Abstract
Objective:
To integrate long-term measures of disease-modifying drug efficacy and risk to guide selection of first-line treatment of multiple sclerosis.
Methods:
We created a Markov decision model to evaluate disability worsening and progressive multifocal leukoencephalopathy (PML) risk in patients receiving natalizumab (NTZ), fingolimod (FGL), or glatiramer acetate (GA) over 30 years. Leveraging publicly available data, we integrated treatment utility, disability worsening, and risk of PML into quality-adjusted life-years (QALYs). We performed sensitivity analyses varying PML risk, mortality and morbidity, and relative risk of disease worsening across clinically relevant ranges.
Results:
Over the entire reported range of NTZ-associated PML risk, NTZ as first-line therapy is predicted to provide a greater net benefit (15.06 QALYs) than FGL (13.99 QALYs) or GA (12.71 QALYs) treatment over 30 years, after accounting for loss of QALYs due to PML or death (resulting from all causes). NTZ treatment is associated with delayed worsening to an Expanded Disability Status Scale score ≥6.0 vs FGL or GA (22.7, 17.0, and 12.4 years, respectively). Compared to untreated patients, NTZ-treated patients have a greater relative risk of death in the early years of treatment that varies according to PML risk profile.
Conclusions:
NTZ as a first-line treatment is associated with the highest net benefit across full ranges of PML risk, mortality, and morbidity compared to FGL or GA. Integrated modeling of long-term treatment risks and benefits informs stratified clinical decision-making and can support patient counseling on selection of first-line treatment options.
Multiple sclerosis (MS) is a leading cause of neurologic disability in young adults, with the relapsing-remitting (RRMS) form as the predominant subtype.1 Disease-modifying drugs (DMDs) for RRMS have been available for >2 decades.2 With the growing choices of DMDs, there is an increasing need for stratified treatment guidance in MS because of the variable responses and adverse events.
Among the approved DMDs for MS, natalizumab (NTZ) has shown high efficacy,2 but association with progressive multifocal leukoencephalopathy (PML), a rare but serious brain disease attributed to the John Cunningham (JC) virus, has limited its use.3 At least 3 factors influence the risk of NTZ-associated PML: JC virus antibody levels, prior immunosuppressant exposure, and duration of NTZ treatment. These factors collectively stratify an individual patient's risk for PML.3,4 However, there is no known randomized controlled trial comparing the long-term efficacy of NTZ monotherapy with other DMDs as first-line treatment. Similarly, the long-term risk of PML due to NTZ is unknown given that the current published data are limited to 6 years of surveillance.4 With recent reports of PML in patients receiving newer oral DMDs,5–7 there is a need for an up-to-date comparison of DMDs that includes a broad range of PML risk profiles and associated outcomes.
Here, we leveraged publicly available data and applied a decision analysis approach to simulate a head-to-head comparison of the long-term benefits and risks across 3 representative DMDs in MS: glatiramer acetate (GA; injectable), fingolimod (FGL; oral), and NTZ (infusion).
METHODS
Decision analysis model.
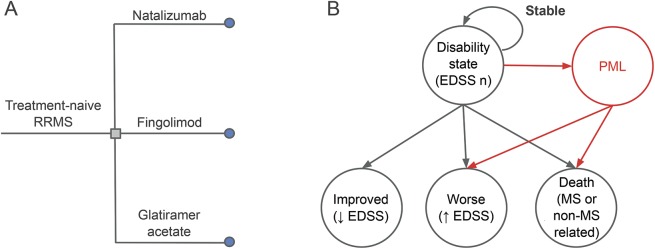
We created a Markov state transition model to simulate a multi-arm comparison of DMDs in MS (figure 1). We conducted decision analyses using TreeAge Pro-2014 (TreeAge Software, Inc, Williamstown, MA). We selected 3 representative DMDs (GA, FGL, NTZ) on the basis of their different treatment efficacies in RRMS and PML risk profiles.
Figure 1. Decision model.
Treatment-naive patients with relapsing-remitting multiple sclerosis (RRMS) choose 1 of the 3 representative disease-modifying drugs: natalizumab, fingolimod, or glatiramer acetate (A, left). From a baseline Expanded Disability Status Scale (EDSS) state, each year, a patient will remain in the same EDSS state (stable), move to an improved state, move to a worse state (disability worsening), or die (B, right). The probability of disability worsening on each drug is calculated with relative risk data from the most recently published randomized controlled trial (appendix e-1 and table e-1). Patients treated with natalizumab or fingolimod have a risk of developing progressive multifocal leukoencephalopathy (PML). Patients who develop PML either die (PML mortality) or survive with an increase in disability (PML morbidity) by the start of the following year.
