Abstract
Background:
The posteromedial cortex (PMC) is a collective term for an anatomically heterogeneous area of the brain constituting a core node of the human default mode network (DMN), which is engaged during internally focused subjective cognition such as autobiographical memory.
Methods:
We explored the effects of causal perturbations of PMC with direct electric brain stimulation (EBS) during presurgical epilepsy monitoring with intracranial EEG electrodes.
Results:
Data were collected from 885 stimulations in 25 patients implanted with intracranial electrodes across the PMC. While EBS of regions immediately dorsal or ventral to the PMC reliably produced somatomotor or visual effects, respectively, we found no observable behavioral or subjectively reported effects when sites within the boundaries of PMC were electrically perturbed. In each patient, null effects of PMC stimulation were observed for sites in which intracranial recordings had clearly demonstrated electrophysiologic responses during autobiographical recall.
Conclusions:
Direct electric modulation of the human PMC produced null effects when standard functional mapping methods were used. More sophisticated stimulation paradigms (e.g., EBS during experimental cognitive tests) will be required for testing the causal contribution of PMC to human cognition and subjective experience. Nonetheless, our findings suggest that some extant theories of PMC and DMN contribution to human awareness and subjective conscious states require cautious re-examination.
The posteromedial cortex (PMC) includes Brodmann areas 23 (posterior cingulate), 29/30 (retrosplenial cortex), 7m (medial parietal cortex), and 311 (figure 1). Early human neuroimaging studies identified PMC as a unique brain region displaying high resting cerebral metabolism and blood flow,2,3 deactivation during goal-directed attention tasks,4 and activation during tasks of internal mentation such as episodic memory retrieval.5,6 This unique profile of functional response occurs consistently in concert with a host of brain regions known as the default mode network (DMN).7
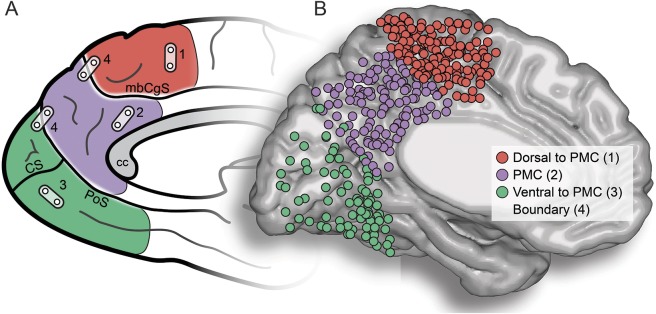
Figure 1. PMC and EBS sites.
(A) Schematic of anatomic regions of interest is shown. Posteromedial cortex (PMC; purple) reflects posterior medial parietal cortex, which is ventral to the marginal branch of the cingulate sulcus (mbCgS) and dorsal to the parieto-occipital sulcus (PoS; calcarine sulcus [CS] also shown). PMC is therefore intermediate between dorsal (red) medial motor/somatomotor cortex and ventral medial occipital cortex (green). For electric brain stimulation (EBS), bipolar pairs of neighboring electrodes are used, and EBS can therefore occur within an anatomic region of interest (1–3) or at/across the boundary of anatomic regions (4). (B) Visualization of all electrode sites used for EBS on a normalized cortical surface (Montreal Neurological Institute; left hemisphere). All right hemisphere sites have been reflected onto the left hemisphere. Colors of electrodes are based on the native anatomic location within participants, making direct anatomic mapping to the normalized surface imprecise. CC = corpus callosum.
Clinical research has focused on changes in the high baseline activity of the PMC across levels of conscious state.3 PMC activity is systematically suppressed during drug-induced loss of consciousness8–12 and sleep13 and is reversed with the restoration of consciousness from the vegetative state.14–16
The extant data have led to suggestions that the PMC may have an essential role in maintaining coherent subjective awareness and therefore may serve as a sensitive locus of conscious state.17–19 However, despite the appeal of this impressive role, the causal contribution of PMC to human subjective experience has remained unknown. A challenge to this endeavor is the hidden anatomic location of the PMC within the longitudinal fissure, limiting noninvasive stimulation studies.
