Abstract
Objective:
To determine the natural history of dissecting aneurysm (DA) and whether DA is associated with an increased recurrent stroke risk and whether type of antithrombotic drugs (antiplatelets vs anticoagulants) modifies the persistence or development of DA.
Methods:
We included 264 patients with extracranial cervical artery dissection (CAD) from the Cervical Artery Dissection in Stroke Study (CADISS), a multicenter prospective study that compared antiplatelet with anticoagulation therapy. Logistic regression was used to estimate age- and sex-adjusted odds ratios. We conducted a systematic review of published studies assessing the natural history of DA and stroke risk in patients with non-surgically-treated extracranial CAD with DA.
Results:
In CADISS, DA was present in 24 of 264 patients at baseline. In 36 of 248 patients with follow-up neuroimaging at 3 months, 12 of the 24 baseline DAs persisted, and 24 new DA had developed. There was no association between treatment allocation (antiplatelets vs anticoagulants) and whether DA at baseline persisted at follow-up or whether new DA developed. During 12 months of follow-up, stroke occurred in 1 of 48 patients with DA and in 7 of 216 patients without DA (age- and sex-adjusted odds ratio 0.84; 95% confidence interval 0.10–7.31; p = 0.88). Published studies, mainly retrospective, showed a similarly low risk of stroke and no evidence of an increased stroke rate in patients with DA.
Conclusions:
The results of CADISS provide evidence suggesting that DAs may have benign prognosis and therefore medical treatment should be considered.
Cervical artery dissection (CAD) is an important cause of stroke in younger adults.1 A common angiographic consequence is dissecting aneurysm (DA), also called false or pseudoaneurysm, occurring in 13%–49% of patients with CAD.2–12 It has been suggested that DAs indicate increased stroke risk, either as a source of embolization or via expansion and compressive symptoms. This has led some specialists to treat DA; in a recent study, 20% were obliterated with stenting and coiling.12 Other authorities suggest the risk of stroke in CAD is low and no treatment is required. Small studies report low stroke risk,4–7,10 but these are retrospective with incomplete case ascertainment. Data from prospective studies with predefined clinical and imaging follow-up protocols are limited.11
The Cervical Artery Dissection in Stroke Study (CADISS) was a randomized controlled trial comparing antiplatelet with anticoagulant therapy in CAD.13,14 In addition, patients who did not meet the inclusion criteria or where the patient or doctor were not prepared to randomize were recruited to the nonrandomized arm.15 Angiographic imaging was reviewed at baseline and repeated in the majority of participants at 3 months. This provides robust data from a prospective study on the prevalence and outcome of DA.
We determined the incidence and risk factors for DA in CADISS, their natural history on angiographic imaging, and whether they were associated with an increased recurrent stroke risk. We also examined whether type of antithrombotic drugs (antiplatelets vs anticoagulants) was associated with the persistence or development of DA. In addition, we performed a systematic review of published studies assessing the natural history of DA and stroke risk in patients with non-surgically-treated extracranial CAD with DA.
METHODS
Participants.
CADISS was a multicenter prospective study comparing anticoagulation with antiplatelet therapy in patients with CAD. Full details with follow-up to the 3-month primary endpoint have been published previously.13,14 A total of 250 patients were randomized 1:1 via an automated 24-hour telephone randomization service to a treatment regimen of antiplatelet agents or anticoagulants for 3 months in an open design with blinded evaluation of endpoints. Inclusion criteria were extracranial carotid or vertebral artery dissection with symptom onset within the last 7 days, in combination with imaging evidence of definite or probable dissection. If the patient had had a stroke or TIA within the last 7 days he or she was eligible even if this was preceded by local symptoms with onset more than 7 days previously. Imaging evidence of definite or probable dissection had to be on MRI/magnetic resonance angiography (MRA), CT angiography (CTA), or intra-arterial angiography. Exclusion criteria were intracranial cerebral artery dissection; contraindications to antiplatelet agents or anticoagulation therapy, including active peptic ulceration or bleeding peptic ulcer within 1 year; patient refusal to consent; patient already taking antiplatelet agents or anticoagulants for other reasons; and pregnancy. Patients not eligible for inclusion in the randomized arm, or where the doctor or patient did not accept randomization, were recruited to the nonrandomized arm (CADISS-NR) if they were within 31 days of symptom onset.15 Patients in CADISS-NR underwent the same imaging and clinical follow-up protocol.
Standard protocol approvals, registrations, and patient consents.
The local ethics committee approved the study, and all patients provided written consent.
Data collection and outcome assessment.