For long-term drug efficacy, we modeled disease worsening (as defined by the Expanded Disability Status Scale [EDSS])8 over a 30-year period (appendix e-1 at Neurology.org). For each annual cycle, disease status was determined as stable (maintaining the same EDSS score), improved (moving to a lower EDSS score), or worsened (moving to a higher EDSS score). To measure the outcome of treatment-associated PML, we assigned a proportion of the patients developing PML each year (based on the known PML risk associated with the DMD). Patients with PML either died or developed further disability by the start of the following year.
The primary outcome was the cumulative treatment utility measured as quality-adjusted life-years (QALYs) for each drug. We used time to severe disability (defined as an EDSS score ≥6 or requirement for walking assistance) and annual mortality for each drug as secondary outcomes. QALYs measure quantity and quality of life; each year is scored on the basis of perceived quality of life, with 0 representing death, 1 representing perfect health, and negative values representing states perceived worse than death. They represent a subjective, population-level measure of perceived disease burden and have been used extensively in previous decision analysis studies.9–11 Annual QALYs for each EDSS state were calculated by multiplying the proportion of patients within the EDSS state by its utility value (table e-1 for definition of utility values). The sum of annual QALYs over a time period measures the cumulative benefit comparable across multiple drugs.
Model inputs.
Model inputs for the decision analysis are presented in table e-1.
Disability worsening.
Transition probabilities that describe the probabilities of moving between EDSS states or remaining in the same EDSS state from 1 year to the next were obtained from the UK Multiple Sclerosis Risk Sharing Scheme.11,12 For a given drug, “forward” EDSS transitions, representing worsening disability (disease worsening), were adjusted according to the relative risk of worsening (RRworse) compared to untreated on the basis of the most recent randomized clinical trial (trials completed between 2005 and 2011, appendix e-1). Transition probabilities from each EDSS state to EDSS 10 (MS-related death) were calculated to provide EDSS-specific mortality rates (appendix e-2).
PML risk.
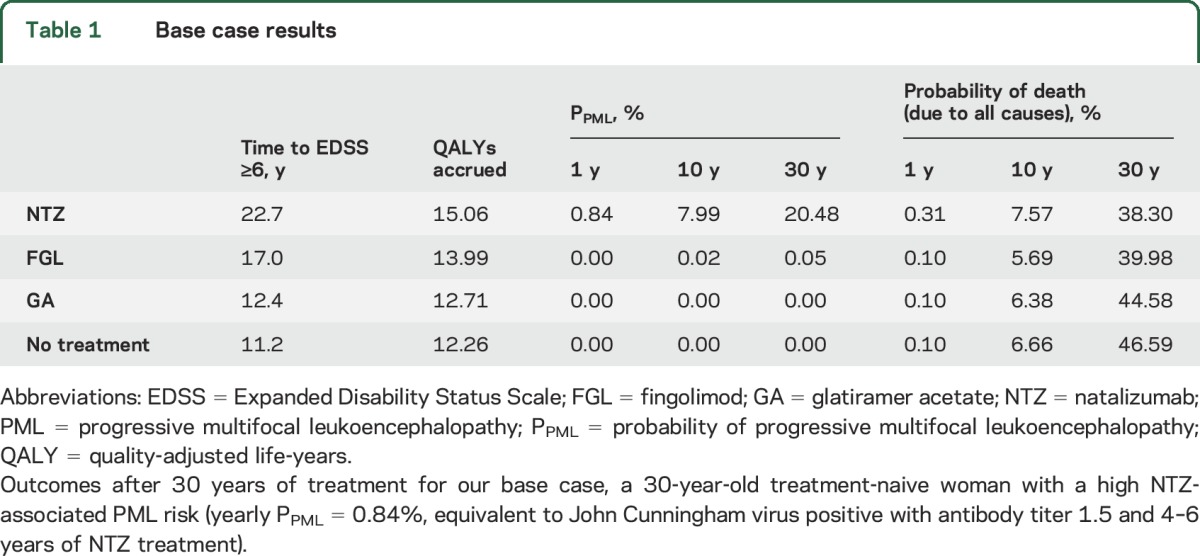
PML risk was measured as the annual probability of developing PML (table 1 and figure e-1). A recent surveillance report provided the annual probability of NTZ-associated PML over a range of PML risk profiles (figure e-1).4 To make a conservative assumption for the base case and to avoid inflating the benefit of NTZ (high efficacy and high risk), we selected an annual NTZ-associated PML probability (PPML) representing the highest risk, equivalent to that of a patient with the highest JC virus antibody titer (≥1.5) receiving NTZ as first-line treatment for 4 to 6 years. Because NTZ-associated PML risk after 6 years of treatment has not been reported, we modeled it as a constant annual probability for the 30-year duration, independently of EDSS score or duration of therapy.4
Table 1.