Recently, our group has used invasive intracranial recordings in patients with epilepsy to explore the cognitive neurophysiology of the PMC.20–24 Across a series of studies, our work has focused on the local and network dynamics of human PMC during conditions previously reported to differentially engage the DMN.7 Given the converging lines of empiric evidence regarding PMC function, its putative clinical relevance, and the paucity of causal data, we designed the following study to quantify the effects of perturbing PMC function by direct electric brain stimulation (EBS).
METHODS
Participants.
Data reported here were obtained from 25 patients undergoing invasive electrophysiologic monitoring for the surgical treatment of refractory epilepsy at the Stanford Medical Center (California). The sample comprised 11 women and 14 men with a mean ± SD age of 36.28 ± 11.41 years and a mean ± SD full-scale IQ of 95.2. ± 19 (obtained from 17 participants for whom the data were available). As discussed below, experimental task data from many of these participants have previously been reported.20–24
Standard protocol approvals, registrations, and patient consents.
All participants provided verbal and written consent before participating in the research presented here, which was approved by the Stanford Institutional Review Board for human experiments.
Electrode implantation/localization.
Participants were surgically implanted with subdural strip/grid electrode arrays (Adtech Medical Instruments, Racine, WI) for electrocortical recording and stimulation. The location and configuration of electrode implantations were completely driven by the clinical requirements unique to each participant. Electrodes were circular platinum with a physical diameter of 4 mm and imbedded in a flexible silicon sheet, with an exposed recording diameter of 2.3 mm (interelectrode distance 10 mm center to center).
To identify electrode locations on each participant's cortical surface, preoperative MRI scans were coregistered with postoperative CT scans. Individual electrodes were identified within the CT scan, and strip/grid arrays were projected to the cortical surface to account for alignment error and brain shift.25 Cortical surface electrode locations and volume MRI/CT images were used to identify participants for the present study. For visualization, electrodes were normalized to Montreal Neurological Institute space (Montreal Neurological Institute Colin 27).
Anatomy.
All participants included here had undergone preresection electric stimulation functional mapping, specifically within regions of the PMC. For our purposes, PMC was defined in each individual with sulcal landmarks used as the selection boundaries (i.e., inferior/ventral to the marginal branch of the cingulate sulcus and anterior/dorsal to the parieto-occipital sulcus). For positive controls, we include electrode sites extending beyond these boundaries if they were physically part of an electrode array falling within the PMC boundary. As shown in figure 1, the PMC neighbors the medial somatomotor and visual cortices. We therefore classified all electric stimulations into 3 gross anatomic regions: dorsal, which is anterior/superior to the marginal branch of the cingulate sulcus; ventral, which is posterior/inferior to the parieto-occipital sulcus; and PMC, our region of interest, which reflects the medial cortex between these 2 landmarks (figure 1). To limit the confound of ictal phenomena, none of the included patients were clinically identified to have a PMC seizure-onset zone or to receive a PMC subregion surgical resection.
Electric brain stimulation.
EBS is routinely preformed in preparation for surgical resection as a functional mapping technique. A common goal of this procedure is to identify eloquent cortex supporting essential sensory-motor functions. Data reported here come from functional mapping procedures performed on each participant typically in one session of EBS. During this procedure, pairs of neighboring electrodes are selected for bipolar stimulation, in which an alternating square wave current is delivered between the 2 electrodes. The clinician performing the procedure decides stimulation parameters, which typically have a duration of 1 to 3 seconds and a frequency of 50 to 100 Hz with current levels ≈2 mA below the threshold for producing after discharges (i.e., 2–10 mA). Stimulations were delivered with either an Ojemann cortical stimulator (Integra, Plainsboro, NJ) or an S12X Grass cortical stimulator (Grass Technologies, Pleasanton, CA).
