Forced aerobic exercise associated with task practice may be optimal in facilitating motor recovery after stroke.
Abstract
OBJECTIVE. To understand how two types of aerobic exercise affect upper-extremity motor recovery post-stroke. Our aims were to (1) evaluate the feasibility of having people who had a stroke complete an aerobic exercise intervention and (2) determine whether forced or voluntary exercise differentially facilitates upper-extremity recovery when paired with task practice.
METHOD. Seventeen participants with chronic stroke completed twenty-four 90-min sessions over 8 wk. Aerobic exercise was immediately followed by task practice. Participants were randomized to forced or voluntary aerobic exercise groups or to task practice only.
RESULTS. Improvement on the Fugl-Meyer Assessment exceeded the minimal clinically important difference: 12.3, 4.8, and 4.4 for the forced exercise, voluntary exercise, and repetitive task practice–only groups, respectively. Only the forced exercise group exhibited a statistically significant improvement.
CONCLUSION. People with chronic stroke can safely complete intensive aerobic exercise. Forced aerobic exercise may be optimal in facilitating motor recovery associated with task practice.
Stroke is a leading cause of long-term disability in the United States, and approximately 795,000 Americans experience a new or recurrent stroke each year (Billinger, Mattlage, et al., 2012). As many as 50% of stroke survivors experience residual hemiparesis, resulting in decreased mobility, decreased social participation, and depression (Kelly-Hayes et al., 2003). Current rehabilitation approaches focus on applying restorative motor learning techniques to promote motor recovery (Knaepen, Goekint, Heyman, & Meeusen, 2010). Neuroplasticity is thought to underlie the relearning of lost motor behavior after stroke via modification and potentiation of neural connectivity, and it is traditionally achieved through motor practice, which engages attention, motivation, and learning networks of the brain (Dobkin & Dorsch, 2013).
An abundance of evidence has shown that in healthy adults, aerobic exercise not only improves cardiovascular health but also has an impact on brain structure and function (Cotman, Berchtold, & Christie, 2007; Knaepen, Goekint, Heyman, & Meeusen, 2010; Mang, Campbell, Ross, & Boyd, 2013; McDonnell, Smith, & Mackintosh, 2011; Poo, 2001). Healthy older adults and adults with cognitive impairments exhibit improvements in learning and memory and a reduction in risk of neurodegeneration after aerobic exercise intervention (Cotman et al., 2007; McDonnell et al., 2011; Petzinger et al., 2011). The positive cardiovascular effects of aerobic exercise in stroke survivors have been well documented, and aerobic exercise has been supported as a potential means to reduce the risk of a subsequent stroke (Billinger, Mattlage, et al., 2012; Ivey, Hafer-Macko, & Macko, 2006). However, a gap exists in understanding the potential neurophysiological role of aerobic exercise in facilitating the motor recovery process after stroke because the potential of aerobic exercise, forced or voluntary, has not been empirically tested in humans.
Aerobic exercise has been theorized to play a key role in neuroplasticity and potentially in motor recovery (for a review, see Knaepen et al., 2010). Although the precise mechanism is unknown, it has been hypothesized that aerobic exercise may promote motor recovery poststroke as a result of angiogenesis, increases in cerebral blood flow, increases in neurotransmitters, or upregulation of neurotrophins (for a review, see Mang et al., 2013). Influencing levels of brain-derived neurotrophic factor (BDNF) through aerobic exercise is of particular interest because it has been identified as a key mediator in facilitating neuroplasticity via long-term potentiation and dendritic growth and remodeling (Poo, 2001). Neuroscientists have hypothesized that aerobic exercise may prime the central nervous system for neuroplastic changes after stroke (Mang et al., 2013). It has been theorized that completing motor task practice immediately after aerobic exercise may capitalize on transient increases in BDNF, which are greatest 10–60 min after aerobic exercise (Knaepen et al., 2010; Mang et al., 2013).
Exercise intensity is a critical factor in facilitating BDNF levels (Knaepen et al., 2010). Neurological disease has been shown to decrease cardiovascular fitness and diminish motor output, serving as a barrier to achieving and maintaining high-intensity aerobic exercise (Billinger, Coughenour, Mackay-Lyons, & Ivey, 2012). Forced exercise (FE), a mode of exercise in which the voluntary efforts of the person are augmented to facilitate sustained exercise of greater intensity, has been shown to overcome these consequences of neurological disease (Alberts, Linder, Penko, Lowe, & Phillips, 2011; Ridgel, Vitek, & Alberts, 2009). People with Parkinson’s disease (PD) participated in a lower-extremity cycling intervention in which the pedaling rate was augmented to 80–90 revolutions per minute (rpm; Alberts et al., 2011; Ridgel et al., 2009). The exercise was not passive, because participants actively contributed to the pedaling to exercise within their prescribed target heart rate (HR) zone. After 8 wk of forced aerobic exercise, global improvements in motor function and improved control of grasping forces were observed among the FE group compared with the voluntary exercise (VE) group, who pedaled at a lower self-selected rate (Alberts et al., 2011; Ridgel et al., 2009). Functional magnetic resonance imaging data indicated increased cortical and subcortical activation after a single session of FE, comparable to activation patterns observed with levodopa therapy (Beall et al., 2013).
The global improvements in motor function, coupled with changes in brain activation patterns, suggest a change in central motor processing as a result of FE (Alberts et al., 2011). These promising results in people with PD, along with the theorized benefits that aerobic exercise may facilitate motor recovery in stroke (Mang et al., 2013), provide a rationale to investigate whether aerobic exercise, and FE in particular, improves upper-extremity (UE) motor outcomes when coupled with a repetitive task practice (RTP) intervention.