Base case results
For FGL, we also assumed a constant annual PML risk, estimated from the reported proportion of PML among all FGL-treated patients (3 cases from 114,000 treated as of late 2015).5,13 Because there is no known report of GA-associated PML, PML risk was not included in GA-treated patients. For patients developing PML, we assumed a 24% mortality rate (consistent with the most recently published literature on PML mortality due to NTZ)14: these patients would die by the start of the following year. All PML survivors were assumed to experience morbidity equivalent to an increase of 3 EDSS states.
Background assumptions.
In a treatment-naive MS cohort with an initial disability state distribution corresponding to early MS (table e-2), patients chose 1 of the 3 DMDs (GA, FGL, NTZ) as an initial treatment. Each treatment arm had the same baseline patient characteristics, including the pretreatment disability state transition probabilities. To represent a clinically probable range of disease evolution, we assumed that patients would undergo annual transitions of 0 to 3 EDSS states above or below their EDSS score from the preceding year.11
We obtained baseline disability states and transition probabilities from the UK Multiple Sclerosis Risk Sharing Scheme.11,12 These studies aimed to evaluate the cost-effectiveness of MS DMDs in the United Kingdom, using the British Columbia Multiple Sclerosis database as a population-based, untreated, natural history control. The cohort has been validated against other MS natural history cohorts, and the derived disability state transition probabilities have been used in recent MS treatment decision models.11,12
Sensitivity analyses.
We conducted 1- and 3-way sensitivity analyses across a clinically plausible range of annual PPML (0%–5% per year), PML survival (0%–100%), and PML morbidity (increase of 0–9 EDSS states in PML survivors) to assess their effect on the primary outcome of QALY accrual over time. We stratified the cohort into 3 groups representing the range of PML risk profiles: low PPML, medium PPML, and high PPML (figure e-1).
We additionally conducted a one-way sensitivity analysis varying EDSS-specific mortality rates and calculated its effect on QALY accrual over 30 years of treatment. An exponential function was used to vary mortality rate across a wide clinical range (corresponding to an annual MS-related mortality rate of 90%–0%, appendix e-2).
Finally, we conducted 3-way sensitivity analyses over the entire range of RRworse (0–1.0) for each drug to assess its effect on cumulative QALY accrual over time (appendix e-1).
RESULTS
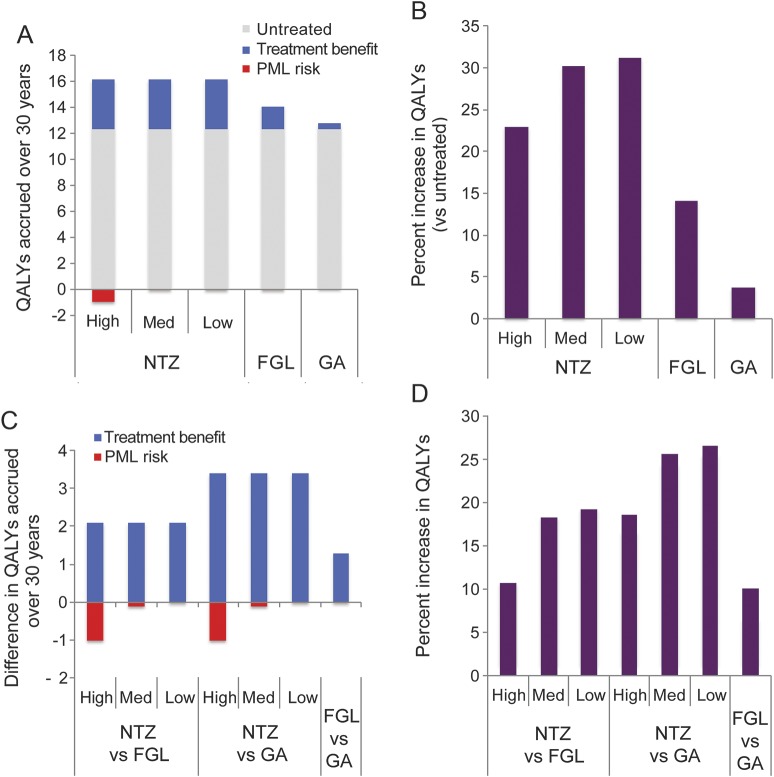
In this decision analysis study, we leveraged publicly available data and simulated a head-to-head comparison of 3 representative DMDs as first-line therapy and investigated their long-term effects on patients with MS. Over the entire reported range of NTZ-associated PML risk in patients without prior immunosuppression (PPML = 0%–0.84% per year),4 NTZ as first-line therapy was predicted to provide a greater net benefit (as measured by cumulative QALYs) over a 30-year period than FGL, GA, or no treatment (table 1, figure 2, A–D). Given the association between lower treatment utility values and higher disability states, patients with slower disability worsening accrued more QALYs over time. For patients with any PML risk profile, NTZ was associated with a net increase in treatment utility or QALYs compared to FGL or GA because of its higher efficacy in delaying disability.