For all EBS procedures, continuous electrocorticography (ECoG) recordings were performed simultaneously, along with continuous video/audio recordings. During the EBS procedure, stimulation parameters were logged along with the observed or reported effect of each stimulation. External raters, blinded to the aims of this project, secondarily screened digitized EBS data logs by watching the recorded procedure to confirm behavior/subjective reports. For a given stimulation, any observed behavior (e.g., hand movement) was noted in addition to any subjective report (e.g., “I saw a bright flash of light”) provided by the participant.
To classify the differing types of observed or reported EBS effects, we used 5 basic categories: motor, somatomotor, visual, auditory, or cognitive. Stimulations were therefore classified as 1 of these 5 categories or listed as no effect. While more nuanced classification categories might allow more refined insight, subsequent analysis showed that additional categories were redundant, as discussed below.
RESULTS
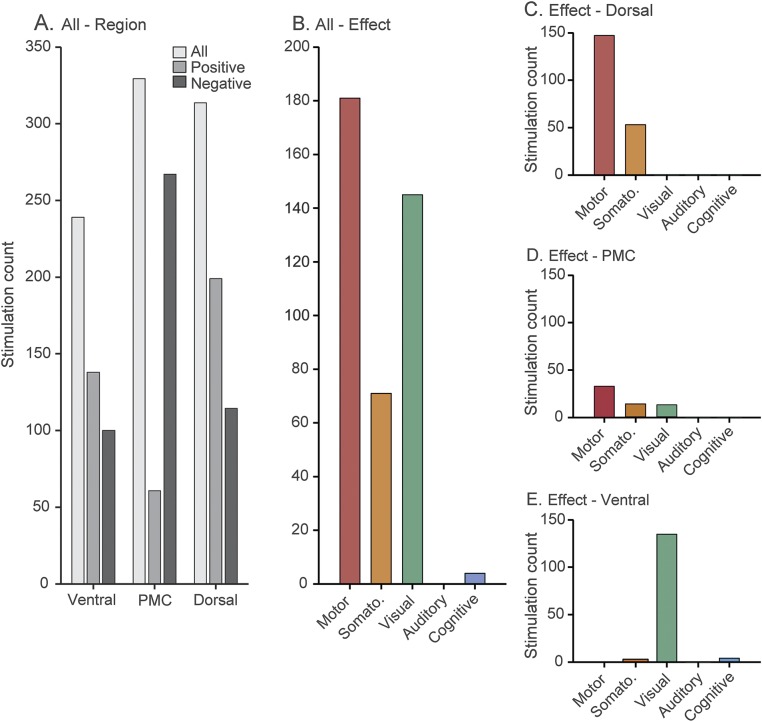
Overall, we obtained 885 cortical stimulations across 25 participants (hemisphere: 7 right/18 left) and classified these data on the basis of their anatomic location (dorsal, ventral, PMC) and behavioral or subjective effect (motor, somatomotor, visual, auditory, cognitive). More stimulations were performed within the PMC (stimulation n = 330) followed by the dorsal (stimulation n = 315) and ventral (stimulation n = 240) regions. Across all stimulations, 401 (45.31%) were classified as producing an observed or reported effect. Figure 2 shows the histogram of effects for all effective stimulation sites. In general, effective stimulations produced only motor (stimulation n = 181, 45.14%), somatomotor (stimulation n = 71, 17.70%), or visual effects (stimulation n = 145, 36.16%).
Figure 2. Count data for regional effects of EBS.
(A) Bar plot shows count data for entire dataset, depicting the rate of observed (positive) and null (negative) stimulations. (B) Bar plot shows count data (all regions) for all effective stimulations across the 5 categories of classification. (C–E) Bar plots show the effects of stimulation for the dorsal (C), posteromedial cortex (PMC; D), and ventral (E) regions of interest. Stimulation in dorsal regions produces the bulk of motor/somatomotor effects, whereas stimulation of ventral regions produces the majority of visual effects. PMC shows minimal stimulation effects; however, all of these observations come from stimulations performed on boundary stimulation pairs, which will jointly engage either dorsal or ventral regions. When these confounded pairs are excluded, no positive effects remain for PMC. EBS = electric brain stimulation.
