Abstract
The cone reconstruction technique, first described by da Silva and modified by Dearani and by others, has become the repair method of choice in patients with Ebstein anomaly of the tricuspid valve. This report details the outcome of the modified cone reconstruction technique in 6 children who underwent surgical correction of Ebstein anomaly at the Tomsk Institute of Cardiology in Siberia.
From 2012 through 2015, 4 boys and 2 girls (age range, 11 mo–12 yr) underwent surgery to correct Ebstein anomaly. All had presented with cyanosis, exertional dyspnea, fatigue, or new-onset atrial arrhythmia, and none had undergone previous cardiac surgery. All survived the operation. One patient needed tricuspid valve replacement with a bioprosthesis after early breakdown of the cone reconstruction. As of December 2016, all the patients had no symptoms, tricuspid stenosis, or arrhythmia. This series indicates that cone reconstruction—the most anatomic repair technique for the dysmorphic Ebstein tricuspid valve—can be successfully performed in pediatric heart centers with a large experience.
Keywords: Cardiac surgical procedures/methods, Ebstein anomaly/surgery, reconstructive surgical procedures/methods, recovery of function, reproducibility of results, treatment outcome, tricuspid valve/abnormalities/surgery, tricuspid valve insufficiency/etiology/surgery
In 1866, Wilhelm Ebstein described autopsy findings of a strikingly abnormal tricuspid valve (TV).1,2 In the 1950s, this lesion was termed Ebstein anomaly or Ebstein malformation. After many surgeons unsuccessfully attempted to repair Ebstein anomaly through the years, da Silva described the cone reconstruction (CR) technique.3,4 Modifications of this technique have been used by Dearani and by others,5–9 and CR has become the preferred repair method for patients with Ebstein anomaly. We describe the procedure and report the outcomes of the initial 6 patients who underwent modified CR at a major pediatric cardiac center in Tomsk, Russia.
Patients and Methods
The Tomsk Institute of Cardiology, a large referral center in Siberia for congenital heart disease surgery, serves patients from Russia and several former Soviet republics. Consent for treatment and data-sharing was obtained for the patients involved in this report.
Patients. From 2012 through 2015, 4 girls and 2 boys (age range, 11 mo–12 yr) underwent corrective surgery for Ebstein anomaly at the Tomsk Institute of Cardiology. All presented with cyanosis, exertional dyspnea, fatigue, or new-onset atrial arrhythmia. None had undergone prior cardiac surgery. One patient with Wolff-Parkinson-White syndrome needed preoperative radiofrequency catheter ablation. The primary surgeon (EVK) performed CR with use of Dearani modifications8,9 that he had learned at the Mayo Clinic in 2012.
Surgical Technique. The modified CR technique involves surgical delamination (dissection) of all viable leaflet tissue from the myocardium and approximation of the sides of the leaflets to form a cone that completely surrounds the tricuspid orifice in the atrioventricular (AV) junction.5,8,9 The reconstructed valve is anchored at the hinge point of the right AV groove.
The most important aspect of CR is to free all fibrous and muscular attachments between the leaflet bodies and the myocardium while preserving the fibrous attachments of the leaflets' leading edges, particularly the chordae tendineae. Surgical fenestrations enable improved leaflet mobility while these chordal attachments are maintained.
Delamination is started at the 12 o'clock position of the anterior leaflet of the TV, just below the true anatomic annulus, and is continued clockwise toward the inferior leaflet. After the septal leaflet has been delaminated, the mobilized inferior leaflet is rotated clockwise and approximated to the leading edge of the septal leaflet. This creates a 360° cone of leaflet tissue, which gives the reconstructed TV hinge points at the true anatomic annulus. Care is taken throughout to avoid injuring the right coronary artery, especially during plication of the atrialized right ventricle (RV), and to prevent heart block. Adding a partial or complete annuloplasty ring can reduce postoperative valvular regurgitation.
Results
All 6 patients survived surgery. Intra-atrial communications were closed in each. Five of the 6 underwent successful CR and were discharged from the hospital with excellent results. A 12-year-old girl had unfavorable leaflet anatomy that led to early breakdown of the CR; her TV was replaced with a bioprosthesis.
The longest follow-up duration was 4 years. As of December 2016, the 5 patients with enduring CR had no symptoms, arrhythmias, right-sided heart failure, or tricuspid stenosis, and only mild tricuspid regurgitation. In 2 patients, RV systolic function remained mildly depressed.
Figures 1 and 2 show the preoperative anatomy in a 3-year-old girl from this cohort. Images obtained before her hospital discharge (Figs. 3 and 4) reveal the excellent surgical result: the TV leaflets residing at the level of the true anatomic annulus, minimal residual tricuspid regurgitation, and a large portion of the RV below the level of leaflet coaptation in comparison with the preoperative findings. Qualitative RV systolic function was good in this patient after CR.
Fig. 1.