Figure 2. Results of decision analysis over 30 years.
Treatment benefit is assessed with the use of quality-adjusted life-years (QALYs) accrued over 30 years for the 3 disease-modifying drug–treated and untreated patients with multiple sclerosis. Comparison was made between patients receiving a given drug and untreated patients (A and B) and among those receiving different treatments (C and D). Patients treated with natalizumab (NTZ) and fingolimod (FGL) have a risk of developing progressive multifocal leukoencephalopathy (PML), a serious brain infection. High, medium, and low denote levels of PML risk due to NTZ treatment (also figure e-1). Unlike NTZ, FGL-associated PML is much rarer, and there is currently no known set of factors that stratify the risk of PML in FGL-treated patients. Patients who develop PML either die and gain no further QALYs in the following year or survive and progress to a higher disability state by the start of the following year. Red bar denotes net loss of utility (or negative values, A and C). The net benefit from each treatment can be calculated by aggregating QALYs gained and lost over the time period and comparing them with untreated patients (B) or other treatments (D). GA = glatiramer acetate.
Our base case (table e-1) was a hypothetical 30-year-old treatment-naive female patient with RRMS with the highest reported annual probability of both NTZ-associated PML (annual PPML = 0.85%, equivalent to 4–6 years of NTZ therapy; highest JC virus antibody titer index; and no prior immunosuppression) and FGL-associated PML (annual PPML = 0.002%). For this hypothetical patient, NTZ as first-line therapy provided greater benefit, resulting in 15.06 QALYs accrued over 30 years of treatment compared to 13.99 QALYs on FGL treatment and 12.71 QALYs on GA treatment (table 1). NTZ resulted in a longer time to EDSS ≥6.0 than FGL or GA (NTZ 22.7 years, FGL 17.0 years, GA 12.4 years, untreated 11.2 years, table 1). The early (1- and 10-year) mortality rates were higher in NTZ-treated patients than in those treated with FGL and GA or the untreated (table 1). By 30 years, NTZ treatment was associated with the lowest mortality (NTZ 38.30%, FGL 39.98%, GA 44.58, untreated 46.59%).