Patients were seen in person for follow-up at 3 months postrandomization. Data on outcome and occurrence of recurrent stroke and TIA were recorded. Repeat imaging with MRA or CTA was performed whenever possible at 3 months to assess vessel recanalization. All radiology images at baseline and 3 months were reviewed by a consultant neuroradiologist in the coordinating center who was blinded to treatment allocation. Telephone follow-up was performed at 6 and 12 months and in cases of possible stroke original records and scans were reviewed. All stroke cases were adjudicated by a committee blinded to patient treatment and the results of angiographic imaging.
Participants included in the present analysis.
On central radiology review, there were confirmatory features of a dissection in 197 of 250 patients, and in 1 additional patient; although the patient was recruited within 7 days, due to a technical problem with the randomization process, randomization itself occurred on day 9. Therefore 197 patients were included in the analysis, in addition to 67 patients with centrally confirmed imaging appearances of dissection in the NR arm. These 264 patients were included in the present analysis. Follow-up was complete at 1 year in all 264 patients.
Statistical analysis.
Characteristics of patients with and without DA were compared using t and χ2 tests. Logistic regression was used to estimate age- and sex-adjusted odds ratios (ORs) with 95% confidence intervals (CIs). The statistical analyses were performed using Stata version 14.1 (StataCorp, College Station, TX). All tests were 2-sided and p values < 0.05 were considered statistically significant.
Systematic review.
Relevant studies were identified by searches of PubMed (including MEDLINE) from inception to October 15, 2016, using the search terms carotid artery, vertebral artery, or extracranial artery combined with pseudoaneurysms, dissecting aneurysms, or false aneurysms. No language or other restrictions were imposed. The reference lists of retrieved publications were reviewed to search for additional studies. Two authors (S.C.L., A.K.) performed the literature search. Inclusion criteria were (1) prospective or retrospective longitudinal study; and (2) reported results on anatomical or clinical outcome of aneurysmal forms of extracranial CAD in medically treated patients. Exclusion criteria were intracranial CAD, invasive treatment of patients, fewer than 5 patients with extracranial CAD with DA, case-report, case-control, or cross-sectional study, nonhuman study, and other nonrelevant reports not meeting the inclusion criteria.
Data were extracted independently by 2 authors (S.C.L., H.S.M.), and any disagreement was resolved by consensus. The following information was extracted: last name of the first author, publication year, study design (prospective or retrospective), number of patients, mean age of patients, mean follow-up time, imaging modality for follow-up, time between symptom and angiography, number of dissecting aneurysms and affected vessel, anatomical findings from follow-up imaging, and clinical outcome events (any fatal or nonfatal stroke).
Data on the presence and anatomical outcome of DA were only taken from studies that performed angiographic imaging at baseline and follow-up with CTA, MRA, or digital subtraction angiography. Studies with Doppler ultrasound alone follow-up were not included as this has a low sensitivity for detecting DA.
RESULTS
CADISS.
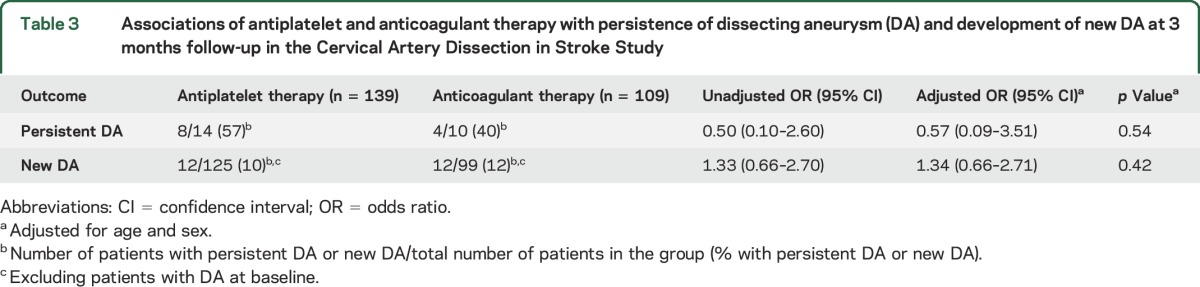
On central imaging review, a DA was present in 24 (9.1%) of the 264 patients at baseline (table 1). Follow-up MRA or CTA at 3 months was present and of adequate quality for central radiologic review for 248 of the 264 patients. Analysis of these 248 patients showed that DA was present at baseline in 24 (9.7%). At a median follow-up of 3.2 months (interquartile range 3.0–3.5 months), 12 (6 internal carotid artery [ICA] and 6 vertebral artery [VA]) of the 24 baseline DAs persisted whereas 12 DAs (7 ICA and 5 VA) had resolved. In addition, 24 new DAs (14 ICA and 10 VA) had developed. Patients with and without DA did not differ significantly with regard to age, sex, vascular risk factors, history of recent trauma, or use of IV thrombolysis (table 2). Treatment allocation (antiplatelets vs anticoagulants) did not modify whether DA at baseline persisted at the 3-month follow-up or whether new DA developed (table 3).
