials.56,57 The purpose was to standardize postoperative sequelae of TAVR—such as myocardial infarction, stroke, bleeding sequelae, acute kidney injury, vascular sequelae, prosthetic valve performance, and other sequelae related to prosthetic valve placement, including death. The clinical benefit endpoints proposed by VARC are exercise performance, the judgment of NYHA functional status, and performance on various quality-of-life and frailty questionnaires. The creatine kinase-myocardial band was recommended as a periprocedural marker for myocardial infarction with a 2nd-sample requirement of a greater-than-20% increase and a 2nd-sample elevation of at least 10 times the upper limit of normal.
Cerebral Embolism. Transcatheter AVR is associated with the highest stroke rates among percutaneous cardiac interventions, ranging from 4% to 5% in the 2 large randomized clinical trials that compared TAVR and SAVR in high-risk patients.21,25 Although cerebral microembolism was detected via ultrasonography and magnetic resonance imaging in the earlier studies in patients undergoing TAVR,58,59 no deleterious effect on neurocognitive function was found at a 3-month follow-up evaluation.60 Variation in the practices regarding antithrombotic therapy at different institutions has reverted to standard since the finding—during the Portico Re-sheathable Transcatheter Aortic Valve System U.S. Investigational Device Exemption (Portico IDE) trial—of reduced aortic-valve-leaflet motion on computed tomography: close scrutiny of the computed tomograms of a patient who had experienced a stroke and of an asymptomatic patient showed that leaflet motion improved upon therapeutic anticoagulation.61 Although the incidence of strokes was not statistically significant in the reduced-leaflet-motion arm (compared with the normal-leaflet-motion arm), a significant difference in the incidence of strokes or transient ischemic attacks was seen in patients with reduced leaflet motion in these pooled cohorts: Subclinical Aortic Valve Bioprosthesis Thrombosis Assessed with Four-Dimensional Computed Tomography (Savory) and Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation (Resolve).61 The FDA now requires all patients undergoing TAVR to undergo follow-up computed tomographic scanning to look for signs of leaflet immobility.
Coronary Obstruction. Transcatheter AVR is associated with low risk of coronary ostial obstruction (<1%) in patients with native-valve AS.62 The risk goes up 3- to 4-fold in patients who have undergone valve-in-valve TAVR.62 Coronary obstruction after TAVR is associated with a high mortality rate. The mechanism is generally speculated to be direct or near-direct contact between the bioprosthetic valve and the coronary ostium.63 Meticulous use of computed tomography and fluoroscopy can detect high-risk cases.63,64 Recognizing high-risk cases and taking measures such as the use of a retrievable prosthesis, the deliberate selection of a small-diameter prosthesis, underexpansion of the balloon-expandable valve,65 or use of active protection (for example, placing wire and stent in the coronary vasculature before valve implantation) are options that should be considered for management of coronary obstruction.63
Conduction Abnormalities and Pacemaker Necessity. The overall rates of permanent pacemaker implantation after TAVR were around 17%, versus 5% with SAVR.66,67 The need for a new permanent pacemaker in the Partner A cohort was not significantly different at 30 days (3.8% vs 3.6%), 1 year (5.7% vs 5%) and 5 years (9.7% vs 9.1%) for patients undergoing TAVR versus SAVR, respectively. Siontis and colleagues66 suggested that male sex, baseline conduction disturbances, and intraprocedural atrioventricular block had roles as predictors of permanent pacemaker implantation after TAVR. They found the sequela to be 2.5 times more prevalent with the self-expandable valve than with the balloon-expandable valve, a finding equivalent to that of meta-analysis as well.31 The valve design and its potential for deeper implantation in the LV outflow tract are possible reasons for these higher rates of permanent pacemaker implantation with the self-expandable valve than with the balloon-expandable valve. However, the rates of permanent pacemaker implantation and conduction abnormalities were also found to be higher with the new Edwards Sapien 3 system because of prosthesis oversizing and the depth of the implantation.67
Other Sequelae. Paravalvular leak after TAVR is a frequent occurrence that is associated with increased mortality rates.50,54,68 The new Edwards Sapien 3 valve, which features a sealing skirt at its lower position and a more accurate mechanism of valve positioning, has shown promise in decreasing the rates of paravalvular regurgitation.69–71 In a recent Partner continued-access registry, the decreased rates of paravalvular regurgitation were attributed to improved case selection, improved procedural techniques, and increased procedural experience.72 Vascular sequelae are independent predictors of death, largely attributed to the wider sheaths (inner diameter, 24F–26F) required by earlier-generation devices.73 As the sheath sizes decrease with the new-generation Edwards Sapien 3 system (inner diameter, 14F–16F) and the CoreValve Evolut R (14 F-equivalent system), the rate of vascular sequelae and the incidence of bleeding will continue to decrease.70,71 Acute kidney injury is frequently seen after TAVR74; however, the rates were lower both in the Partner 2 trial and in a recently published meta-analysis.75,76 Other sequelae—like valve embolization, mitral regurgitation, prosthetic-valve endocarditis, and ventricular septal defect—are also known.