The aim of this pilot study was to determine the feasibility and initial efficacy of using aerobic exercise to enhance UE motor recovery after stroke. Two modes of aerobic exercise, FE and VE, were paired with an abbreviated RTP session to determine the effects on motor recovery compared with a time-matched RTP intervention without an aerobic exercise component. The fundamental hypothesis being evaluated was that patients who have incurred a stroke require assistance to exercise at a rate and duration of sufficient intensity for aerobic exercise to facilitate motor recovery. Therefore, we predicted that the FE + RTP group would demonstrate greater improvements in UE motor function than the VE + RTP and time-matched RTP group.
Method
Design
A single-center, three-arm, rater-blind study was conducted at the Cleveland Clinic. The study was approved by the Cleveland Clinic institutional review board, and all participants completed the informed consent process.
Participants
The inclusion criteria were within 6–12 mo of unilateral stroke confirmed by neuroimaging, Fugl-Meyer Assessment (FMA) UE Motor score of 19–55, physician approval to undergo a cardiac stress test, and age 18–85 yr. The exclusion criteria were hospitalization for myocardial infarction, congestive heart failure, or heart surgery within 3 mo of enrollment; cardiac arrhythmia; hypertrophic cardiomyopathy; severe aortic stenosis; cardiac pacemaker; pulmonary embolus; other contraindication to exercise; inability to follow two-step commands; or antispasticity injection in the affected UE within 3 mo of enrollment.
A phone screen was completed by a physical therapist for those who met the basic inclusion criteria on the basis of chart review. A home visit was subsequently conducted that included a medical history, medication reconciliation, and a physical examination.
Cardiopulmonary Testing
A cardiopulmonary exercise test (CPX) was completed on a Lode upright cycle ergometer using a MedGraphics CardiO2/CP system with Breeze software (MGC Diagnostics, St. Paul, MN) before randomization. The CPX data were used to establish baseline measures of cardiorespiratory function and to evaluate cardiac response to high workload. Initial workload was 20 W and increased by 20 W every 2 min; CPX was terminated using American College of Sports Medicine criteria (Swain, 2014). A 12-lead electrocardiogram and gas analysis were used throughout the test. Participants with normal physiological responses to exercise were cleared for participation by a cardiologist.
Randomization
Participants were randomized to one of the following groups: FE + RTP, VE + RTP, or time-matched RTP only. All participants attended exercise sessions 3 times per week for 8 wk for a total of 24 sessions, each 90 min long.
Forced Exercise + Repetitive Task Practice.
Participants in the FE + RTP group cycled on a stationary recumbent bicycle that was customized with a motor to increase their cadence by approximately 30% more than their self-selected rate (Alberts et al., 2011). The motor within the cycle systematically adjusted the contribution in real time relative to the participant’s voluntary efforts, contributing the minimum necessary output to maintain the determined cadence. Each exercise session consisted of a 5-min warm-up, a 35-min main exercise set, and a 5-min cool-down. Participants not able to tolerate the exercise duration were permitted to take a 2-min break every 10 min as needed, building up to an uninterrupted 45-min session. Clip-in shoes, seat settings, and affected UE positioning were individualized to ensure optimal biomechanics. HR was monitored continuously using a Garmin™ Edge® 800 heart rate monitor (Garmin Ltd., Olathe, KS), and each participant was encouraged to maintain a target HR between 60% and 80% of heart rate reserve (HRR), calculated using the Karvonen method on the basis of CPX results (Swain, 2014). Manual blood pressure was obtained every 10 min, and cadence was measured and stored continuously at 100 Hz.
Within 10 min of exercise cessation, a 45-min RTP session was initiated under the guidance of a physical or occupational therapist. Each RTP session focused on highly repetitious UE motor tasks graded by the therapist as the participant progressed. Tasks were based on each participant’s UE functional abilities and stated motor recovery goals and modeled after Birkenmeier, Prager, and Lang (2010). A typical RTP session consisted of three to four different tasks administered during a 45-min session and has been described in detail in Linder, Rosenfeldt, Rasanow, and Alberts (2015).
Voluntary Exercise + Repetitive Task Practice.
Participants in the VE + RTP group were prescribed the identical aerobic exercise duration and target HR zone. However, they cycled at a self-selected cadence on an identical (non-motorized) stationary cycle. Participants were monitored in a manner identical to those in the FE + RTP group, and the same exercise parameters were measured and recorded (HR, blood pressure, and cadence). The RTP session was administered in the same manner as described for the FE + RTP group.
Time-Matched Repetitive Task Practice Only.
Participants randomized to the RTP-only group underwent two 45-min sessions of RTP (for a total of 90 min of RTP) administered in the same manner as for the exercise groups. The RTP-only group did not perform any aerobic exercise.
Outcomes
Outcomes were measured at baseline, end of treatment (EOT), and 4 wk after EOT (EOT + 4), with the exception of CPX, which was administered at baseline and EOT only. The primary UE outcome was change in the UE Motor portion of the FMA (Gladstone, Danells, & Black, 2002). Change in the Wolf Motor Function Test (WMFT; Lin et al., 2009) served as a secondary outcome. The primary outcome for cardiorespiratory function was the change in peak volume of oxygen uptake (VO2). Variables assessing exercise performance were as follows: RTP dosage (repetitions), aerobic exercise intensity measured as percentage of HRR, and exercise rate measured as cadence in rpm.
Statistical Analyses
Outcomes quantifying training performance variables (mean percentage of HRR during FE and VE, cadence during FE and VE, and RTP dose) were analyzed using an analysis of variance (ANOVA) to determine any significant difference among the three groups (Shavelson, 1996). As a follow-up, a Welch’s t test (Welch, 1947) with Bonferroni correction (Dunn, 1961) was used to identify pairwise differences between the groups. An ANOVA was used to quantify significant difference in peak VO2 from baseline to EOT between the groups, and Welch’s t test followed for pairwise comparison between groups. FMA scores represented the primary motor outcome variable.