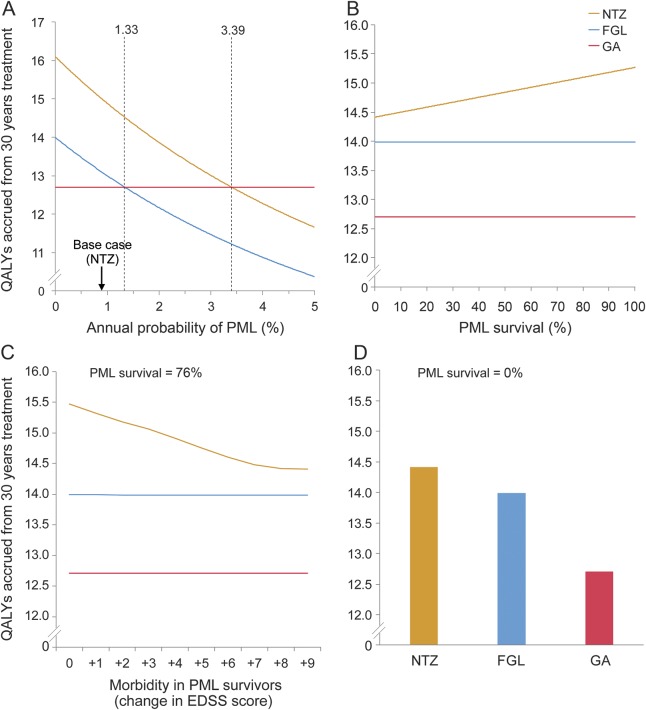
To further investigate the utility and applicability of our model, we performed a series of sensitivity analyses. First, we conducted one-way sensitivity analysis varying a wide range of NTZ-associated PML risk (annual PPML = 0%–3%, figure 3A). By varying the annual PPML due to NTZ treatment, we examined the equivalence threshold beyond which a comparator drug would have a greater utility than NTZ. The NTZ-GA equivalence threshold (QALYs = 12.71) would occur at an annual PPML of 3.39%, which exceeds the risk in our hypothetical patient, who already had the highest known annual probability of NTZ-associated PML (PPML = 0.85% in patients without prior immunosuppression). Similarly, the FGL-GA equivalence threshold would occur at a PPML of 1.33%, far exceeding the highest reported annual PML risk in FGL-treated patients (PPML = 0.0002%).
Figure 3. Sensitivity analyses varying PML risk profile.
One-way sensitivity analysis showed the effect of varying the annual probability (P) of progressive multifocal leukoencephalopathy (PML) over a clinically probable range of quality-adjusted life-years (QALYs) accrued over 30 years (A). An equivalence threshold is defined as the crossing point between colored lines (A) and represents the annual PPML that is associated with the same QALY value resulting from both treatments. The equivalence thresholds for fingolimod (FGL)–glatiramer acetate (GA) (PPML = 1.33%) and for natalizumab (NTZ)-GA (PPML = 3.39%) are displayed (dashed lines, A). In patients developing PML, one-way sensitivity analyses showed the effect of varying PML survival (B) and PML morbidity (C) on QALYs accrued over 30 years. PML morbidity is defined as an increase in Expanded Disability Status Scale (EDSS) in survivors of PML. Using the scenario representing the worst PML survival (PML survival = 0%, D), NTZ treatment accrues more QALYs over 30 years compared to FGL or GA.
Second, we conducted one-way sensitivity analysis varying mortality rates at each EDSS state across a wide range (corresponding to MS-related mortality rate of 90%–0%). Varying mortality rates across this entire range did not affect the primary outcome. For all mortality rates, NTZ treatment was associated with the highest QALY values over 30 years, followed by FGL and then GA treatment (figure e-2).
Third, with the use of RRworse values from the most recent randomized clinical trials for each drug, NTZ as first-line therapy was associated with the highest QALY accrual over 30 years across a full range of PML survival rates and morbidity rates (figure 3, B–D).15–17 In additional 3-way sensitivity analyses, we found that NTZ conferred the maximal number of QALYs over 30 years for all 3 predefined PML risk profiles: high, medium, and low (figure e-3).
The increase in treatment utility due to NTZ came at the cost of higher mortality from PML early in the treatment course. Although NTZ-treated patients with high PML risk had a modest reduction in QALYs due to PML-related morbidity and mortality (1.03 QALYs lost over 30 years, figure 2A), they had higher mortality than age-matched untreated patients for the first 13 years of treatment (figure 4C). An early increase in death was also present in all other PML risk groups: for 4 years after treatment onset for NTZ-treated patients with medium PML risk and during the first year of treatment for NTZ-treated patients with low PML risk and FGL-treated patients (figure 4C).
Figure 4. A clinical decision support tool comparing treatment benefits and risks over time.
