Abstract
Objective:
To identify limbic sites of respiratory control in the human brain, and by extension, the symptomatogenic zone for central apnea.
Methods:
We used direct stimulation of anatomically, precisely placed stereotactic EEG electrodes to analyze breathing responses. We prospectively studied 3 patients who were explored with stereotactically implanted depth electrodes. The amygdala and hippocampus, as well as extralimbic sites (orbitofrontal, temporal tip, and temporal neocortex), were investigated.
Results:
Individual stimulation of the amygdala and hippocampal head consistently elicited central apnea in the expiratory phase, as did exquisitely focal hippocampal seizures.
Conclusions:
These findings confirm that hippocampus and amygdala are limbic breathing control sites in humans, as well as the symptomatogenic zone for central apneic seizures.
Video-monitored sudden unexpected death in epilepsy (SUDEP) cases suggest breathing dysfunction following generalized tonic-clonic seizures is involved in mechanisms of death, and terminal apnea precedes asystole.1 Some SUDEP and near SUDEP cases have occurred with complex partial seizures without secondarily generalized tonic-clonic seizures.1 Apnea was seen in 16 of 35 (48%) complex partial seizures2 and seizure-associated O2 desaturations were seen in 127 out of 253 (50.3%) nongeneralized focal seizures (temporal > extratemporal).3 Apnea and O2 desaturation mechanisms during partial seizures, and their relation to SUDEP, is not understood.4–6 The role of cortical breathing control sites, through seizure-induced, disordered, or inhibited function, may be the key. Although several animal studies identify respiratory cortical control structures, human studies of cortical breathing are few. Invasive studies of brains of patients with refractory epilepsy undergoing assessment for epilepsy surgery provide opportunities for mapping cortical functions. Their identification may help us understand why people die after an epileptic seizure.
METHODS
Patients and clinical setting.
Patients aged >18 years with medically intractable epilepsy were undergoing stereotactic EEG evaluations for epilepsy surgery in the epilepsy monitoring unit at University Hospital Cleveland Medical Center. We prospectively studied 3 such consecutive patients. All patients were consented participants in a University Hospitals Case Medical Center institutional review board–approved research project evaluating the role of cortical structures in human respiratory and autonomic function.
Procedure.
Platinum-iridium depth electrodes measuring 1.1 mm in diameter and 2.5 mm in length, evenly spaced at 5-mm intervals, were implanted stereotactically in the hippocampus, amygdala, and lateral temporal, orbitofrontal, temporal tip, and posterior cingulate neocortex under general anesthesia. Implantation trajectories were simulated using iplan-stereotaxy 2.6 software (Brainlab, Munich, Germany) based on recent 3T MRI of the brain. X-ray and cranial CT were performed within 24 hours postsurgically. Using iplan-stereotaxy 2.6 software, postsurgical cranial CT and presurgical brain MRI were superimposed for precise localization of single electrode contacts within the patient's presurgical MRI.
Stimulation.
Bedside cortical electrical stimulation was carried out using the same parameters (bipolar stimulation, 50 Hz, and pulse width 0.2 ms) as for clinical brain mapping, with train durations of up to 30 seconds, using a Grass S-88X Stimulator (Astro-Med, Inc., West Warwick, RI). Current intensity started at 1 mA, and increased in 1 mA increments to a maximum of 10 mA. In case a seizure was induced, stimulation was discontinued and IV lorazepam (Ativan) was given to stop the seizure. Resuscitation equipment was always kept in intimate proximity to the patient in case of need.
Cardiorespiratory and EEG monitoring.
Nasal airflow was recorded using a nasal thermistor (Pro-Tech airflow sensor). Chest and abdominal excursions were recorded using inductance plethysmography (Ambu [Ballerup, Denmark] Sleepmate). Arterial oxygen saturation (SpO2) and heart rate were monitored using pulse oximetry (Nellcor OxiMax N600x; Medtronic, Langhorne, PA) and end tidal carbon dioxide (ETCO2) using a capnograph (model 7900; Philips, Best, the Netherlands). EEG and ECG were acquired using a Nihon Kohden (Tokyo, Japan) EEG-1200 with 256-channel amplifier. We defined apnea as involuntary cessation of breathing where at least 2 breaths were missed, compared to baseline breathing rate, with a drop in peak signal excursion by ≥90% of pre-event baseline. Apnea onset was measured from the nadir of the preceding breath (that was clearly reduced) to the beginning of the first breath that approximated baseline amplitude. During stimulation, all patients had nasal thermal sensors to confirm presence and type of apnea. EEG was then analyzed for seizures and afterdischarges during the stimulation.