The Future of TAVR
The results of the recently published Partner 2 trial, which compared TAVR outcomes (in this instance, outcomes of the Sapien XT valve system) with SAVR have shown similar overall outcomes with respect to primary endpoints (death and stroke) at 2 years between the 2 groups, although the results favored TAVR in patients who underwent TAVR through the transfemoral route.76 Transcatheter AVR also resulted in fewer bleeding sequelae, lower rates of acute kidney injury and new-onset atrial fibrillation, shorter hospitalization and intensive-care stay, and larger aortic valve areas than did surgery. In comparison, TAVR had a higher rate of paravalvular regurgitation and vascular sequelae. The encouraging results of the Partner 2 trial come despite the drawbacks of the Edwards Sapien XT valve system, which has largely been replaced by the newer-generation Edwards Sapien 3 system.
The results of the Edwards Sapien 3 observational study included a reduced rate of vascular sequelae because of the smaller sheath size and a lower rate of paravalvular regurgitation because of the skirt mechanism.70 The Surgical Replacement and Transcatheter Aortic Valve Implantation (Surtavi) trial (NCT01586910), which is Medtronic's CoreValve version of the Partner 2 trial, will further extend the debate about the possibility of TAVR's use as a first-line treatment for intermediate-risk patients. In the meantime, the Sapien 3 prosthesis has been approved for use in intermediate-surgical-risk patients in the U.S. Moreover, the FDA has recently approved clinical trials to test the efficacy of TAVR in low-risk patients, both for the Medtronic arm using the CoreValve Evolut R System (NCT02701283) and the Edwards Sapien arm using the Edwards Sapien 3 valve system (Partner 3 trial, NCT02675114). The newer-generation valves have the potential to improve the success of the procedure and to reduce sequelae. The ability to reposition and recapture the Evolut R valve has given operators extra confidence during the procedure and has led to a CE mark for use in intermediate-surgical-risk patients in Europe. The Repositionable Percutaneous Replacement of Stenotic Aortic Valve Through Implantation of Lotus Valve System Randomized Clinical Evaluation (Reprise III) trial is currently enrolling patients to test the safety and efficacy of the Lotus Edge™ Valve (Boston Scientific Corporation; Natick, Mass) for TAVR. Finally, the Direct Flow Medical® Transcatheter Aortic Valve System (Direct Flow Medical, Inc.; Santa Rosa, Calif), a part of the TranScatheter Aortic Valve RepLacement System US Feasibility (Salus) trial, is designed to minimize aortic regurgitation while replacing the faulty native aortic valve in high- and extremely high-surgical-risk patients. Rapid technological advances such as these are reinforcing the future of TAVR.