Prognostic factors that predict motor recovery include age and baseline UE function. To evaluate the impact of age and baseline UE function, an ANOVA was performed using age and baseline FMA scores for each group. Age was significantly different between groups; however, no difference was found in FMA scores at baseline across the groups. To correct for age, a pairwise t test with Bonferroni correction was conducted; the results indicated that age between the groups was no longer significant. All subsequent analyses were completed without adjustment for age and baseline FMA score. An ANOVA was subsequently used to determine significant differences in FMA scores among the three groups from baseline to EOT and EOT + 4. For within-group comparisons of FMA scores, a paired Welch’s t test was performed.
The secondary motor outcome was score on the WMFT. An explicit, group-specific numerical exploratory data analysis was performed on the 15 timed tasks and the 2 non-timed tasks, and an ANOVA was performed on scores from the WMFT Functional Ability Scale. All hypothesis testing was performed at the .05 level of significance. The statistical analysis was conducted using R (Version 3.1.1; R Core Team, Vienna, Austria).
Results
Of the 147 people screened, 21 met all criteria for participation and consented to enrollment. Two exhibited abnormal CPX results; 1 sought further cardiology testing and was cleared to participate, and the other did not consent to additional follow-up. Twenty participants were randomized; 3 withdrew (1 because of randomization to the RTP-only group, 1 for an inability to commit to the time requirement, and 1 because of recurrent stroke), and 17 completed all aspects of study intervention and testing. The EOT + 4 motor outcomes of 1 participant in the FE group were not included, because she received botulinum toxin immediately prior to EOT + 4 testing. Participant demographics are provided in Table 1. The most common medications participants were taking were cholesterol-lowering medications (n = 15), anticoagulants (n = 13), anti-hypertensives (n = 12), vitamins (n = 8), antacids (n = 7), anti-spasticity medications or muscle relaxants (n = 5), and antidepressants (n = 4).
Table 1.
Participant Demographics
| Characteristic | FE + RTP (n = 6) | VE + RTP (n = 6) | RTP Only (n = 5) | p |
| Age, yr, M (SD) | 44.8 (11.7) | 60.7 (12.1) | 61.6 (8.3) | nsa |
| Gender, n | ||||
| Female | 1 | 1 | 0 | |
| Male | 5 | 5 | 5 | NA |
| Race | NA | |||
| White | 3 | 5 | 4 | |
| African-American | 2 | 1 | 1 | |
| Asian | 1 | 0 | 0 | |
| Left-sided/right-sided lesion | 5/1 | 4/2 | 1/4 | NA |
| Months since stroke, M (SD) | 8.7 (2.7) | 9.9 (1.5) | 9.1 (2.1) | ns |
| Baseline Fugl-Meyer score, M (SD) | 36.1 (7.4) | 30.5 (12.9) | 25.4 (7.8) | ns |
Note. FE = forced exercise; M = mean; NA = not applicable; ns = not significant; RTP = repetitive task practice; SD = standard deviation; VE = voluntary exercise.
ns with Bonferroni correction.
Exercise and Repetitive Task Practice Training Outcomes
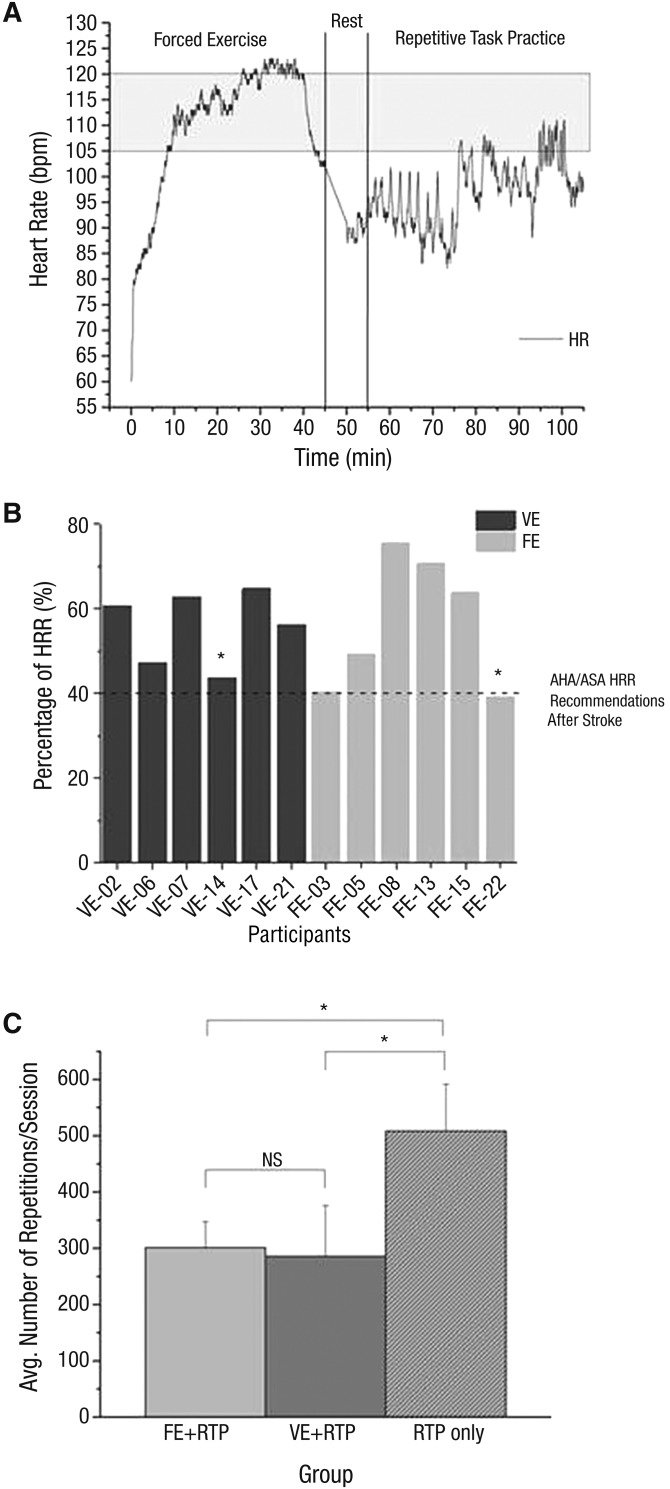
HR was monitored continuously for both groups. Figure 1A displays HR response for a typical participant in the FE + RTP group, indicating that target HR was achieved for the majority of the aerobic exercise session. Both groups were able to exercise ≥40% of their HRR, the minimum recommended by the American Stroke Association (Figure 1B). The FE + RTP and VE + RTP groups exercised at mean intensities of 56.5% HRR (standard deviation [SD] = 15.7) and 55.9% HRR (SD = 8.7), respectively. Participants in the FE + RTP group pedaled at a cadence of 80.7 rpm (SD = 4.0), which was significantly higher than the VE + RTP group (mean [M] = 67.2 rpm, SD = 10.0), t(6.8) = 2.9, p = .02.
