Abstract
Introduction
Community-based and other epidemiologic studies within the United States have identified substantial disparities in health care among adults with epilepsy. However, few data analyses addressing their health-care access are representative of the entire United States. This study aimed to examine national survey data about adults with epilepsy and to identify barriers to their health care.
Materials and methods
We analyzed data from U.S. adults in the 2010 and the 2013 National Health Interview Surveys, multistage probability samples with supplemental questions on epilepsy. We defined active epilepsy as a history of physician-diagnosed epilepsy either currently under treatment or accompanied by seizures during the preceding year. We employed SAS-callable SUDAAN software to obtain weighted estimates of population proportions and rate ratios (RRs) adjusted for sex, age, and race/ethnicity.
Results
Compared to adults reporting no history of epilepsy, adults reporting active epilepsy were significantly more likely to be insured under Medicaid (RR = 3.58) and less likely to have private health insurance (RR = 0.58). Adults with active epilepsy were also less likely to be employed (RR = 0.53) and much more likely to report being disabled (RR = 6.14). They experience greater barriers to health-care access including an inability to afford medication (RR = 2.40), mental health care (RR = 3.23), eyeglasses (RR = 2.36), or dental care (RR = 1.98) and are more likely to report transportation as a barrier to health care (RR = 5.28).
Conclusions
These reported substantial disparities in, and barriers to, access to health care for adults with active epilepsy are amenable to intervention.
Keywords: Epilepsy, Epidemiology, Surveillance, United States, Insurance, Access to care
1. Introduction
The Institute of Medicine has recently affirmed the paramount importance of quality health-care access for people with epilepsy and has identified deficiencies therein [1]. At nearly the same time, the U.S. Department of Health and Human Services published new public health objectives, Healthy People 2020, for the first time listing among its priorities the improvement of access to appropriate epilepsy care [2]. Several sources provide evidence that many people with epilepsy in the United States currently lack such care. Community-based and other epidemiologic studies within the United States have identified substantial disparities in health care among people with epilepsy: variable quality and timeliness of evaluations following the onset of the disorder, variable qualifications among physicians providing principal care for epilepsy, variable use of newer medications with fewer side effects, and varying extents to which patients with treatment-resistant epilepsy are referred to specialty centers that have advanced diagnostic and treatment resources [1,3–5]. These disparities are manifest especially among people of different socioeconomic strata, race, and locality — characteristics which are, of course, closely interrelated in the United States [3,6].
Few data addressing health-care access among people with epilepsy have been analyzed that are representative of the United States as a whole. One analysis of data from a 2005 survey conducted in 13 states examined epilepsy prevalence and access to specialty care [7]. More recently, we reported preliminary data on epilepsy from the 2010 National Health Interview Survey (NHIS) [8]. People with active epilepsy represented 1.0% of the U.S. civilian noninstitutionalized population aged 18 years or more but comprised a disproportionately higher percentage (1.9%) of those in households of low income (less than $35,000 annually). A salient finding of that study was the unexpectedly low proportion (52.8%) of those with active epilepsy who reported seeing a neurologist or epilepsy specialist during the past year.
The purpose of this study was to examine the 2010 and 2013 NHIS data pertaining to people with epilepsy in greater depth, to identify barriers to their health care, and to assess these findings in the context of other relevant studies.
2. Materials and methods
The National Health Interview Survey is an annual cross-sectional household interview survey using a multistage area probability sampling design intended to represent the civilian noninstitutionalized U.S. population [9]. In 2010 and 2013, the interviewed samples for the Sample Adult component were 27,157 and 34,557 adults 18 years of age and older, respectively, 98.6% of whom provided direct self-reported answers to questions; for the remainder, answers were provided by knowledgeable proxies [10]. In these two years, the survey included supplemental questions on epilepsy. A screening question asked whether a doctor or other health professional had ever diagnosed the person with epilepsy or a seizure disorder. For those with affirmative responses, follow-up questions asked whether they were currently taking medication to control the condition, whether they had one or more seizures in the past year, and whether they had seen a neurologist or epilepsy specialist in the past year [7].