References
- 1. Ross J Jr, Braunwald E. . Aortic stenosis. Circulation 1968; 38( 1 Suppl): 61– 7. [DOI] [PubMed] [Google Scholar]
- 2. Varadarajan P, Kapoor N, Bansal RC, Pai RG. . Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged > or =80 years. Eur J Cardiothorac Surg 2006; 30( 5): 722– 7. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, . et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published erratum appears in Circulation 2014;129(23): e650]. Circulation 2014; 129( 23): 2440– 92. [DOI] [PubMed] [Google Scholar]
- 4. Bouma BJ, van Den Brink RB, van Der Meulen JH, Verheul HA, Cheriex EC, Hamer HP, . et al. To operate or not on elderly patients with aortic stenosis: the decision and its consequences. Heart 1999; 82( 2): 143– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iung B, Cachier A, Baron G, Messika-Zeitoun D, Delahaye F, Tornos P, . et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26( 24): 2714– 20. [DOI] [PubMed] [Google Scholar]
- 6. Sharony R, Grossi EA, Saunders PC, Schwartz CF, Ciuffo GB, Baumann FG, . et al. Aortic valve replacement in patients with impaired ventricular function. Ann Thorac Surg 2003; 75( 6): 1808– 14. [DOI] [PubMed] [Google Scholar]
- 7. Kvidal P, Bergstrom R, Malm T, Stahle E. . Long-term follow-up of morbidity and mortality after aortic valve replacement with a mechanical valve prosthesis. Eur Heart J 2000; 21( 13): 1099– 111. [DOI] [PubMed] [Google Scholar]
- 8. Hufnagel CA, Harvey WP, Rabil PJ, McDermott TF. . Surgical correction of aortic insufficiency. Surgery 1954; 35( 5): 673– 83. [PubMed] [Google Scholar]
- 9. Harken DE, Taylor WJ, Lefemine AA, Lunzer S, Low HB, Cohen ML, Jacobey JA. . Aortic valve replacement with a caged ball valve. Am J Cardiol 1962; 9: 292– 9. [DOI] [PubMed] [Google Scholar]
- 10. Duran CG, Gunning AJ. . A method for placing a total homologous aortic valve in the subcoronary position. Lancet 1962; 2( 7254): 488– 9. [DOI] [PubMed] [Google Scholar]
- 11. Binet JP, Duran CG, Carpenter A, Langlois J. . Heterologous aortic valve transplantation. Lancet 1965; 2( 7425): 1275. [DOI] [PubMed] [Google Scholar]
- 12. Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. . Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg 1984; 87( 3): 394– 402. [PubMed] [Google Scholar]
- 13. Andersen HR, Knudsen LL, Hasenkam JM. . Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13( 5): 704– 8. [DOI] [PubMed] [Google Scholar]
- 14. Bonhoeffer P, Boudjemline Y, Saliba Z, Hausse AO, Aggoun Y, Bonnet D, . et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation 2000; 102( 7): 813– 6. [DOI] [PubMed] [Google Scholar]
- 15. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, . et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106( 24): 3006– 8. [DOI] [PubMed] [Google Scholar]
- 16. Sakata Y, Syed Z, Salinger MH, Feldman T. . Percutaneous balloon aortic valvuloplasty: antegrade transseptal vs. conventional retrograde transarterial approach. Catheter Cardiovasc Interv 2005; 64( 3): 314– 21. [DOI] [PubMed] [Google Scholar]
- 17. Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, . et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113( 6): 842– 50. [DOI] [PubMed] [Google Scholar]
- 18. Lichtenstein SV, Cheung A, Ye J, Thompson CR, Carere RG, Pasupati S, Webb JG. . Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114( 6): 591– 6. [DOI] [PubMed] [Google Scholar]
- 19. Ye J, Webb JG, Cheung A, Soon JL, Wood D, Thompson CR, . et al. Transapical transcatheter aortic valve-in-valve implantation: clinical and hemodynamic outcomes beyond 2 years. J Thorac Cardiovasc Surg 2013; 145( 6): 1554– 62. [DOI] [PubMed] [Google Scholar]
- 20. Agarwal S, Tuzcu EM, Krishnaswamy A, Schoenhagen P, Stewart WJ, Svensson LG, Kapadia SR. . Transcatheter aortic valve replacement: current perspectives and future implications. Heart 2015; 101( 3): 169– 77. [DOI] [PubMed] [Google Scholar]
- 21. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, . et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364( 23): 2187– 98. [DOI] [PubMed] [Google Scholar]
- 22. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, . et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363( 17): 1597– 607. [DOI] [PubMed] [Google Scholar]