Figure 1.
(A) Continuous HR during a session of FE exercise and RTP. (B) Exercise intensity measured as mean percentage of HRR for each FE and VE participant’s cycling sessions during the main 35-min exercise set. (C) Mean number of RTP repetitions per session for all participants in each group.
The shaded area in 1A represents the target HR zone for this participant. The dashed line in 1B represents the AHA/ASA recommendations for minimum intensity measured by HRR during aerobic exercise after stroke.
Note. *Participant was taking beta blockers. AHA/ASA = American Heart Association/American Stroke Association; Avg. = average; bpm = beats per min; FE = forced exercise; HR = heart rate; HRR = heart rate reserve; NS = not significant; RTP = repetitive task practice; VE = voluntary exercise.
The RTP-only group completed significantly more repetitions (M = 506.0, SD = 83.4), t(4.0) = 13.6, p < .001, than both the FE + RTP and VE + RTP groups (Figure 1C). The average number of repetitions did not differ between the FE + RTP and VE + RTP groups (Ms = 301.0 and 285.0 repetitions, SDs = 46.0 and 90.0, respectively), t(7.4) = 0.4, p = .70.
Cardiovascular Outcomes
The VE + RTP group demonstrated a significant improvement in mean peak VO2 of 2.4 mL/kg/min (SD = 1.0), t(5.0) = 5.6, p < .01, and the FE + RTP group improved by a mean of 1.3 mL/kg/min (SD = 3.0), which did not reach significance. The RTP-only group exhibited a slight decrease in mean peak VO2 of −0.4 mL/kg/min (SD = 1.6).
Motor Outcomes
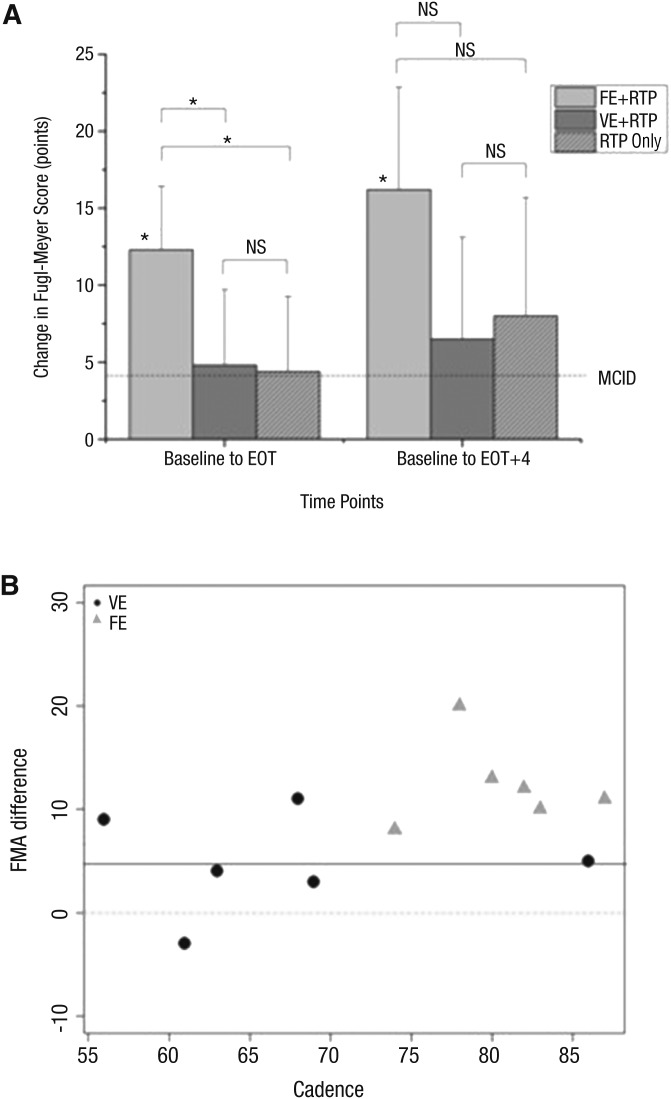
As shown in Figure 2A, all three groups exhibited improvements in FMA that exceeded the minimal clinically important difference (MCID; Page, Fulk, & Boyne, 2012) from baseline to EOT. These values further improved at EOT + 4. Only the FE + RTP group exhibited a statistically significant improvement in FMA score from baseline to EOT (M = 12.3, SD = 4.1; range = 8–20), t(5) = 7.3, p < .001; and from baseline to EOT + 4 (M = 16.4, SD = 5.8), t(4) = 6.3, p = .003; whereas the VE + RTP group (M = 4.8, SD = 4.9), t(5) = 2.4, p = .06, and RTP group (M = 4.4, SD = 4.9), t(4) = 2.0, p = .11, did not. Overall, the FE + RTP group improved significantly at EOT compared with the VE + RTP group, t(9.7) = 2.8, p = .01, and RTP-only group, t(7.9) = 2.89, p = .02.