Given this clustering of stimulation effects, we next separated all effective stimulations by anatomic location. Figure 2, C–E shows the histograms of stimulation effects for each anatomic location of interest. While dorsal and ventral regions show an expected dissociation of motor/somatomotor and visual effects, respectively, minimal effects are observed in PMC. Indeed, when stimulation cases that were considered a boundary pair (on or crossing a boundary sulcus) are taken into account, no stimulation effects were observed within the PMC from a total of 248 stimulations. This distribution of stimulation effects across anatomic locations was similar between the left and right hemispheres. We quantified these differences in count data using a mixed-effects Poisson regression with participant as a random effect. For this analysis, we combined left and right hemisphere data and excluded the auditory condition because there were no observed effects for this category. As clearly shown in figure 2, a significant difference in the stimulation effects was observed between the PMC and dorsal/ventral regions (p < 0.001) and between the dorsal and ventral regions (p < 0.01).
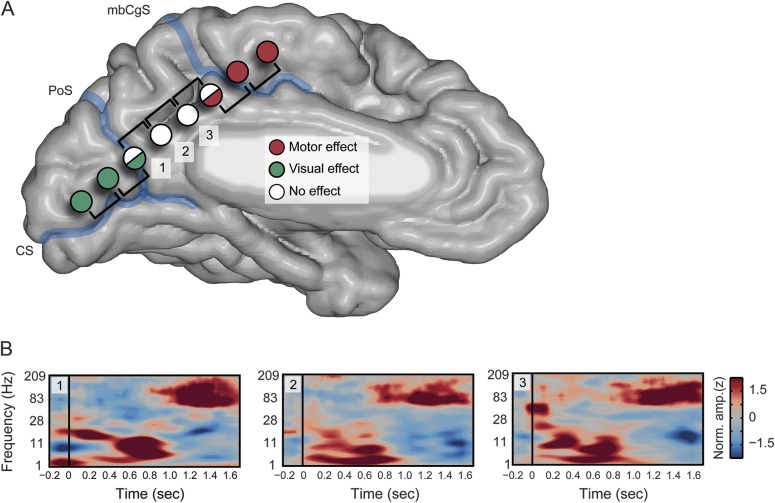
Together, the stimulation data show predictable stimulation effects ventral and dorsal to the PMC but a lack of any specific observed or subjective effect of stimulation within the PMC when boundary stimulations are excluded. Importantly, failure of PMC stimulation was not due to erroneous or null stimulations unique to this region because many participants had stimulations performed sequentially along electrode arrays spanning the PMC and either the ventral or dorsal regions (figure 3). Furthermore, in these cases, the transition to observed/subjective effects closely matches the anatomic boundaries used, consistent with our previous work21,22 (figure 3). Critically, many of the PMC sites stimulated in this study show clear ECoG high-frequency task responses, reflecting DMN function, as previously reported.20,21,23,24 As shown in figure 3, recordings from the PMC produce clear functional responses during episodic retrieval; however, only stimulation of the ventral and dorsal regions caused any subjective or observed effect.
Figure 3. PMC null EBS effects observed at task positive sites.