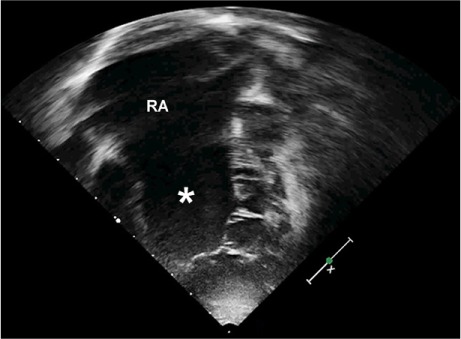
Two-dimensional transthoracic echocardiogram (4-chamber view, in diastole) shows a severe form of Ebstein anomaly of the tricuspid valve in a 3-year-old girl: tethering of the septal and anterior leaflets, impaired delamination of the septal leaflet, and displacement of the valve's hinge point to the level of the moderator band. There is severe right atrial (RA) enlargement, and a sizable atrialized portion of the severely enlarged right ventricle (asterisk).
Fig. 2.
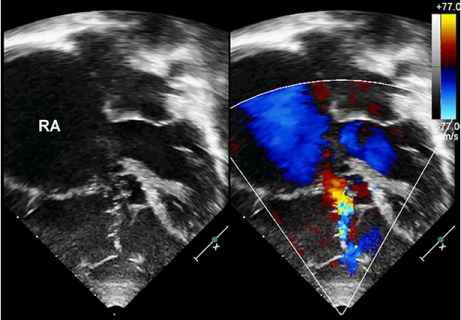
Two-dimensional transthoracic echocardiogram (4-chamber view and color-flow Doppler mode, in systole), shows a lack of leaflet coaptation and severe tricuspid regurgitation in the 3-year-old girl.
RA = right atrium
Fig. 3.
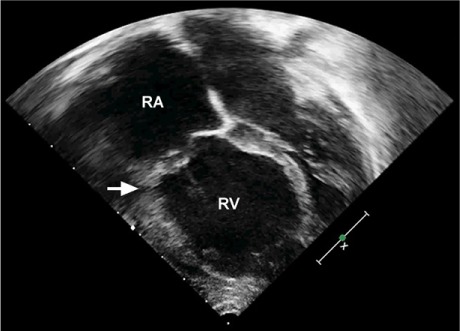
Two-dimensional transthoracic echocardiogram (4-chamber view) after cone reconstruction in the 3-year-old girl shows leaflets residing at the level of the anatomic annulus (arrow). A large portion of the right ventricle (RV) has been reclaimed.
RA = right atrium
Fig. 4.
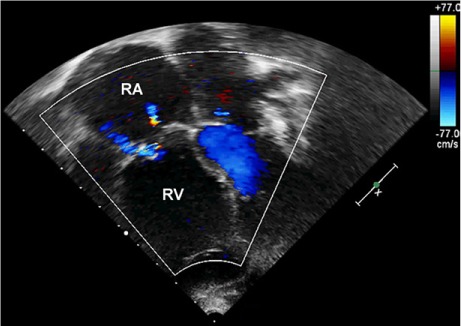
Two-dimensional transthoracic echocardiogram (4-chamber view in color-flow Doppler mode) after cone reconstruction in the 3-year-old girl shows mild residual tricuspid regurgitation and a mean inflow gradient of 3 mmHg.
RA = right atrium; RV = right ventricle
Discussion
In correcting Ebstein anomaly, surgical goals include complete or subtotal closure of intra-atrial communications, TV repair or replacement, elimination of arrhythmia, plication of the atrialized RV, right reduction atrioplasty, and repair of associated defects (such as closing ventricular septal defects and relieving RV outflow tract obstruction). Cone reconstruction, the most anatomic repair technique described to date, has revolutionized TV repair in patients with Ebstein anomaly.
In 2007, da Silva and colleagues3 reported the cases of 40 patients who underwent correction of Ebstein anomaly by means of CR. In 2013, Anderson and colleagues10 reported that 98% of Mayo Clinic patients younger than 21 years of age who had undergone CR were discharged from the hospital with good results. To date, reports of postoperative arrhythmias have been rare in this patient population.
No one in our series needed a bidirectional cavopulmonary anastomosis (BDCPA) at the time of CR. A BDCPA is indicated when there is severe RV enlargement or dysfunction, compression of the left ventricle because of a shift of the interventricular septum, or moderate TV stenosis after CR (mean gradient, ≥8 mmHg); or when the right atrial-to-left atrial pressure ratio is >1.5, indicating poor RV function.
In the Mayo Clinic series,10 approximately 30% of patients who underwent CR also needed a BDCPA, which is not ideal because it can cause pulsatility of the head and neck veins, facial swelling, and the development of venovenous collateral vessels and pulmonary artery venous fistulae. In addition, BDCPA procedures preclude access to the heart from the internal jugular vein in future cardiac catheterization or electrophysiologic procedures.11,12
One patient, a 12-year-old girl, underwent TV replacement with a bioprosthesis after the CR failed. Her anterior leaflet was heavily muscularized, and there was no septal leaflet delamination. Patients with Ebstein anomaly and this valvular morphology are challenging candidates for any form of TV repair. However, CR can be viable for other patients who have recurrent, severe tricuspid regurgitation after previously attempted TV repairs.8
This case series indicates that other pediatric cardiac centers can adopt the CR technique for Ebstein anomaly with reproducible results. However, mentoring in the nuances of Ebstein-anomaly repair is essential. Care needs to be given to the right posterior AV groove to avoid injury to the right coronary artery, and one must meticulously avoid causing heart block. The Tomsk Institute's lead surgeon (EVK) spent considerable time studying CR techniques at the Mayo Clinic. Despite the steep learning curve, CR has greatly improved the treatment of children with Ebstein anomaly, providing the most anatomic repair of the dysmorphic TV.