Figure 2.
(A) Changes in upper-extremity FMA motor scores from baseline to EOT and from baseline to EOT + 4. (B) The relationship between average cadence (pedaling rate in revolutions per minute) during the intervention and change in FMA motor scores.
The dashed line in 2A represents MCID on the FMA. All participants in the FE group (gray triangles, 2B) exhibited improvement greater than the MCID for the FMA, whereas the VE group (black circles) had 3 participants who did not achieve the MCID.
Note. EOT = end of treatment; EOT + 4 = 4 wk after EOT; FE = forced exercise; FMA = Fugl-Meyer Assessment; MCID = minimal clinically important difference; NS = not significant; RTP = repetitive task practice only; VE = voluntary exercise.
*p < .05.
Changes in motor function measured by the WMFT are shown in Table 2. Improvements were observed in the two non-timed tasks (grip strength and weight to box) and in Functional Ability Scale scores. Overall, no differences were observed in timed tasks across the groups.
Table 2.
Effect of FE + RTP, VE + RTP, and RTP Only on Outcomes at Each Evaluation Time Point
| Outcome Measure | FE + RTP (n = 6) | VE + RTP (n = 6) | RTP Only (n = 5) | ||||||
| Baseline | EOT | EOT + 4a | Baseline | EOT | EOT + 4 | Baseline | EOT | EOT + 4 | |
| FMA score, M (SD) | 36.2 (7.4) | 48.5b (6.8) | 52.5 (0.6) | 30.5 (12.9) | 35.3 (14.6) | 37.0 (17.6) | 25.4 (7.8) | 29.8 (12.0) | 33.4 (15.0) |
| WMFT | |||||||||
| Total performance time, s, M (SD) | 3.8 (6.0) | 4.4 (10.74) | 3.9 (7.0) | 11.6 (25.0) | 11.6 (22.4) | 14.8 (28.8) | 13.0 (23.2) | 14.2 (27.3) | 10.5 (20.2) |
| Gross motor performance time—tasks, s (Timed Tasks 1–7), M (SD) | 1.8 (3.3) | 0.8 (0.5) | 0.7 (0.4) | 5.5 (12.1) | 6.8 (15.2) | 6.3 (21.4) | 2.7 (6.1) | 2.4 (4.7) | 2.3 (4.6) |
| Performance time—fine motor tasks, s (Timed Tasks 8–15), M (SD) | 5.7 (7.2) | 7.4 (14.0) | 6.7 (8.6) | 20.3 (34.2) | 18.8 (28.4) | 25.0 (33.6) | 23.5 (29.0) | 25.9 (34.5) | 18.6 (25.8) |
| Weight to box, lb, M (SD) | 17.3 (2.0) | 18.7 (1.3) | 20.0 (0.0) | 8.7 (3.2) | 12.3 (2.4) | 14.3 (2.0) | 8.0 (3.5) | 8.4 (4.0) | 9.2 (4.1) |
| Grip, kg, M (SD) | 21.5 (2.9) | 23.8 (3.5) | 28.1 (3.3) | 10.3 (3.0) | 12.0 (2.9) | 11.7 (3.1) | 9.0 (1.9) | 11.3 (1.8) | 12.3 (1.6) |
| No. of tasks not completed within 120 s, total, M (SD) | 0.3 (0.8) | 0.0 (0.0) | 0.0 (0.0) | 3.8 (4.6) | 3.3 (4.4) | 2.7 (3.3) | 3.4 (4.7) | 2.8 (3.8) | 3.2 (4.4) |
| No. of gross motor tasks not completed within 120 s, (SD) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.7 (1.2) | 0.5 (1.2) | 0.5 (0.8) | 1.2 (1.8) | 1.0 (1.4) | 1.2 (1.8) |
| No. of fine motor tasks not completed within 120 s, M (SD) | 0.3 (0.8) | 0.0 (0.0) | 0.0 (0.0) | 3.2 (3.6) | 2.8 (3.4) | 2.2 (2.6) | 2.2 (3.0) | 1.8 (2.5) | 2.0 (2.7) |
| Functional Ability Score | 4.0 (0.6) | 4.6 (0.4) | 4.8 (0.2) | 2.9 (1.3) | 3.3 (1.2) | 3.4 (1.3) | 2.8 (1.0) | 3.3 (1.3) | 3.4 (1.4) |
| Peak VO2 | 21.9 (7.5) | 23.2 (5.1) | N/A | 16.3 (3.5) | 18.7 (4.1)b | NA | 16.0 (3.3) | 15.5 (3.6) | NA |
Note. EOT = end of treatment; EOT + 4 = end of treatment + 4 wk; FE = forced exercise; FMA = Fugl–Meyer Assessment; M = mean; NA = not applicable; RTP = repetitive task practice; SD = standard deviation; VE = voluntary exercise; VO2 = volume of oxygen uptake; WMFT = Wolf Motor Function Test.
n = 5, secondary to 1 participant receiving botulinum toxin before EOT + 4.
Significant change from baseline, p < .05.