Table 1.
Number (%) of patients with dissecting aneurysm (DA) at baseline and 3 months in the Cervical Artery Dissection in Stroke Study
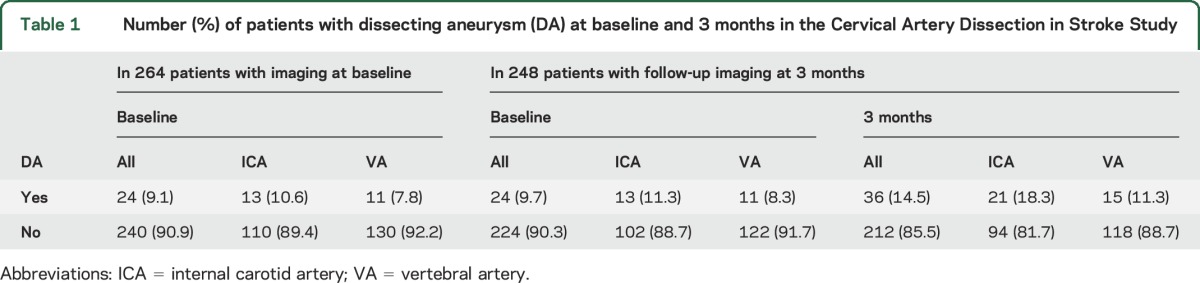
Table 2.
Characteristics of patients with and without dissecting aneurysm (DA) at baseline or 3 months in the Cervical Artery Dissection in Stroke Study
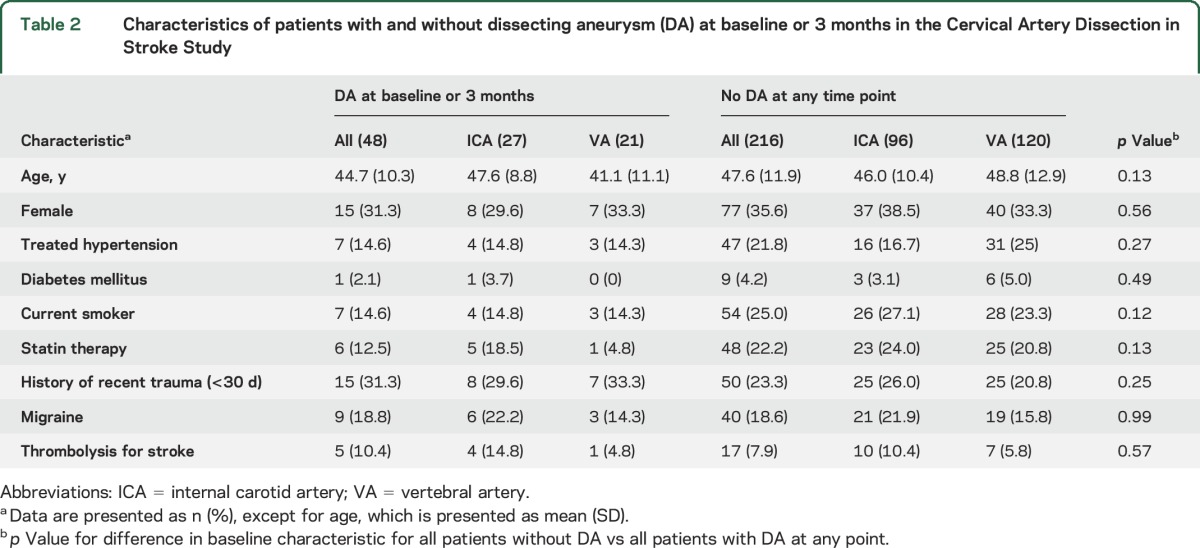
Table 3.
Associations of antiplatelet and anticoagulant therapy with persistence of dissecting aneurysm (DA) and development of new DA at 3 months follow-up in the Cervical Artery Dissection in Stroke Study
Follow-up data to the final follow-up of 12 months were obtained in all patients. Eight strokes (all ipsilateral) occurred during follow-up in the 264 patients. One of the events occurred in the 48 patients with DA at baseline or 3 months, and 7 occurred in the 216 patients without DA (age- and sex-adjusted OR 0.84; 95% CI 0.10–7.31, p = 0.88). There were too few events to determine whether antiplatelets or anticoagulants were more effective at preventing recurrent stroke in patients with DA: no stroke in 26 DA patients treated with antiplatelets and 1 stroke in 22 DA patients treated with anticoagulants.