RESULTS
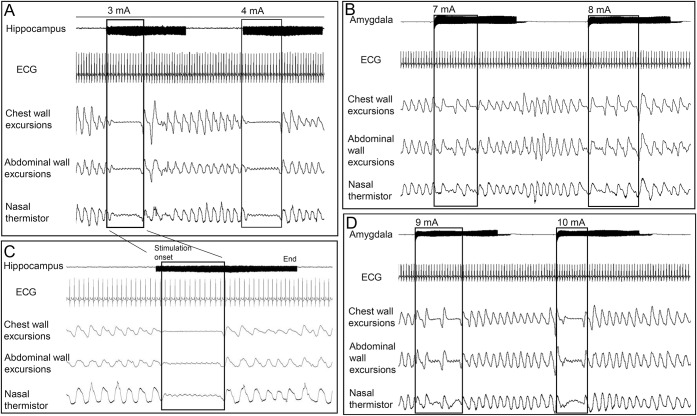
Patient characteristics are shown in table 1. Stimulation of the amygdala and hippocampal head elicited consistent (reproduction of the same response at the same or higher stimulation intensity at the same site) apnea in all 3 patients (table 2); no breathing responses were seen from the temporal tip, lateral temporal, or orbitofrontal neocortex. At stimulation onset, immediate inhibition of respiration was clearly evident on belt/plethysmography signal and video with the thorax assuming the expiratory position. Patients were able to breathe out once but unable to breathe in again. Apnea periods ended before stimulation ended in all cases. When amygdalo-hippocampal seizures were induced, apnea duration was prolonged, but always ended before seizures ended. Slight increases in ETCO2 were noted, but with no clear relationship to onset of rebreathing. Patients were always agnostic of apnea. In subsequent experiments, patient 3 was instructed to breathe during hippocampal stimulation–induced apnea and was immediately able to do so. Heart rate decreased or remained constant during apnea periods but significantly increased when clinical seizures were induced (table 2). In patient 2, whereas amygdala stimulation at 10 mA induced apnea, intensities of 6, 7, and 8 mA produced clear and instant disordered breathing pattern, more evident in the initial phase of stimulation, without fulfilling our definition of apnea (figures 1 and 2).
Table 1.
Patient characteristics

Table 2.
Characteristics of stimulation-induced apnea
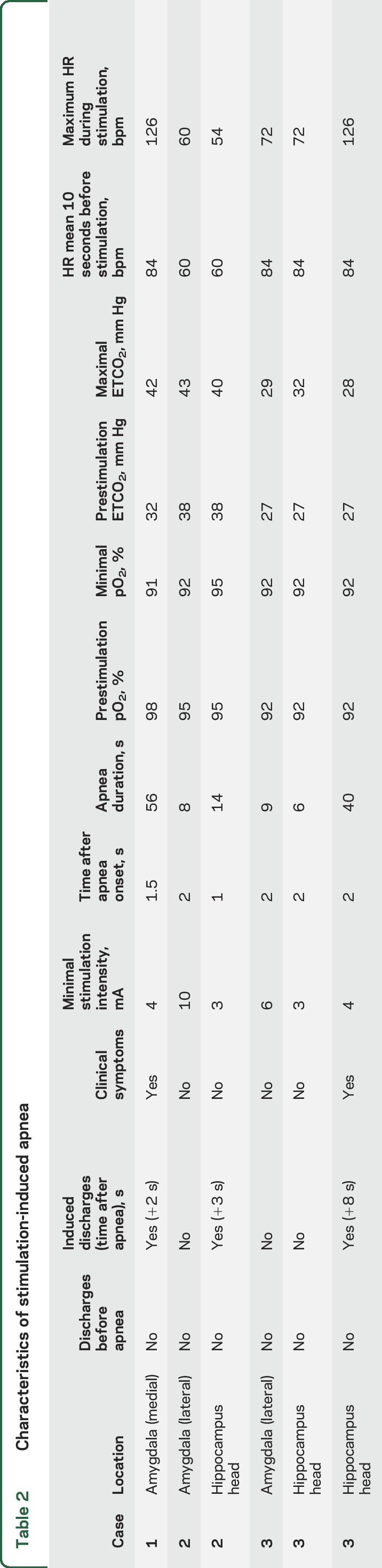
Figure 1. Apnea after hippocampus and amygdala stimulation.
(A) Hippocampus head stimulation at intensities of 3 and 4 mA induced instant central apnea in patient 2. Apnea periods ended before stimulation was discontinued. (C) Apnea with 3 mA intensity stimulation of the hippocampus head in patient 2. (B, D) Stimulation in patient 2 at 6, 7, and 8 mA current intensities induced immediate change in respiratory rhythm. Central apnea was elicited at 10 mA current intensity in the same patient.
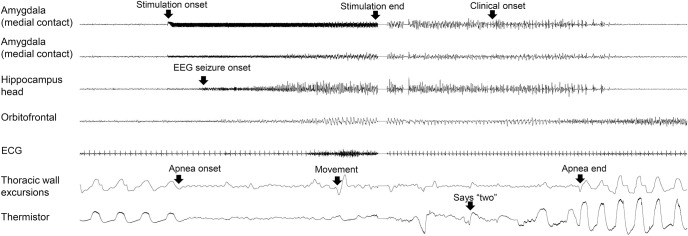
Figure 2. Apnea induced by amygdala stimulation and hippocampal seizure.