(A) Stimulation effects are shown for an example participant (P6), with a linear continuous electrocorticography (ECoG) array spanning all anatomic regions of interest. Within this participant, subsequent stimulation across the array shows that ventral stimulations produce reliable visual effects (e.g., “I see a bunch of waves,” right lower visual field) followed by no effects on stimulation of the posteromedial cortex (PMC) and then the observation of motor effects (e.g., “Right leg jerk”) with the transition to dorsal regions. The transition of effects closely matches our anatomic boundaries (marginal branch of the cingulate sulcus [mbCgS] and parieto-occipital sulcus [PoS]) and helps to highlight the influence of boundary pair stimulations on observed/reported effects. While the PMC sites display no effects, it is not because this region is pathologic or otherwise nonfunctional. (B) Time-frequency plots are shown for 3 PMC electrodes from panel A (1–3). Each spectrogram displays characteristic properties of ECoG response such as high-frequency power increase and concomitant low-frequency suppression. These responses are during task conditions of autobiographical retrieval in which the participant must respond if an autobiographical statement is true or false (e.g., “I ate fruit this morning”). This spectral response is selective to episodic retrieval conditions and has a replicable late onset, consistent with the time course of retrieval search. These data have previously been published21 and replicated elsewhere.23,24 CS = calcarine sulcus; EBS = electric brain stimulation.
DISCUSSION
Direct causal perturbation of the PMC produced no pronounced change in participants' reported conscious state or observed behavior. These stimulations included PMC sites where clear electrocortical responses had been observed during memory retrieval experiments.20–24 While the mapping results reported here are consistent with basic anatomic predictions within sensory cortices, the differing efficacy of EBS across neighboring cortical regions and the discrepancies between EBS and functional ECoG data are concerning. Together these findings have important implications for the cognitive neurology of PMC and clinical practice more generally.
Our data strongly temper the possibility of the PMC being a sensitive locus of the subjective state or level of awareness.18,19,26,27 Along these lines, our findings are in contrast to previous reports of disrupting conscious awareness by stimulating tumor bed sites in human PMC.28,29 Moreover, our data also limit the involvement of PMC in early sensory-motor function, consistent with its pattern of anatomic connectivity in the nonhuman primate.1,30 These observations still leave many unanswered questions for the functional and therefore clinical consequences of PMC subregion disruption. Selective lesions to the PMC region are rare and often include white matter tracts, clouding deficit interpretation.31 Similarly, the semiology of PMC subregion seizures is highly diverse and often confounded by common secondary propagation pathways.32 Collectively, neurologic data on PMC function are both sparse and inconsistent, highlighting an important research domain for clinicians and scientists.
Of clinical relevance is our contrasting observation for the effects of EBS in associative (PMC) vs somatomotor and visual cortices. While electric stimulation to the latter clearly leads to strong changes in the participant's sensory domain, the former leads to no subjective or pronounced behavioral changes. While strong empiric data are lacking, we speculate that the effect of EBS in the PMC can be functionally mapped only under conditions of task-specific perturbation (i.e., during associative/integrative functional engagement). Such an approach is more closely aligned with procedures in other high-order regions where language mapping in frontotemporal cortices is explored during active speech.33
More generally, EBS is used regularly to help tailor surgical resection, seeking to identify and preserve eloquent brain regions. However, associative cortices like the PMC produce a challenge for effective functional mapping of a large mantle of the human brain. As our data clearly show, standard EBS mapping will provide limited insight, and possibly false negatives, for higher associative cortices. This argument draws support from recent efforts to leverage the high signal-to-noise ratio of ECoG recordings for rapid task-based functional mapping.34 However, as recently noted,35 it is of future importance to better understand the deficits associated with resection guided by ECoG task-positive regions. Along these lines, an important caveat for task response–based ECoG mapping is the fundamental distinction between correlational and causal functional significance. While ECoG recordings sensitively capture evoked task responses, this alone does not provide causal evidence that the observed region is necessary or critical for the function interrogated. It is in this regard that EBS methods will continue to serve a critical role in functional mapping.
As we have noted above and emphasize here again, our limited effects of standard EBS mapping suggest that more subtle experimental task paradigms are required for association cortices to observe the effects of stimulation on behavior (e.g., episodic memory retrieval). However, achieving a sufficiently sampled behavioral effect under EBS task conditions is both technically challenging and time-consuming. For example, unlike speech arrest, the behavioral correlates for higher cognitive functions such as memory retrieval require pretask performance benchmarking, multiple trial repetitions for behavioral inference, and an unwieldy array of potential stimulation protocols. Regarding stimulation protocols, we note that our own null findings may reflect the stimulation parameters used and that using greater stimulation levels/durations may be necessary to cause observable or reported effects. However, our previous observations from higher-order parietal36 and temporal37–39 cortices in similar patients suggest that the current levels used are sufficient to cause reliable subjective effects.