Systematic review.
The literature search identified 4,725 articles, of which 12 studies met the inclusion criteria (figure). Among the included studies, 9 provided data on anatomical outcome4–7,12,16–19 and 9 provided data on clinical outcome4–7,10–12,18,19 in patients with CAD with DA.
Figure. Flowchart of study selection.
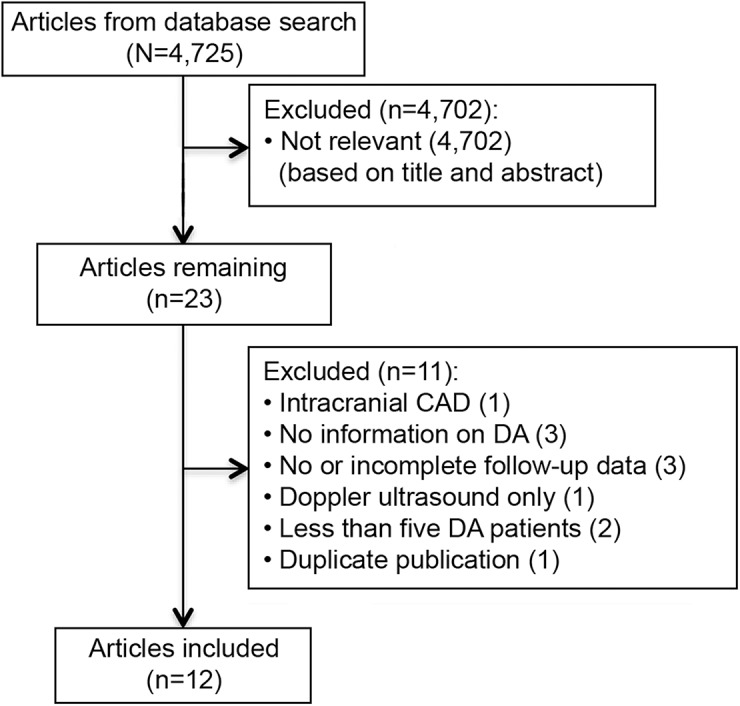
CAD = cervical artery dissection; DA = dissecting aneurysm.
The definition used to define complete and partial resolution differed between studies but the results showed a very low rate of DA expansion with no cases of expansion in 8 of the 9 studies (table 4). The studies confirmed that many DAs resolved completely on follow-up imaging. The resolution rate appeared to be higher in patients initially imaged shortly after