- 23. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, . et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385( 9986): 2477– 84. [DOI] [PubMed] [Google Scholar]
- 24. Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, . et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015; 385( 9986): 2485– 91. [DOI] [PubMed] [Google Scholar]
- 25. Adams DH, Popma JJ, Reardon MJ. . Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 371( 10): 967– 8. [DOI] [PubMed] [Google Scholar]
- 26. Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, . et al. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv 2015; 8( 5): 645– 53. [DOI] [PubMed] [Google Scholar]
- 27. Meredith IT, Walton A, Walters DL, Pasupati S, Muller DW, Worthley SG, . et al. Mid-term outcomes in patients following transcatheter aortic valve implantation in the CoreValve Australia and New Zealand Study. Heart Lung Circ 2015; 24( 3): 281– 90. [DOI] [PubMed] [Google Scholar]
- 28. Barbanti M, Petronio AS, Ettori F, Latib A, Bedogni F, De Marco F, . et al. 5-Year outcomes after transcatheter aortic valve implantation with CoreValve prosthesis. JACC Cardiovasc Interv 2015; 8( 8): 1084– 91. [DOI] [PubMed] [Google Scholar]
- 29. Avanzas P, Munoz-Garcia AJ, Segura J, Pan M, Alonso-Briales JH, Lozano I, . et al. Percutaneous implantation of the CoreValve self-expanding aortic valve prosthesis in patients with severe aortic stenosis: early experience in Spain [in Spanish]. Rev Esp Cardiol 2010; 63( 2): 141– 8. [DOI] [PubMed] [Google Scholar]
- 30. Abdel-Wahab M, Neumann FJ, Mehilli J, Frerker C, Richardt D, Landt M, . et al. 1-Year outcomes after transcatheter aortic valve replacement with balloon-expandable versus self-expandable valves: results from the CHOICE randomized clinical trial. J Am Coll Cardiol 2015; 66( 7): 791– 800. [DOI] [PubMed] [Google Scholar]
- 31. Agarwal S, Parashar A, Kumbhani DJ, Svensson LG, Krishnaswamy A, Tuzcu EM, Kapadia SR. . Comparative meta-analysis of balloon-expandable and self-expandable valves for transcatheter aortic valve replacement. Int J Cardiol 2015; 197: 87– 97. [DOI] [PubMed] [Google Scholar]
- 32. Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, . et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011; 58( 20): 2130– 8. [DOI] [PubMed] [Google Scholar]
- 33. Chieffo A, Buchanan GL, Van Mieghem NM, Tchetche D, Dumonteil N, Latib A, . et al. Transcatheter aortic valve implantation with the Edwards SAPIEN versus the Medtronic CoreValve Revalving system devices: a multicenter collaborative study: the PRAGMATIC Plus Initiative (Pooled-RotterdAm-Milano-Toulouse In Collaboration). J Am Coll Cardiol 2013; 61( 8): 830– 6. [DOI] [PubMed] [Google Scholar]
- 34. Sabate M, Canovas S, Garcia E, Hernandez Antolin R, Maroto L, Hernandez JM, . et al. In-hospital and mid-term predictors of mortality after transcatheter aortic valve implantation: data from the TAVI National Registry 2010–2011. Rev Esp Cardiol (Engl Ed) 2013; 66( 12): 949– 58. [DOI] [PubMed] [Google Scholar]
- 35. Collas VM, Dubois C, Legrand V, Kefer J, De Bruyne B, Dens J, . et al. Midterm clinical outcome following Edwards SAPIEN or Medtronic CoreValve transcatheter aortic valve implantation (TAVI): results of the Belgian TAVI registry. Catheter Cardiovasc Interv 2015; 86( 3): 528– 35. [DOI] [PubMed] [Google Scholar]
- 36. Janosi RA, Kahlert P, Plicht B, Wendt D, Eggebrecht H, Erbel R, Buck T. . Measurement of the aortic annulus size by real-time three-dimensional transesophageal echocardiography. Minim Invasive Ther Allied Technol 2011; 20( 2): 85– 94. [DOI] [PubMed] [Google Scholar]
- 37. Leipsic J, Gurvitch R, Labounty TM, Min JK, Wood D, Johnson M, . et al. Multidetector computed tomography in transcatheter aortic valve implantation. JACC Cardiovasc Imaging 2011; 4( 4): 416– 29. [DOI] [PubMed] [Google Scholar]
- 38. Andreini D, Pontone G, Mushtaq S, Bartorelli AL, Ballerini G, Bertella E, . et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am Heart J 2014; 168( 3): 332– 9. [DOI] [PubMed] [Google Scholar]
- 39. Arora A, Bahekar AA. . Staged high-risk percutaneous coronary intervention with Impella support after on-pump transcatheter aortic valve replacement. Tex Heart Inst J 2016; 43( 5): 423– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, . et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50( 1): 69– 76. [DOI] [PubMed] [Google Scholar]