Given clinical constraints, focusing on task-based cortical mapping via event-related ECoG responses may be an efficient and complementary approach. Progress in quantifying common spectral features of electrocortical response suggests that tracking high-frequency broadband activity (e.g., 70–200 Hz) is a promising metric for rapid task-based functional mapping.34,40,41 This frequency range shows desirable spatiotemporal precision and a general invariance across associative and primary cortical regions.42,43 An added benefit for this approach is that it fosters close collaborations between clinical teams and cognitive neuroscientists who are interested in collecting intracranial data. The development of task-based ECoG functional mapping techniques allows researchers benefiting from volunteering intracranial monitoring patients to, in turn, use their science to help improve patient care.
GLOSSARY
- DMN
default mode network
- EBS
electric brain stimulation
- ECoG
continuous electrocorticography
- PMC
posteromedial cortex
AUTHOR CONTRIBUTIONS
Dr. Brett Foster: designed and conducted the study, analyzed the data, wrote the manuscript. Dr. Josef Parvizi: designed and conducted the study, wrote the manuscript.
STUDY FUNDING
Funding was provided by the National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, and National Science Foundation.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA 2006;103:1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2001;2:685–694. [DOI] [PubMed] [Google Scholar]
- 4.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage 2007;37:1083–1090; discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ. The mind's eye: precuneus activation in memory-related imagery. Neuroimage 1995;2:195–200. [DOI] [PubMed] [Google Scholar]
- 6.Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci 2004;24:10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 8.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science 2008;322:876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkire MT, Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res 2005;150:229–244. [DOI] [PubMed] [Google Scholar]
- 10.Langsjo JW, Alkire MT, Kaskinoro K, et al. Returning from oblivion: imaging the neural core of consciousness. J Neurosci 2012;32:4935–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boly M, Moran R, Murphy M, et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci 2012;32:7082–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie G, Deschamps A, Backman SB, et al. Critical involvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br J Anaesth 2011;106:548–557. [DOI] [PubMed] [Google Scholar]
- 13.Maquet P. Functional neuroimaging of normal human sleep by positron emission tomography. J Sleep Res 2000;9:207–231. [DOI] [PubMed] [Google Scholar]
- 14.Laureys S, Lemaire C, Maquet P, Phillips C, Franck G. Cerebral metabolism during vegetative state and after recovery to consciousness. J Neurol Neurosurg Psychiatry 1999;67:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 2004;3:537–546. [DOI] [PubMed] [Google Scholar]
- 16.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014;137:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boly M, Phillips C, Tshibanda L, et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann NY Acad Sci 2008;1129:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boveroux P, Bonhomme V, Boly M, Vanhaudenhuyse A, Maquet P, Laureys S. Brain function in physiologically, pharmacologically, and pathologically altered states of consciousness. Int Anesthesiol Clin 2008;46:131–146. [DOI] [PubMed] [Google Scholar]
- 19.Tononi G, Koch C. The neural correlates of consciousness: an update. Ann NY Acad Sci 2008;1124:239–261. [DOI] [PubMed] [Google Scholar]