presentation and was lower in asymptomatic DAs, suggesting that if DAs are to resolve they tend to do so shortly after formation.
Table 4.
Previous studies of anatomic outcome of dissecting aneurysms (DAs) due to cervical artery dissection in medically treated patients
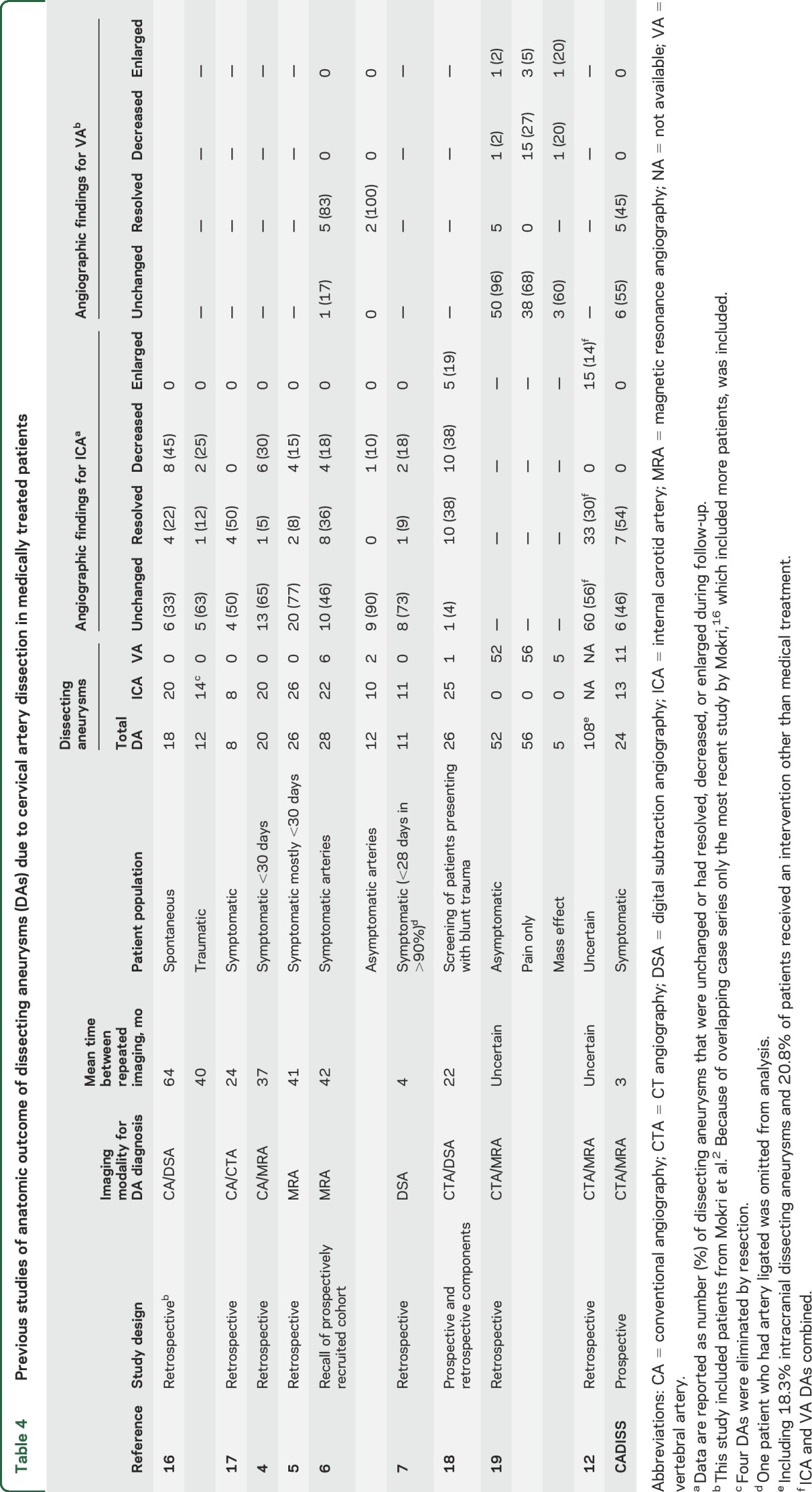
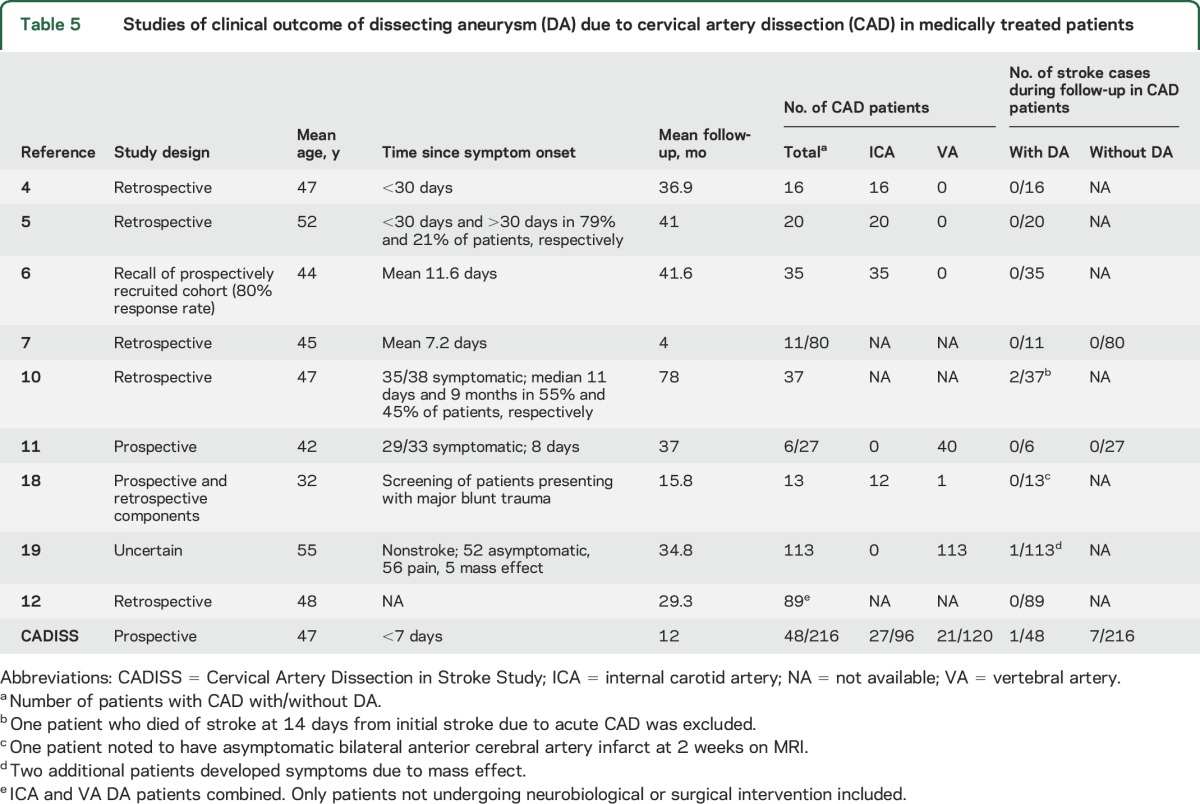
Seven studies provided outcome data in patients with CAD with DA but had no comparison group without DA4–6,10,12,18,19; combined, these studies included 323 patients with DAs, of whom 3 had a stroke (1 fatal and 1 capsular stroke in 1 study10 and 1 nonfatal ischemic stroke in another study19) during follow-up (table 5). Only 2 studies compared clinical outcome in patients with CAD and without DA7,11; no strokes occurred in either group (table 5).