Acknowledgments
The authors acknowledge the echocardiographic contributions of Galina Martsinkevich, MD, and the translation contributions of Ms Irma Ozashvilli of the Tomsk Institute of Cardiology. The partnership of Heart to Heart International Children's Medical Alliance (Oakland, Calif) with the Tomsk Institute of Cardiology enabled this international education effort.
References
- 1. Ebstein W. . Uber einen sehr seltenen Fall von Insufficienz der Valvula tricuspidalis, bedingt durch eine angeborene hochgradige Misshildung derselben [in German]. Arch Anat Physiol 1866; 33: 238– 54. Available from: https://babel.hathitrust.org/cgi/pt?id=mdp.39015033391502;view=1up;seq=250. [Google Scholar]
- 2. Schiebler GL, Gravenstein JS, Van Merop LH. . Ebstein's anomaly of the tricuspid valve. Translation of original description with comments. Am J Cardiol 1968; 22( 6): 867– 73. Available in part from: http://www.ajconline.org/article/0002-9149(68)90185-9/fulltext. [DOI] [PubMed] [Google Scholar]
- 3. da Silva JP, Baumgratz JF, da Fonseca L, Franchi SM, Lopes LM, Tavares GM, . et al. The cone reconstruction of the tricuspid valve in Ebstein's anomaly. The operation: early and mid-term results. J Thorac Cardiovasc Surg 2007; 133( 1): 215– 23. [DOI] [PubMed] [Google Scholar]
- 4. Silva JP, Silva Lda F, Moreira LF, Lopez LM, Franchi SM, Lianza AC, . et al. Cone reconstruction in Ebstein's anomaly repair: early and long-term results [in Portuguese]. Arq Bras Cardiol 2011; 97( 3): 199– 208. [DOI] [PubMed] [Google Scholar]
- 5. Dearani JA, Bacha E, da Silva JP. . Cone reconstruction of the tricuspid valve for Ebstein's anomaly: anatomic repair. Oper Tech Thoracic Cardiovasc Surg 2008; 13: 109– 25. Available from: http://www.optechtcs.com/article/S1522-2942(08)00028-7/pdf. [Google Scholar]
- 6. Sata S, Murin P, Hraska V. . Cone reconstruction of Ebstein's anomaly in a neonate. Ann Thorac Surg 2012; 94( 4): e99– 100. [DOI] [PubMed] [Google Scholar]
- 7. Vogel M, Marx GR, Tworetzky W, Cecchin F, Graham D, Meyer JE, . et al. Ebstein's malformation of the tricuspid valve: short-term outcomes of the “cone procedure” versus conventional surgery. Congenit Heart Dis 2012; 7( 1): 50– 8. [DOI] [PubMed] [Google Scholar]
- 8. Dearani JA, Said SM, Burkhart HM, Pike RB, O'Leary PW, Cetta F. . Strategies for tricuspid re-repair in Ebstein malformation using the cone technique. Ann Thorac Surg 2013; 96( 1): 202– 10. [DOI] [PubMed] [Google Scholar]
- 9. Dearani JA, Said SM, O'Leary PW, Burkhart HM, Barnes RD, Cetta F. . Anatomic repair of Ebstein's malformation: lessons learned with cone reconstruction. Ann Thorac Surg 2013; 95( 1): 220– 8. [DOI] [PubMed] [Google Scholar]
- 10. Anderson HN, Dearani JA, Said SM, Norris MD, Pundi KN, Miller AR, . et al. Cone reconstruction in children with Ebstein anomaly: the Mayo Clinic experience. Congenit Heart Dis 2014; 9( 3): 266– 71. [DOI] [PubMed] [Google Scholar]
- 11. Quinonez LG, Dearani JA, Puga FJ, O'Leary PW, Driscoll DJ, Connolly HM, Danielson GK. . Results of the 1.5-ventricle repair for Ebstein anomaly and the failing right ventricle. J Thorac Cardiovasc Surg 2007; 133( 5): 1303– 10. [DOI] [PubMed] [Google Scholar]
- 12. Chauvaud S, Fuzellier JF, Berrebi A, Lajos P, Marino JP, Mihaileanu S, Carpentier A. . Bi-directional cavopulmonary shunt associated with ventriculo and valvuloplasty in Ebstein's anomaly: benefits in high risk patients. Eur J Cardiothorac Surg 1998; 13( 5): 514– 9. [DOI] [PubMed] [Google Scholar]