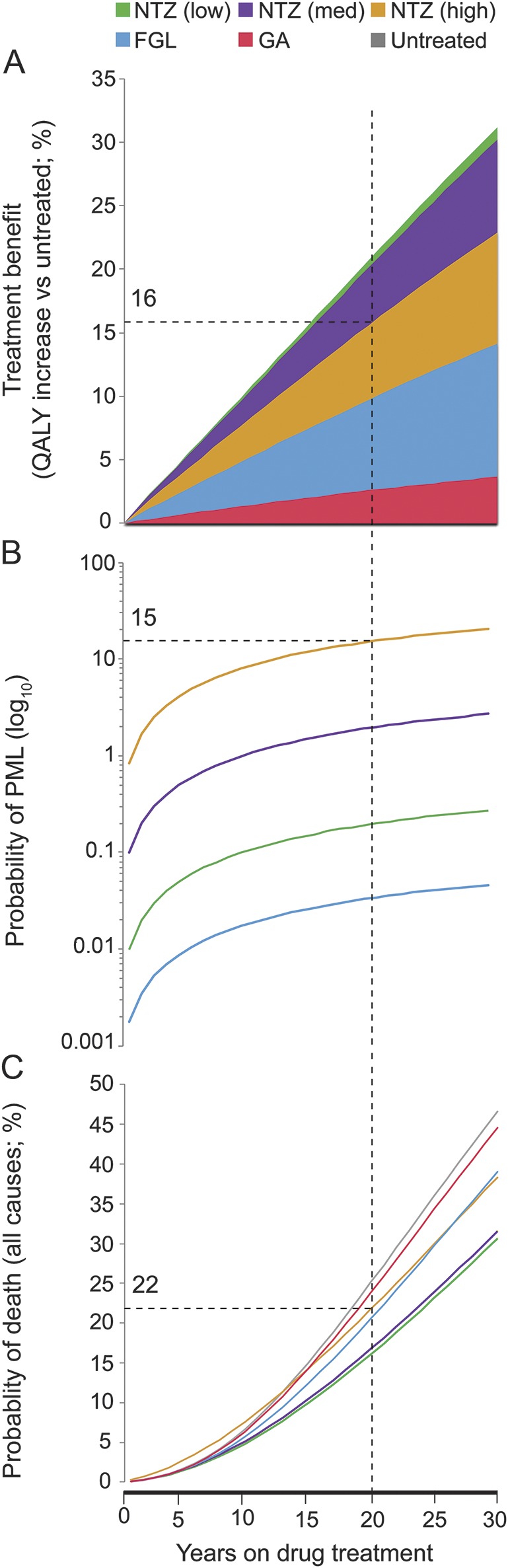
In chronic neurologic conditions such as multiple sclerosis (MS), choice of drug treatment requires an assessment of the tradeoff between long-term benefit and risks. Here, we integrated treatment benefit (measured as quality-adjusted life-years [QALYs] accrued compared to the untreated, A); the probability of progressive multifocal leukoencephalopathy (PML), one of the most severe risks due to MS disease-modifying drugs (B); and the probability of death due to all causes (C). Using these figures, we can examine the benefit, PML risk, and mortality risk for each drug at specific time points. For example, after 20 years of natalizumab (NTZ) treatment (dashed line in figures), a hypothetical patient with high PML risk (as defined in figure e-1) has a 16% higher QALY accrued than the untreated, 15% probability of developing PML, and 22% mortality risk. FGL = fingolimod; GA = glatiramer acetate.
To illustrate potential clinical applicability, we created a decision support tool by integrating measures of treatment benefit, PML risk, and risk of death (all causes) across a 30-year course (figure 4). For a given time point, one can compare the benefits and risks for each DMD. In a patient with high PML risk (dashed gray line), for example, there would be a 16% increase in QALYs (vs an untreated patient), a 15% probability of developing PML, and a 22% probability of dying after 20 years of NTZ treatment. We additionally performed 3-way sensitivity analyses varying the annual RRworse for the 3 DMDs, illustrating the feasibility of including additional DMD options in future versions of the model (figure e-4).
DISCUSSION
In this decision analysis study, we simulated a head-to-head comparison of the long-term net efficacy of 3 representative DMDs in treatment-naive patients with MS. We modeled the consequence of multiple PML risk profiles both within (high, medium, and low NTZ-associated PML risk) and among (NTZ- vs FGL-associated PML risk) DMDs in MS. Furthermore, our study includes a method of incorporating EDSS-specific mortality rates to account for an increasing risk of death in patients with MS at higher disability states. This approach enabled the examination of a wide range of MS-related mortality rates in the context of DMD treatment. Given the challenges of performing multi-arm comparisons in randomized clinical trials, our approach provides a complementary framework by deploying extensive sensitivity analyses and incorporating a wide range of clinically probable variations (PML risk, survival, and related morbidity) to investigate the basis of assumptions given the limited existing data. To create a clinical decision support tool for stratification, we included clinically meaningful metrics that are important for a patient's long-term quality of life and compared them across 3 DMDs to highlight the interesting changes in net outcome over time.