Amygdala stimulation induced an immediate apnea in patient 1. Three seconds after apnea onset, a hippocampal seizure was seen. Breathing resumed when the seizure ended in the hippocampus and amygdala, but continued in the orbitofrontal cortex for a few seconds.
DISCUSSION
Breathing responses have been described with electrical stimulation in cats, dogs, and monkeys in a variety of brain regions including amygdala, hippocampus, anterior cingulate, and anterior insular cortices. In humans, where opportunities for similar experiments are limited, few studies of cortical stimulation targeting breathing control structures exist. To our knowledge, there are only 2 studies in the literature describing amygdalar stimulation–induced apnea7,8 and none of hippocampal stimulation–induced apnea in the modern era.
Our study, through electrical stimulation, definitively establishes the role of major limbic structures, confirming the amygdala stimulation results of Dlouhy et al.8 and confirming the role of the hippocampus in breathing control. Inspiration was preferentially affected, and as an active process, is likely immediately inhibited, whereas expiration is allowed to occur as a passive phenomenon. The most likely mechanism for immediate apnea is stimulation-induced inhibition or disruption of brainstem inspiratory neuronal function. Inspiratory breathing rhythm is thought to be generated in the pre-Bötzinger complex, a group of neurons in the ventrolateral medulla of the brainstem. Perturbation of neural function in and around this area severely disrupts breathing rhythm.9 The different types of breathing responses (apnea and disordered, irregular breathing) seen in our studies likely represent varying degrees of magnitude of response, depending on stimulus intensity.
In all stimulation periods producing apnea, patients started rebreathing before stimulation ended. Similar findings have been described in electrical stimulation studies in animals. Brain adaptation may explain this phenomenon. Several mechanisms for the overriding of apnea are possible. A previous study assessing breathing responses after amygdala stimulation postulated increased pCO2 as an override mechanism that compelled rebreathing. It is indeed well-known that small changes in CO2/pH affect breathing. Another plausible explanation lies in a lessening of seizure discharge intensity. In our patients, there was distinct devolution in discharge frequency or amplitude coincident with rebreathing.
The central apnea induced by amygdalohippocampal stimulation is of particular interest given its potential role as a SUDEP pathomechanism. It seems plausible that some apneic seizures produce prolonged, potentially fatal apnea. Asphyxia in animal models results in decline of heart rate and cardiac arrest within 5 minutes.10 Based on our study and the most current observations, patients are able to override ictal apnea when instructed to talk, and asking patients with preserved consciousness to breathe may be helpful. However, since patients frequently lose consciousness or comprehension during seizures, active attempts to abort seizures producing apnea may be more crucial. It is worth emphasizing that respiratory monitoring of seizures in the epilepsy monitoring unit is an important facet to safety and the understanding of seizure, near-SUDEP, and SUDEP phenomena.
One of the limitations of our study is the small number of patients and the few structures that were stimulated. Involuntary suprapontine control of respiration may not be limited to the amygdala and hippocampus. Further studies including other brain regions are necessary for complete understanding of suprapontine breathing control.
GLOSSARY
- SUDEP
sudden unexpected death in epilepsy
AUTHOR CONTRIBUTIONS
Dr. Lhatoo: study supervision and critical revision of the manuscript for important intellectual content. Dr. Lacuey: study concept and design, analysis and interpretation. Dr. Zonjy: acquisition of data. Dr. Londono: acquisition of data.
STUDY FUNDING
This work was supported in part by the Universitat Autonoma de Barcelona as part of a Doctorate thesis in Medicine.
DISCLOSURE
N. Lacuey, B. Zonjy, and L. Londono report no disclosures relevant to the manuscript. S. Lhatoo is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405-01 and NIH/NINDS U01-NS090407-01. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 2.Nadkarni MA, Friedman D, Devinsky O. Central apnea at complex partial seizure onset. Seizure 2012;21:555–558. [DOI] [PubMed] [Google Scholar]
- 3.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008;131:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuele SU, Afshari M, Afshari ZS, et al. Ictal central apnea as a predictor for sudden unexpected death in epilepsy. Epilepsy Behav 2011;22:401–403. [DOI] [PubMed] [Google Scholar]
- 5.Tezer FI, Remi J, Noachtar S. Ictal apnea of epileptic origin. Neurology 2009;72:855–857. [DOI] [PubMed] [Google Scholar]
- 6.Nashef L, Walker F, Allen P, et al. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry 1996;60:297–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. AMA Arch Neurology Psychiatry 1952;68:609–619. [DOI] [PubMed] [Google Scholar]
- 8.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci 2015;35:10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 2003;26:239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D, Mabrouk OS, Liu T, et al. Asphyxia-activated corticocardiac signaling accelerates onset of cardiac arrest. Proc Natl Acad Sci USA 2015;112:E2073–E2082. [DOI] [PMC free article] [PubMed] [Google Scholar]