Table 5.
Studies of clinical outcome of dissecting aneurysm (DA) due to cervical artery dissection (CAD) in medically treated patients
DISCUSSION
In the prospective CADISS study, our data demonstrate that DA is a relatively common sequel to extracranial vessel dissection, has a benign prognosis, and the presence of a DA does not indicate that an individual with dissection is at higher risk of recurrent stroke. Our data further suggest that DA is a relatively dynamic process with a significant proportion of aneurysms either healing or developing over the initial 3 months following clinical diagnosis of vessel dissection. There was no difference in the persistence of DA or development of new DA for antiplatelet vs anticoagulant therapy.
CADISS provides some of the most robust evidence on the prognosis of DA. Its prospective design and complete outcome ascertainment during follow-up makes it much less susceptible to bias than previous retrospective studies. During the 1-year follow-up, there were few recurrent strokes, and stroke risk in patients who had a DA on either initial imaging or 3-month imaging was no higher than those without DA. CADISS also provides data on the anatomical outcome of DA. The results showed that approximately half of all DA resolved entirely within the first 3 months, but that additional aneurysms appeared after the initial angiographic imaging.
The results of both the follow-up angiographic imaging and clinical follow-up in CADISS were broadly in agreement with those from our systematic review. This also showed a very low risk of recurrent strokes in patients with DA. However, most previous studies had significant limitations, including retrospective design and the potential for ascertainment bias and variable inclusion criteria. In addition, in some studies a proportion of patients had interventions other than medical therapy, for example coiling or surgical intervention.12
Despite their benign prognosis, a significant number of patients with DA are treated with interventions, the most common being coiling, which has an associated risk of stroke. Our data suggest that such interventions may not be warranted and medical treatment alone is sufficient, and indeed is likely to be safer. In CADISS, all patients were on either antiplatelet drugs or anticoagulants, and in the systematic review most patients were on antithrombotic medication. It is therefore impossible to determine from the available data whether patients with DA need long-term antithrombotic medication. There are occasional cases of DA where expansion occurs, resulting in compressive symptoms. There were no such cases in CADISS, and such complications appeared to be very rare, but in these exceptional cases intervention may be required.12
The results of CADISS provide robust evidence that DAs may have a benign prognosis and therefore medical treatment should be considered. This finding is consistent with those from a systematic review of previous largely retrospective observational studies.