Adverse Events
One participant in the VE + RTP group who was 9.5 mo poststroke experienced a recurrent hemorrhagic stroke approximately 36 hr after Day 11 of the intervention. The participant’s medical history, screen, baseline CPX test (which was unremarkable), and exercise session parameters during training were provided to a five-member Patient Safety Monitoring Board that concluded unanimously that the event was not due to study participation.
Discussion
Aerobic exercise has been theorized to facilitate motor recovery after stroke (Mang et al., 2013). This is the first study to systematically test that concept and demonstrate that people who have had a stroke, when properly screened and monitored, can safely complete an intensive aerobic exercise intervention and that aerobic exercise can facilitate motor recovery when coupled with an RTP program. Overall, all groups demonstrated improvements in motor recovery, meeting or exceeding MCID values for the FMA (Page et al., 2012); thus, all interventions could be considered efficacious. Considering that those in the aerobic exercise groups completed approximately half the number of repetitions as the RTP group, aerobic exercise appears to have a favorable impact on brain function. On the basis of our preliminary data, FE appears to be the ideal mode of aerobic exercise to facilitate motor recovery in patients who have had a stroke.
Identification of Appropriate Candidates
Critical to ensuring the safety of participants completing any aerobic exercise intervention is the cardiovascular screen (Linder et al., 2015). The primary cardiovascular reasons for exclusion prior to CPX testing were active or uncontrolled cardiac arrhythmia, congestive heart failure, and severely low ejection fraction. The screen was effective in determining which participants could safely complete the intensive aerobic exercise intervention and proved to be more cost-effective than formal CPX testing for all potential candidates. This process is critical for the clinical use of aerobic exercise in general, and it can be used in conjunction with submaximal tests, as recommended by the American Stroke Association (Billinger et al., 2014).
Although our primary aim was to investigate the effects of aerobic exercise training on motor recovery after stroke, cardiovascular improvements after aerobic exercise can also have an impact from a chronic disease management standpoint. Stroke is considered an endpoint of cardiovascular disease, with approximately 30% of people experiencing a recurrent stroke in their lifetime (Billinger et al., 2014). Risk factors that are modifiable and responsive to aerobic exercise include improved control of hypertension, diabetes mellitus, hyperlipidemia, obesity, and physical inactivity.
Participants presented with diminished cardiopulmonary function, with a mean peak VO2 of 18.0 mL/kg/min at baseline. Peak VO2 values of ≤ 20 mL/kg/min have been associated with limited physical function in the performance of instrumental activities of daily living (IADLs; Letombe et al., 2010). Therefore, even modest improvements in peak VO2 may have meaningful implications for improving endurance and ease of IADL performance. Despite diminished cardiopulmonary function at baseline, as shown in Figure 1B, participants in both aerobic exercise groups achieved cardiovascular intensity levels of ≥40% HRR as recommended by the American Stroke Association (Billinger et al., 2014), demonstrating feasibility of the prescribed aerobic exercise approach. Adherence to exercise prescription indicates that, when properly screened, patients who have had a stroke can complete a relatively intensive aerobic exercise intervention.
Aerobic Exercise Facilitates Motor Recovery
Although all groups exhibited improvements on the FMA, gains made by the VE + RTP group were similar to gains observed in the RTP-only group. Similar gains in recovery with VE + RTP, despite completing 40% fewer repetitions than the RTP-only group, suggests that aerobic exercise facilitates motor recovery. These results support the potential neuroplastic effects of aerobic exercise training in stroke recovery theorized by Knaepen et al. (2010) and Mang et al. (2013), suggesting that aerobic exercise enhances learning when paired with motor task practice. Although both modes of aerobic exercise training appeared to enhance motor recovery compared with the RTP-only group, which did not partake in aerobic exercise, our FE approach was superior to VE in reducing impairment in the hemiparetic UE.
Forced Exercise May Be Optimal for Motor Recovery
The FE + RTP group demonstrated significantly greater improvement on the FMA than the VE + RTP and RTP-only groups. Participants in both aerobic exercise groups completed approximately the same number of RTP repetitions; thus, the mode of exercise may be responsible for differential gains in motor recovery. Both the VE + RTP and FE + RTP groups exercised at a similar level of intensity in terms of percentage of HRR and duration. The only difference between the two groups in terms of exercise prescription was that the FE + RTP group pedaled approximately 20% faster than those in the VE + RTP group. On the basis of preliminary data, it appears that pedaling rate may be an important variable in facilitating the therapeutic effects of RTP (Figure 2B) because the participants pedaling at higher rates tended to exhibit greater gains on the FMA.
Our previous experience with FE with participants with PD (Alberts et al., 2011; Ridgel et al., 2009) and the current results support the hypothesis that assisting voluntary efforts during cycling may improve afferent input to the central nervous system. From a neuromuscular perspective, sustained physical activity is achieved through the effective recruitment of motor units and the resultant generation of efficient mechanical movement (Billinger, Coughenour, et al., 2012). Because somatosensory input is often diminished after stroke, assisted cycling may facilitate efficient mechanical movement and increased motor output.
In addition, after stroke people experience difficulty generating the power necessary for sustained physical activity, because a profound shift toward a higher proportion of fast-twitch fibers, which are significantly more prone to fatigue, has been observed in hemiparetic leg muscle (Billinger, Coughenour, et al., 2012). Therefore, the direct sequelae of stroke, which include altered afferent feedback from the periphery, hemiparesis, and impaired motor control, combined with secondary changes to muscle physiology, may contribute to limitations in sustained physical activity. It is hypothesized that with FE, the consistent pedaling rate and torque production provided by the motor decreases muscle fatigue, allowing people to overcome the physiological barriers to sustained exercise training, similar to our studies with participants with PD (Alberts et al., 2011; Ridgel et al., 2009).