Our findings showed a marked difference in mortality among differing PML risk groups. Although both are JC virus positive, NTZ-treated patients with medium PML risk had an increased mortality (vs untreated) during each of the first 4 years of NTZ treatment, while NTZ-treated patients with high PML risk had an increased mortality during each of the first 13 years of treatment. Quantitative information emerging from the model can help facilitate physician-patient discussion during treatment selection.
In contrast to previous studies,9,10,18 we did not include QALYs lost (or disutility) as a result of either relapses or drug adverse events. The variable frequency and severity of relapses (clinical or radiographic) and adverse events make assignment of a single yearly disutility value less realistic or generalizable. Because we derived the transition probabilities from a natural history cohort that included patients undergoing relapses, the baseline accumulation and improvement in disability associated with disease relapse and subsequent recovery were captured on a yearly basis and incorporated into the model. Other factors such as fatigue, cognitive impairment, depression, and pain are also crucial to treatment efficacy. These parameters are more difficult to quantify and have variable frequency in patients with MS.19–22 However, we were able to indirectly capture their effects on quality of life through the utility values associated with each disability state.
Our approach has several limitations. First, QALYs have been used extensively to measure treatment utility in decision models, but their translation into the clinical arena may not be intuitive. Clinicians will need to advise patients on the implication of an increase in QALYs according to quality and quantity of life gained. Second, the QALYs accrued with each drug are dependent on defined utility values assigned to each disability state that were selected from a broad, diverse range of surveys in which the perceived state of health is determined from patient-scored quality-of-life parameters.11,23 While the real-life experiences in a disability state vary, these values remain useful estimates and have been used in MS treatment decision analyses.11 Third, because the surveillance data for NTZ-associated PML are limited to the first 6 years of treatment, we assumed a stable PPML for the remainder of the 30-year period, corresponding to extension of the year 4 to 6 PML risk surveillance data. To be conservative, for our base case, we maintained a constant annual PPML representing the highest reported NTZ-associated PML risk in treatment-naive patients (PPML = 0.0084, or 1 in 118). The assumption that annual PML risk would be constant throughout the 30-year period likely overestimated the risk early in treatment and underestimated the risk in later years. Future efforts in model development would incorporate change in PML risk and JC virus serostatus over time. Finally, our model included only treatment-naive patients to simulate consideration for first-line treatment and assumed full adherence to their allocated first-line drug. Future efforts that model the influence of previous therapies and incorporate variable adherence patterns may inform decisions on switching treatments, but this is beyond the scope of this study, which focuses on selection of first-line treatment. To indirectly capture the adherence to each drug, we used values from the intention-to-treat analysis when deriving the RRworse. A recent network meta-analysis further showed no significant difference in tolerability among DMDs.24
Our framework paves the way for a clinical decision support tool to guide stratified selection of first-line MS treatment. Standard counseling and monitoring of patients will remain a crucial part of clinical care. In the future, we will incorporate newer treatments with reported risk (e.g., dimethyl fumarate: 4 PML cases in 175,000 treated patients with MS reported to date).6,7 Our estimation of PML risk can be refined with the use of future NTZ-associated PML surveillance data. Estimates of disability worsening on each drug can be updated from new randomized trials as they become available. We also plan to investigate the effect of incorporating baseline patient characteristics (e.g., sex, age at onset) into the model by leveraging longitudinal cohorts of well-characterized patient population. These dynamic models, with regularly updated risk-benefit inputs, provide a cost-effective estimation of multi-arm drug comparisons in chronic neurologic diseases, for which long-term randomized controlled trials are often not feasible, and can assist clinicians during discussions of treatment selection with patients with MS.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge Feng Zhu and Drs. Helen Tremlett, Martin Duddy, and Jacqueline Palace for sharing the transition probability and EDSS state distribution tables from the UK Multiple Sclerosis Risk Sharing Scheme analyses. They also acknowledge Alison Thomson and Professor Gavin Giovannoni for allowing the use and adaptation of their PML risk data illustration.
GLOSSARY
- DMD
disease-modifying drug
- EDSS
Expanded Disability Status Scale
- FGL
fingolimod
- GA
glatiramer acetate
- JC
John Cunningham
- MS
multiple sclerosis
- NTZ
natalizumab
- PML
progressive multifocal leukoencephalopathy
- PPML
probability of progressive multifocal leukoencephalopathy
- QALY
quality-adjusted life-years
- RRMS
relapsing-remitting multiple sclerosis
- RRworse
relative risk of worsening
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Bargiela: study design, analysis and interpretation of data, and writing of manuscript. Drs. Bianchi and Westover: study design, analysis and interpretation of data, and revision of manuscript. Drs. Chibnik, Healy, and De Jager: interpretation of data and revision of manuscript. Dr. Xia: study design, analysis and interpretation of data, revision of manuscript, and study supervision.