- 41. Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, . et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA 2013; 310( 19): 2069– 77. [DOI] [PubMed] [Google Scholar]
- 42. Ross J Jr. . Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J Am Coll Cardiol 1985; 5( 4): 811– 26. [DOI] [PubMed] [Google Scholar]
- 43. Poulin F, Carasso S, Horlick EM, Rakowski H, Lim KD, Finn H, . et al. Recovery of left ventricular mechanics after transcatheter aortic valve implantation: effects of baseline ventricular function and postprocedural aortic regurgitation. J Am Soc Echocardiogr 2014; 27( 11): 1133– 42. [DOI] [PubMed] [Google Scholar]
- 44. Di Bello V, Giannini C, De Carlo M, Delle Donne MG, Nardi C, Palagi C, . et al. Acute improvement in arterial-ventricular coupling after transcatheter aortic valve implantation (CoreValve) in patients with symptomatic aortic stenosis. Int J Cardiovasc Imaging 2012; 28( 1): 79– 87. [DOI] [PubMed] [Google Scholar]
- 45. Ben-Dor I, Goldstein SA, Pichard AD, Satler LF, Maluenda G, Li Y, . et al. Clinical profile, prognostic implication, and response to treatment of pulmonary hypertension in patients with severe aortic stenosis. Am J Cardiol 2011; 107( 7): 1046– 51. [DOI] [PubMed] [Google Scholar]
- 46. Kodali SK, O'Neill WW, Moses JW, Williams M, Smith CR, Tuzcu M, . et al. Early and late (one year) outcomes following transcatheter aortic valve implantation in patients with severe aortic stenosis (from the United States REVIVAL trial). Am J Cardiol 2011; 107( 7): 1058– 64. [DOI] [PubMed] [Google Scholar]
- 47. Yared K, Garcia-Camarero T, Fernandez-Friera L, Llano M, Durst R, Reddy AA, . et al. Impact of aortic regurgitation after transcatheter aortic valve implantation: results from the REVIVAL trial [published erratum appears in JACC Cardiovasc Imaging 2013;6(6):747]. JACC Cardiovasc Imaging 2012; 5( 5): 469– 77. [DOI] [PubMed] [Google Scholar]
- 48. Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, . et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol 2012; 60( 6): 548– 58. [DOI] [PubMed] [Google Scholar]
- 49. Mack MJ. . Risk scores for predicting outcomes in valvular heart disease: how useful? Curr Cardiol Rep 2011; 13( 2): 107– 12. [DOI] [PubMed] [Google Scholar]
- 50. Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, . et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation 2011; 123( 3): 299– 308. [DOI] [PubMed] [Google Scholar]
- 51. Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, . et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation 2013; 127( 23): 2316– 26. [DOI] [PubMed] [Google Scholar]
- 52. Panchal HB, Ladia V, Amin P, Patel P, Veeranki SP, Albalbissi K, Paul T. . A meta-analysis of mortality and major adverse cardiovascular and cerebrovascular events in patients undergoing transfemoral versus transapical transcatheter aortic valve implantation using Edwards valve for severe aortic stenosis. Am J Cardiol 2014; 114( 12): 1882– 90. [DOI] [PubMed] [Google Scholar]
- 53. Urena M, Webb JG, Eltchaninoff H, Munoz-Garcia AJ, Bouleti C, Tamburino C, . et al. Late cardiac death in patients undergoing transcatheter aortic valve replacement: incidence and predictors of advanced heart failure and sudden cardiac death. J Am Coll Cardiol 2015; 65( 5): 437– 48. [DOI] [PubMed] [Google Scholar]
- 54. O'Neill BP, Guerrero M, Thourani VH, Kodali S, Heldman A, Williams M, . et al. Prognostic value of serial B-type natriuretic peptide measurement in transcatheter aortic valve replacement (from the PARTNER Trial). Am J Cardiol 2015; 115( 9): 1265– 72. [DOI] [PubMed] [Google Scholar]
- 55. Walther T, Hamm CW, Schuler G, Berkowitsch A, Kotting J, Mangner N, . et al. Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY registry. J Am Coll Cardiol 2015; 65( 20): 2173– 80. [DOI] [PubMed] [Google Scholar]
- 56. Leon MB, Piazza N, Nikolsky E, Blackstone EH, Cutlip DE, Kappetein AP, . et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol 2011; 57( 3): 253– 69. [DOI] [PubMed] [Google Scholar]
- 57. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, . et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012; 60( 15): 1438– 54. [DOI] [PubMed] [Google Scholar]