The current study was not designed to identify the precise mechanism underlying differential effects of FE versus VE. However, animal and human studies investigating the effects of intensive aerobic exercise training have provided insight into why FE may be ideally suited to facilitate motor recovery. Previous studies have shown that FE increases levels of BDNF, insulin-like growth factor–1, and dopamine, all of which have been implicated in neuroplasticity and enhanced learning (Pareja-Galeano et al., 2013). Petzinger et al. (2011) reported increased dopaminergic neurotransmission as a result of FE, with the greatest response in the dorsolateral striatum, a region that contributes significantly to motor function and that suffers extensive neuronal loss after stroke. In PD animal models, FE has been found to alter glutamate and glutamatergic receptors, which have been shown to be critical in facilitating neuroplasticity by restoring normal synaptic function (Petzinger et al., 2011). Although PD and stroke differ neuropathologically, the proposed dopaminergic response and alterations in glutamate and glutaminergic function as a result of FE may have a similar neurorestorative effect in stroke models, as has been shown in PD. Human models investigating the effects of aerobic exercise on learning and neurorestoration have identified BDNF and insulin-like growth factor–1 as key mediators and potential mechanisms associated with improved cognitive performance. Collectively, these neurophysiological responses in the central nervous system, when paired with motor task practice, may be exploited to optimize motor learning and may have contributed to the improved motor outcomes observed as a result of our FE intervention (Mang et al., 2013).
Study Limitations and Future Directions
It is important that these results not be over-interpreted because this was a preliminary trial with a small, heterogeneous sample. Although baseline FMA scores were not significantly different statistically, clinical intuition acknowledges that the differences in baseline function and age across the groups may have contributed to changes in motor recovery. Future studies will use a stratified randomization scheme to account for potentially confounding variables that may have an impact on recovery. In addition, sensory function, which may influence motor recovery, was not quantitatively evaluated. Last, understanding total exertion in terms of exercise watts would enhance the interpretation of results regarding exercise variables that may contribute to motor recovery. The variation in initial UE function compromised the utility of the WMFT because of floor and ceiling effects across the sample. Future studies will include biomechanical and kinematic measures quantifying UE dexterity to better characterize changes in motor function.
Implications for Occupational Therapy Practice
The results of this trial have the following implications for occupational therapy practice:
With proper screening and supervision, people who have had a stroke can safely participate in an intensive aerobic exercise intervention.
A 45-min session of intensive aerobic exercise on a stationary bicycle may facilitate UE motor recovery when paired with an abbreviated session of RTP.
Conclusion
We have demonstrated that it is feasible for a heterogeneous cohort of people who have had a stroke to safely complete an intensive aerobic exercise intervention and hundreds of RTP repetitions despite hemiparesis and cardiovascular deconditioning. Initial data indicate the efficacy of two aerobic exercise interventions to optimize the benefits associated with motor task practice and improve motor recovery after stroke. Our innovative approach in pairing FE with motor training answers the call by Ivey et al. (2006) for “novel exercise interventions . . . to realize the potential for long-term functional recovery and cardiovascular health in the chronic hemiparetic condition” (p. 443). Our combined approach is clinically translatable and may serve to optimize motor outcomes while simultaneously improving cardiovascular fitness in this at-risk population.
Acknowledgments
We thank Cindy Clark, Mandy Penko, Matthew Rasanow, Andrew Bazyk, and Mike Crawford for their contributions in clinical testing, data collection, and data analysis. This study was supported by the National Institute of Neurological Disorders and Stroke (Grant R03HD073566 to Susan M. Linder and Jay L. Alberts) and is registered with clinicaltrials.gov (NCT02076776). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Preliminary results of this trial were presented at the Society for Neuroscience conference in November 2014, the American Physical Therapy Association Combined Sections Meeting in February 2015, and the International Stroke Conference in February 2015. Jay L. Alberts has authored intellectual property associated with the algorithm used in the control of the FE bicycle. The remaining authors declare no conflict of interest.
Contributor Information
Susan M. Linder, Susan M. Linder, DPT, NCS, is Project Staff, Department of Biomedical Engineering and Cleveland Clinic Concussion Center, Cleveland Clinic, Cleveland, OH; linders@ccf.org
Anson B. Rosenfeldt, Anson B. Rosenfeldt, DPT, MBA, is Research Physical Therapist, Department of Biomedical Engineering, Cleveland Clinic, Cleveland, OH
Tanujit Dey, Tanujit Dey, PhD, is Associate Staff, Cleveland Clinic Concussion Center and Quantitative Health Sciences, Cleveland Clinic, Cleveland, OH.