ACKNOWLEDGMENT
Trial steering committee: Hugh S. Markus, University of Cambridge (co-PI); John Norris, University of London (co-PI); Graham S. Venables, Royal Hallamshire Hospital, Sheffield; Sally Kerry, St George's, London; Ahamed Hassan, Leeds General Infirmary, Leeds; Christopher Levi, John Hunter Hospital, New South Wales, Australia. Trial managers: Jennifer Peycke, Melina Willson, Cara Hicks, Elizabeth Hayter. Study neuroradiologists: Jeremy Madigan, Andrew Clifton, St Georges NHS Foundation Trust. Imaging review: Alice King, Jeremy Madigan, St Georges NHS Foundation Trust. Data monitoring committee: Gary A. Ford, University of Newcastle (Chair); Philip M.W. Bath, University of Nottingham; Chris Weir, University of Glasgow. Adjudication committee: Prof. Lalit Kalra, Kings College London (chair); Dr. Denis Briley, Stoke Mandeville Hospital; Dr. Ajay Bhalla, Guys and St Thomas NHS Trust. CADISS participating centers (number of enrolled per center; principal investigator): UK total: 232: Aberdeen Royal Infirmary, Aberdeen (12; John Reid), Aintree University Hospital, Liverpool (13; Raj Kumar), Airedale General Hospital, Keighley (3; Samantha Mawer, Matthew Smith), Brighton and Sussex University Hospital, Brighton (3; Khalid Ali), Charing Cross Hospital, London (5; Pankaj Sharma), Cheltenham General and Gloucester Royal Hospitals, Cheltenham and Gloucester (1; Dipankar Dutta), Derriford Hospital, Plymouth (1; Azlisham Mohd Nor), Frenchay Hospital, Bristol (1; Rose Boswell, Neil Baldwin), Guy's and St Thomas' (6; Anthony Rudd), Heartlands Hospital, Birmingham (0; David Stanley), Hope Hospital, Kent and Canterbury Hospital, Canterbury (3; Isle Burger), King's College Hospital, London (9; Lalit kalra), Leeds General Hospital, Leeds (6; Ahamed Hassan), Musgrove Park Hospital, Taunton (1; Christopher Price), Newcastle Hospitals NHS Foundation Trust, Newcastle upon Tyne (5; Anand Dixit), Ninewells Hospital, Dundee (6; Ronald MacWalter), Northwick Park, Harrow (1; David Cohen), Pinderfields General Hospital, Wakefield (2; Richard Davey), Queen Elizabeth Hospital, Gateshead (1; Tim Cassidy), Queen Elizabeth Queen Mother Hospital, Margate (6; Gunarathnam Gunathilagan), Royal Bournemouth Hospital, Bournemouth (2; Damian Jenkinson), Royal Cornwall Hospitals NHS Trust, Truro (5; Frances Harrington), Royal Devon and Exeter Hospital, Exeter (7; Martin James), Royal Hallamshire Hospital, Sheffield (15; Graham Venables), Royal Hampshire Hospital, Winchester (1; Nigel Smyth), Royal Preston Hospital, Preston (1; Hedley Emsley), Royal United Hospital, Bath (4; Louise Shaw), Southampton General Hospital, Southampton (2; Joanna Lovett), Southend Hospital, Southend (11; Paul Guyler), St George's Hospital, London (58; Hugh Markus), Royal London Hospital (1; Patrick Gompertz), Torbay Hospital, Torbay (2; Debs Kelly, Isam Salih), University Hospital North Staffordshire, Stoke-on Trent (9; Brendan Davies), University Hospital Wales, Cardiff (1; Hamsaraj Shetty), University Hospitals of Leicester (2; Amit Mistri), Western General Hospital, Edinburgh 3 (Malcolm Macleod, Bridget Colam, Rustam Al-Shahi Salman), William Harvey Hospital, Ashford (2; David Hargroves), Yeovil District Hospital (2; Khalid Rashed) Frimley Park Hospital, Frimley (2; Brian Clarke), Watford General Hospital, Watford, (18; David Collas). Australia total: 18: Austin Hospital, Melbourne (2; Richard Gerraty), Gosford Hospital, Gosford (3; Jon Sturm), John Hunter Hospital, New Lambton (4: Chris Levi), Royal Adelaide Hospital, Adelaide (6; Tim Kleinig), Royal Brisbane and Women's Hospital, Brisbane (1; Andrew Wong), Royal Melbourne Hospital, Melbourne (1; Peter Hand), Royal Prince Alfred Hospital (1; Candice Delcourt).
GLOSSARY
- CAD
cervical artery dissection
- CADISS
Cervical Artery Dissection in Stroke Study
- CI
confidence interval
- CTA
CT angiography
- DA
dissecting aneurysm
- ICA
internal carotid artery
- MRA
magnetic resonance angiography
- NR
nonrandomized
- OR
odds ratio
- VA
vertebral artery
AUTHOR CONTRIBUTIONS
Susanna Larsson performed the literature search, assessed the eligibility and extracted the data of identified studies, analyzed and interpreted the data, and drafted the manuscript. Alice King conducted the literature search, contributed to interpretation of the data, and revised the article critically for important intellectual content. Jeremy Madigan, Christopher Levi, and John Norris were involved in interpretation of the data and revised the article critically for important intellectual content. Hugh Markus conceived and designed the study, assessed the eligibility and extracted the data of identified studies, interpreted the data, and drafted the manuscript. The corresponding author attests that the authors had access to all the study data, takes responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. All authors gave final approval of the version to be published. The corresponding author affirms that he has listed everyone who contributed substantially to the work.