STUDY FUNDING
No targeted funding reported. No funding source plays any role in the writing of the manuscript or the decision to submit for publication. The authors were not paid to write this article by a pharmaceutical company or other agency. The corresponding author has full access to all the data in the study and final responsibility for the decision to submit for publication.
DISCLOSURE
D. Bargiela and M. Bianchi report no disclosures relevant to the manuscript. M. Westover is supported by NIH K23NS090900, the Andrew David Heitman Neuroendovascular Research Fund, and the Rappaport Foundation (none relevant to this study). L. Chibnik reports no disclosures relevant to the manuscript. B. Healy has received research funding from Merck Serono S.A., Verily Life Sciences, Genzyme, and Novartis and has served on an advisory board for Biogen Idec. P. De Jager was a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society (JF2138A1) and has research funding from Biogen and Sanofi/Genzyme (none relevant to this study). Z. Xia was a recipient of the Clinician Scientist Development Award from the National Multiple Sclerosis Society and the American Academy of Neurology (FAN 1755-A-1) and is supported by NIH K08-NS079493 and NIH R01-NS098023 (not relevant to this study). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–1517. [DOI] [PubMed] [Google Scholar]
- 2.Filippini G, Del Giovane C, Vacchi L, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2013;6:CD008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–1880. [DOI] [PubMed] [Google Scholar]
- 4.Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014;76:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medscape. Third Case of PML with Fingolimod (Gilenya) in MS. Available at: http://www.medscape.com/viewarticle/849677. Accessed December 26, 2016. [Google Scholar]
- 6.US Food & Drug Administration. Drug safety communication: FDA warns about case of rare brain infection PML with MS drug Tecfidera (dimethyl fumarate). 2014. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm424625.htm. Accessed November 3, 2015. [Google Scholar]
- 7.MedPageToday. Biogen report another Tecfidera PML case. 2015. Available at: http://www.medpagetoday.com/Neurology/MultipleSclerosis/52622. Accessed November 3, 2015. [Google Scholar]
- 8.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JP, Noyes K, Dorsey ER, Schwid SR, Holloway RG. Quantitative risk-benefit analysis of natalizumab. Neurology 2008;71:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell JD, McQueen RB, Miravalle A, Corboy JR, Vollmer TL, Nair K. Comparative effectiveness of early natalizumab treatment in JC virus-negative relapsing-remitting multiple sclerosis. Am J Manag Care 2013;19:278–285. [PubMed] [Google Scholar]
- 11.Palace J, Duddy M, Bregenzer T, et al. Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator. Lancet Neurol 2015;14:497–505. [DOI] [PubMed] [Google Scholar]
- 12.Palace J, Bregenzer T, Tremlett H, et al. UK multiple sclerosis risk-sharing scheme: a new natural history dataset and an improved Markov model. BMJ Open 2014;4:e004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novartis. Gilenya website. 2015. Available at: http://www.gilenya.com/. Accessed November 3, 2015. [Google Scholar]
- 14.Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015;21:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 16.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:545–556. [DOI] [PubMed] [Google Scholar]
- 17.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. [DOI] [PubMed] [Google Scholar]
- 18.Walker A, Watson C, Alexopoulos ST, Deniz B, Arnold R, Bates D. A benefit-risk analysis of natalizumab in the treatment of patients with multiple sclerosis when considering the risk of progressive multifocal leukoencephalopathy. Curr Med Res Opin 2014;30:629–635. [DOI] [PubMed] [Google Scholar]
- 19.Marrie RA, Reingold S, Cohen J, et al. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler 2015;21:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiland TJ, Jelinek GA, Marck CH, et al. Clinically significant fatigue: prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the Internet. PLoS One 2015;10:e0115541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foley PL, Vesterinen HM, Laird BJ, et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain 2013;154:632–642. [DOI] [PubMed] [Google Scholar]
- 22.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol 2008;7:1139–1151. [DOI] [PubMed] [Google Scholar]
- 23.Orme M, Kerrigan J, Tyas D, Russell N, Nixon R. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value Health 2007;10:54–60. [DOI] [PubMed] [Google Scholar]
- 24.Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 2015;9:CD011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.