Jay L. Alberts, Jay L. Alberts, PhD, is Staff, Department of Biomedical Engineering, Center for Neurological Restoration, and Cleveland Clinic Concussion Center, Cleveland Clinic, Cleveland, OH
References
- Alberts J. L., Linder S. M., Penko A. L., Lowe M. J., & Phillips M. (2011). It is not about the bike, it is about the pedaling: Forced exercise and Parkinson’s disease. Exercise and Sport Sciences Reviews, 39, 177–186. https://doi.org/10.1097/JES.0b013e31822cc71a [DOI] [PubMed] [Google Scholar]
- Beall E. B., Lowe M. J., Alberts J. L., Frankemolle A. M., Thota A. K., Shah C., & Phillips M. D. (2013). The effect of forced-exercise therapy for Parkinson’s disease on motor cortex functional connectivity. Brain Connectivity, 3, 190–198. https://doi.org/10.1089/brain.2012.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinger S. A., Arena R., Bernhardt J., Eng J. J., Franklin B. A., Johnson C. M., . . . Tang A.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, and Council on Clinical Cardiology. (2014). Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 45, 2532–2553. https://doi.org/10.1161/STR.0000000000000022 [DOI] [PubMed] [Google Scholar]
- Billinger S. A., Coughenour E., Mackay-Lyons M. J., & Ivey F. M. (2012). Reduced cardiorespiratory fitness after stroke: Biological consequences and exercise-induced adaptations. Stroke Research and Treatment, 2012, 959120 https://doi.org/10.1155/2012/959120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinger S. A., Mattlage A. E., Ashenden A. L., Lentz A. A., Harter G., & Rippee M. A. (2012). Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. Journal of Neurologic Physical Therapy, 36, 159–165. https://doi.org/10.1097/NPT.0b013e318274d082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier R. L., Prager E. M., & Lang C. E. (2010). Translating animal doses of task-specific training to people with chronic stroke in 1-hour therapy sessions: A proof-of-concept study. Neurorehabilitation and Neural Repair, 24, 620–635. https://doi.org/10.1177/1545968310361957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Berchtold N. C., & Christie L. A. (2007). Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends in Neurosciences, 30, 464–472. https://doi.org/10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Dobkin B. H., & Dorsch A. (2013). New evidence for therapies in stroke rehabilitation. Current Atherosclerosis Reports, 15, 331 https://doi.org/10.1007/s11883-013-0331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn O. J. (1961). Multiple comparisons among means. Journal of the American Statistical Association, 56, 52–64. [Google Scholar]
- Gladstone D. J., Danells C. J., & Black S. E. (2002). The Fugl–Meyer Assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabilitation and Neural Repair, 16, 232–240. https://doi.org/10.1177/154596802401105171 [DOI] [PubMed] [Google Scholar]
- Ivey F. M., Hafer-Macko C. E., & Macko R. F. (2006). Exercise rehabilitation after stroke. NeuroRx, 3, 439–450. https://doi.org/10.1016/j.nurx.2006.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Hayes M., Beiser A., Kase C. S., Scaramucci A., D’Agostino R. B., & Wolf P. A. (2003). The influence of gender and age on disability following ischemic stroke: The Framingham study. Journal of Stroke and Cerebrovascular Diseases, 12, 119–126. https://doi.org/10.1016/S1052-3057(03)00042-9 [DOI] [PubMed] [Google Scholar]
- Knaepen K., Goekint M., Heyman E. M., & Meeusen R. (2010). Neuroplasticity—Exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Medicine, 40, 765–801. https://doi.org/10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- Letombe A., Cornille C., Delahaye H., Khaled A., Morice O., Tomaszewski A., & Olivier N. (2010). Early post-stroke physical conditioning in hemiplegic patients: A preliminary study. Annals of Physical and Rehabilitation Medicine, 53, 632–642. https://doi.org/10.1016/j.rehab.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Lin J. H., Hsu M. J., Sheu C. F., Wu T. S., Lin R. T., Chen C. H., & Hsieh C. L. (2009). Psychometric comparisons of 4 measures for assessing upper-extremity function in people with stroke. Physical Therapy, 89, 840–850. https://doi.org/10.2522/ptj.20080285 [DOI] [PubMed] [Google Scholar]
- Linder S. M., Rosenfeldt A. B., Rasanow M., & Alberts J. L. (2015). Forced aerobic exercise enhances motor recovery after stroke: A case report. American Journal of Occupational Therapy, 69, 6904210010 https://doi.org/10.5014/ajot.2015.015636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang C. S., Campbell K. L., Ross C. J., & Boyd L. A. (2013). Promoting neuroplasticity for motor rehabilitation after stroke: Considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Physical Therapy, 93, 1707–1716. https://doi.org/10.2522/ptj.20130053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell M. N., Smith A. E., & Mackintosh S. F. (2011). Aerobic exercise to improve cognitive function in adults with neurological disorders: A systematic review. Archives of Physical Medicine and Rehabilitation, 92, 1044–1052. https://doi.org/10.1016/j.apmr.2011.01.021 [DOI] [PubMed] [Google Scholar]
- Page S. J., Fulk G. D., & Boyne P. (2012). Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Physical Therapy, 92, 791–798. https://doi.org/10.2522/ptj.20110009 [DOI] [PubMed] [Google Scholar]
- Pareja-Galeano H., Brioche T., Sanchis-Gomar F., Montal A., Jovaní C., Martínez-Costa C., . . . Viña J. (2013). Impact of exercise training on neuroplasticity-related growth factors in adolescents. Journal of Musculoskeletal and Neuronal Interactions, 13, 368–371. [PubMed] [Google Scholar]
- Petzinger G. M., Fisher B. E., Akopian G., Holschneider D. P., Wood R., Walsh J. P., . . . Jakowec M. W. (2011). The role of exercise in facilitating basal ganglia function in Parkinson’s disease. Neurodegenerative Disease Management, 1, 157–170. https://doi.org/10.2217/nmt.11.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. M. (2001). Neurotrophins as synaptic modulators. Nature Reviews Neuroscience, 2, 24–32. https://doi.org/10.1038/35049004 [DOI] [PubMed] [Google Scholar]
- Ridgel A. L., Vitek J. L., & Alberts J. L. (2009). Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabilitation and Neural Repair, 23, 600–608. https://doi.org/10.1177/1545968308328726 [DOI] [PubMed] [Google Scholar]
- Shavelson R. (1996). Statistical reasoning for the behavioral sciences (3rd ed.). Boston: Allyn & Bacon. [Google Scholar]
- Swain D. P. (Ed.). (2014). ACSM’s resource manual for guidelines for exercise testing and prescription (7th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. [Google Scholar]
- Welch B. L. (1947). The generalisation of student’s problems when several different population variances are involved. Biometrika, 34, 28–35. [DOI] [PubMed] [Google Scholar]