- 58. Drews T, Pasic M, Buz S, Unbehaun A, Dreysse S, Kukucka M, . et al. Transcranial Doppler sound detection of cerebral microembolism during transapical aortic valve implantation. Thorac Cardiovasc Surg 2011; 59( 4): 237– 42. [DOI] [PubMed] [Google Scholar]
- 59. Hynes BG, Rodes-Cabau J. . Transcatheter aortic valve implantation and cerebrovascular events: the current state of the art. Ann N Y Acad Sci 2012; 1254: 151– 63. [DOI] [PubMed] [Google Scholar]
- 60. Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, . et al. Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study. Circulation 2010; 121( 7): 870– 8. [DOI] [PubMed] [Google Scholar]
- 61. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, de Backer O, . et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med 2015; 373( 21): 2015– 24. [DOI] [PubMed] [Google Scholar]
- 62. Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, . et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013; 62( 17): 1552– 62. [DOI] [PubMed] [Google Scholar]
- 63. Dvir D, Leipsic J, Blanke P, Ribeiro HB, Kornowski R, Pichard A, . et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv 2015; 8( 1): e002079. [DOI] [PubMed] [Google Scholar]
- 64. Achenbach S, Delgado V, Hausleiter J, Schoenhagen P, Min JK, Leipsic JA. . SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc Comput Tomogr 2012; 6( 6): 366– 80. [DOI] [PubMed] [Google Scholar]
- 65. Barbanti M, Leipsic J, Binder R, Dvir D, Tan J, Freeman M, . et al. Underexpansion and ad hoc post-dilation in selected patients undergoing balloon-expandable transcatheter aortic valve replacement. J Am Coll Cardiol 2014; 63( 10): 976– 81. [DOI] [PubMed] [Google Scholar]
- 66. Siontis GC, Juni P, Pilgrim T, Stortecky S, Bullesfeld L, Meier B, . et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol 2014; 64( 2): 129– 40. [DOI] [PubMed] [Google Scholar]
- 67. Husser O, Pellegrini C, Kessler T, Burgdorf C, Thaller H, Mayr NP, . et al. Predictors of permanent pacemaker implantations and new-onset conduction abnormalities with the SAPIEN 3 balloon-expandable transcatheter heart valve. JACC Cardiovasc Interv 2016; 9( 3): 244– 54. [DOI] [PubMed] [Google Scholar]
- 68. Kodali S, Pibarot P, Douglas PS, Williams M, Xu K, Thourani V, . et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards Sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J 2015; 36( 7): 449– 56. [DOI] [PubMed] [Google Scholar]
- 69. Husser O, Pellegrini C, Kessler T, Burgdorf C, Thaller H, Mayr NP, . et al. Outcomes after transcatheter aortic valve replacement using a novel balloon-expandable transcatheter heart valve: a single-center experience. JACC Cardiovasc Interv 2015; 8( 14): 1809– 16. [DOI] [PubMed] [Google Scholar]
- 70. Amat-Santos IJ, Dahou A, Webb J, Dvir D, Dumesnil JG, Allende R, . et al. Comparison of hemodynamic performance of the balloon-expandable SAPIEN 3 versus SAPIEN XT transcatheter valve. Am J Cardiol 2014; 114( 7): 1075– 82. [DOI] [PubMed] [Google Scholar]
- 71. Webb J, Gerosa G, Lefevre T, Leipsic J, Spence M, Thomas M, . et al. Multicenter evaluation of a next-generation balloon-expandable transcatheter aortic valve. J Am Coll Cardiol 2014; 64( 21): 2235– 43. [DOI] [PubMed] [Google Scholar]
- 72. Beohar N, Kirtane AJ, Blackstone E, Waksman R, Holmes D Jr, Minha S, . et al. Trends in complications and outcomes of patients undergoing transfemoral transcatheter aortic valve replacement: experience from the PARTNER continued access registry. JACC Cardiovasc Interv 2016; 9( 4): 355– 63. [DOI] [PubMed] [Google Scholar]
- 73. Genereux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, . et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol 2012; 59( 25): 2317– 26. [DOI] [PubMed] [Google Scholar]
- 74. Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochelliere R, Doyle D, . et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J 2010; 31( 7): 865– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arora S, Misenheimer JA, Jones W, Bahekar A, Caughey M, Ramm CJ, . et al. Transcatheter versus surgical aortic valve replacement in intermediate risk patients: a meta-analysis. Cardiovasc Diagn Ther 2016; 6( 3): 241– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, . et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374( 17): 1609– 20. [DOI] [PubMed] [Google Scholar]