STUDY FUNDING
CADISS was supported by a project grant from the Stroke Association. Recruitment was supported by the English National Institute for Health Research (NIHR) Stroke Research Network. Hugh Markus is supported by an NIHR Senior Investigator award and his work is supported by the Cambridge University Hospital Comprehensive Biomedical Research Centre.
DISCLOSURE
S. Larsson, A. King, J. Madigan, C. Levi, and J. Norris report no disclosures relevant to the manuscript. H. Markus is supported by an NIHR Senior Investigator award. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 2009;8:668–678. [DOI] [PubMed] [Google Scholar]
- 2.Mokri B, Sundt TM Jr, Houser OW, Piepgras DG. Spontaneous dissection of the cervical internal carotid artery. Ann Neurol 1986;19:126–138. [DOI] [PubMed] [Google Scholar]
- 3.Sturzenegger M. Spontaneous internal carotid artery dissection: early diagnosis and management in 44 patients. J Neurol 1995;242:231–238. [DOI] [PubMed] [Google Scholar]
- 4.Guillon B, Brunereau L, Biousse V, Djouhri H, Levy C, Bousser MG. Long-term follow-up of aneurysms developed during extracranial internal carotid artery dissection. Neurology 1999;53:117–122. [DOI] [PubMed] [Google Scholar]
- 5.Djouhri H, Guillon B, Brunereau L, et al. MR angiography for the long-term follow-up of dissecting aneurysms of the extracranial internal carotid artery. AJR Am J Roentgenol 2000;174:1137–1140. [DOI] [PubMed] [Google Scholar]
- 6.Touze E, Randoux B, Meary E, Arquizan C, Meder JF, Mas JL. Aneurysmal forms of cervical artery dissection: associated factors and outcome. Stroke 2001;32:418–423. [DOI] [PubMed] [Google Scholar]
- 7.Pelkonen O, Tikkakoski T, Leinonen S, Pyhtinen J, Lepojarvi M, Sotaniemi K. Extracranial internal carotid and vertebral artery dissections: angiographic spectrum, course and prognosis. Neuroradiology 2003;45:71–77. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo L, Crasto SG, Savio D, et al. Dissection of cervicocephalic arteries: early diagnosis and follow-up with magnetic resonance imaging. Emerg Radiol 2006;12:254–265. [DOI] [PubMed] [Google Scholar]
- 9.Lee VH, Brown RD Jr, Mandrekar JN, Mokri B. Incidence and outcome of cervical artery dissection: a population-based study. Neurology 2006;67:1809–1812. [DOI] [PubMed] [Google Scholar]
- 10.Benninger DH, Gandjour J, Georgiadis D, Stockli E, Arnold M, Baumgartner RW. Benign long-term outcome of conservatively treated cervical aneurysms due to carotid dissection. Neurology 2007;69:486–487. [DOI] [PubMed] [Google Scholar]
- 11.Wessels T, Mosso M, Krings T, Klotzsch C, Harrer JU. Extracranial and intracranial vertebral artery dissection: long-term clinical and duplex sonographic follow-up. J Clin Ultrasound 2008;36:472–479. [DOI] [PubMed] [Google Scholar]
- 12.Daou B, Hammer C, Chalouhi N, et al. Dissecting pseudoaneurysms: predictors of symptom occurrence, enlargement, clinical outcome, and treatment. J Neurosurg 2016;125:936–942. [DOI] [PubMed] [Google Scholar]
- 13.Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol 2015;14:361–367. [DOI] [PubMed] [Google Scholar]
- 14.Cervical Artery Dissection in Stroke Study Trial I. Antiplatelet therapy vs. anticoagulation in cervical artery dissection: rationale and design of the Cervical Artery Dissection in Stroke Study (CADISS). Int J Stroke 2007;2:292–296. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy F, Lanfranconi S, Hicks C, et al. Antiplatelets vs anticoagulation for dissection: CADISS nonrandomized arm and meta-analysis. Neurology 2012;79:686–689. [DOI] [PubMed] [Google Scholar]
- 16.Mokri B. Traumatic and spontaneous extracranial internal carotid artery dissections. J Neurol 1990;237:356–361. [DOI] [PubMed] [Google Scholar]
- 17.Leclerc X, Lucas C, Godefroy O, et al. Helical CT for the follow-up of cervical internal carotid artery dissections. AJNR Am J Neuroradiol 1998;19:831–837. [PMC free article] [PubMed] [Google Scholar]
- 18.Foreman PM, Griessenauer CJ, Falola M, Harrigan MR. Extracranial traumatic aneurysms due to blunt cerebrovascular injury. J Neurosurg 2014;120:1437–1445. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi N, Murayama Y, Yuki I, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol 2014;35:1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]