- 20.Dastjerdi M, Foster BL, Nasrullah S, et al. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci USA 2011;108:3023–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster BL, Dastjerdi M, Parvizi J. Neural populations in human posteromedial cortex display opposing responses during memory and numerical processing. Proc Natl Acad Sci USA 2012;109:15514–15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster BL, Parvizi J. Resting oscillations and cross-frequency coupling in the human posteromedial cortex. Neuroimage 2012;60:384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster BL, Kaveh A, Dastjerdi M, Miller KJ, Parvizi J. Human retrosplenial cortex displays transient theta phase locking with medial temporal cortex prior to activation during autobiographical memory retrieval. J Neurosci 2013;33:10439–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster BL, Rangarajan V, Shirer WR, Parvizi J. Intrinsic and task-dependent coupling of neuronal population activity in human parietal cortex. Neuron 2015;86:578–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermes D, Miller KJ, Noordmans HJ, Vansteensel MJ, Ramsey NF. Automated electrocorticographic electrode localization on individually rendered brain surfaces. J Neurosci Methods 2010;185:293–298. [DOI] [PubMed] [Google Scholar]
- 26.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron 2011;70:200–227. [DOI] [PubMed] [Google Scholar]
- 27.Qin P, Duncan N, Northoff G. Why and how is the self-related to the brain midline regions? Front Hum Neurosci 2013;7:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbet G, Lafargue G, de Champfleur NM, et al. Disrupting posterior cingulate connectivity disconnects consciousness from the external environment. Neuropsychologia 2014;56:239–244. [DOI] [PubMed] [Google Scholar]
- 29.Herbet G, Lafargue G, Duffau H. The dorsal cingulate cortex as a critical gateway in the network supporting conscious awareness. Brain 2016;139:e23. [DOI] [PubMed] [Google Scholar]
- 30.Balestrini S, Francione S, Mai R, et al. Multimodal responses induced by cortical stimulation of the parietal lobe: a stereo-electroencephalography study. Brain 2015;138:2596–2607. [DOI] [PubMed] [Google Scholar]
- 31.Pflugshaupt T, Nosberger M, Gutbrod K, Weber KP, Linnebank M, Brugger P. Bottom-up visual integration in the medial parietal lobe. Cereb Cortex 2016;26:943–949. [DOI] [PubMed] [Google Scholar]
- 32.Enatsu R, Bulacio J, Nair DR, Bingaman W, Najm I, Gonzalez-Martinez J. Posterior cingulate epilepsy: clinical and neurophysiological analysis. J Neurol Neurosurg Psychiatry 2014;85:44–50. [DOI] [PubMed] [Google Scholar]
- 33.Ojemann GA. Functional mapping of cortical language areas in adults: intraoperative approaches. Adv Neurol 1993;63:155–163. [PubMed] [Google Scholar]
- 34.Wang Y, Fifer MS, Flinker A, et al. Spatial-temporal functional mapping of language at the bedside with electrocorticography. Neurology 2016;86:1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asano E, Gotman J. Is electrocorticography-based language mapping ready to replace stimulation? Neurology 2016;86:1174–1176. [DOI] [PubMed] [Google Scholar]
- 36.Rauschecker AM, Dastjerdi M, Weiner KS, et al. Illusions of visual motion elicited by electrical stimulation of human MT complex. PLoS One 2011;6:e21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parvizi J, Jacques C, Foster BL, et al. Electrical stimulation of human fusiform face-selective regions distorts face perception. J Neurosci 2012;32:14915–14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rangarajan V, Hermes D, Foster BL, et al. Electrical stimulation of the left and right human fusiform gyrus causes different effects in conscious face perception. J Neurosci 2014;34:12828–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rangarajan V, Parvizi J. Functional asymmetry between the left and right human fusiform gyrus explored through electrical brain stimulation. Neuropsychologia 2016;83:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schalk G, Leuthardt EC, Brunner P, Ojemann JG, Gerhardt LA, Wolpaw JR. Real-time detection of event-related brain activity. Neuroimage 2008;43:245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung C, Chang EF. Real-time, time-frequency mapping of event-related cortical activation. J Neural Eng 2012;9:046018. [DOI] [PubMed] [Google Scholar]
- 42.Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog Neurobiol 2012;98:279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller KJ, Honey CJ, Hermes D, Rao RP, denNijs M, Ojemann JG. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. Neuroimage 2014;85(pt 2):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]