For this report, as in the preceding report [8], we defined active epilepsy as a history of physician-diagnosed epilepsy that was either currently under treatment with medication or was accompanied by seizures during the year preceding the survey. In the 2010 and 2013 NHIS, 1066 adult respondents reported a history of physician-diagnosed epilepsy, 644 (59%) of whom were classified as active epilepsy. Mental health needs of the survey recipients were assessed based on responses to 6 items of the Kessler Screening Scale for Psychological Distress (K6), which assesses symptom severity for feelings during the past 30 days described as ‘sad’, ‘nervous’, ‘restless or fidgety’, ‘hopeless’, ‘everything is an effort’, and ‘worthless’ [11]. A total K6 symptom severity score of 13 or more, summed across 6 items scored from 0 to 4, indicates serious psychological distress. The K6 is an efficient screen for “serious mental illness” in the general population [12]. Other standardized 2010 and 2013 NHIS questions on type of health insurance coverage, access to a primary care physician, vision care, dental care, mental health care, barriers to care, and sociodemographic questions examined in this study are available at: http://www.cdc.gov/nchs/nhis/nhis_questionnaires.htm.
We employed SAS® (version 9.3)-callable SUDAAN® (version 11) statistical software to account for the complex survey design, using stratification, clustering, and weighting to obtain estimates of population proportions and rate ratios (RRs) adjusted for age, sex, and race/ ethnicity with 95% confidence intervals (CIs). We used the SUDAAN MULTILOG procedure for two-group comparisons [13]. We examined relative standard errors (RSEs) to judge reliability of estimates. Where the estimates are less than 50%, the RSE is the standard error divided by the estimate and then multiplied by 100 to obtain a percent. Where the estimates are ≥50%, the RSE is the standard error of the estimate / [(100% – value of the estimate)] * 100. Relative standard errors ≥ 30% were considered unreliable. Only more reliable estimates with RSEs < 30% are presented in the tables.
3. Findings
The estimated number of adults having active epilepsy constituted 0.99% [95% CI: 0.90%–1.09%] of the U.S. adult population. Of this estimated number, 17% [14%–21%] were aged 65 years or older.
3.1. Comparisons between persons with active epilepsy and without epilepsy
We first examined health insurance coverage, the preeminent determinant of access to health care. Table 1 shows the distribution of types of health insurance coverage among adults with active epilepsy and among adults with no history of epilepsy, stratified by age group. Among all adults with active epilepsy, 36% were covered under private health insurance, 33% under Medicaid, 15% under other types including Medicare, and 16% were uninsured. Compared to adults with no history of epilepsy, adults reporting active epilepsy were significantly more likely to be insured under Medicaid (RR = 3.58 [3.09–4.15]) and less likely to have private health insurance coverage (RR = 0.58 [0.51–0.66]). Among adults aged 18–64 years, those with active epilepsy were much less likely to have private health insurance (RR = 0.52 [0.44–0.60]) and much more likely to have Medicaid (RR = 3.89 [3.35–4.52]) than those without epilepsy. Among adults aged ≥65 years, although those with active epilepsy were twice as likely to have Medicaid as those without epilepsy (RR = 1.98 [1.34–2.91]), nearly all persons in both groups had some type of insurance.
Table 1.
Health insurance coverage and employment status by age group among people with and without epilepsy.
| Active epilepsy | No epilepsy | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n | % | 95% CI | n | % | 95% CI | RR | 95% CI | |
| Insurance type | ||||||||
| ≥18 years | ||||||||
| Private | 216 | 36.1 | 31.7–40.8 | 35,199 | 62.6 | 61.9–63.3 | 0.58 | 0.51–0.66 |
| Medicaid | 234 | 32.7 | 28.3–37.4 | 6756 | 9.1 | 8.8–9.5 | 3.58 | 3.09–4.15 |
| Other | 115 | 15.1 | 12.4–18.4 | 7613 | 10.7 | 10.3–11.0 | 1.42 | 1.16–1.73 |
| Uninsured | 77 | 15.9 | 12.5–20.2 | 10,846 | 17.2 | 16.7–17.7 | 0.93 | 0.73–1.18 |
| 18–64 years | ||||||||
| Private | 157 | 33.6 | 28.8–38.7 | 28,920 | 65.0 | 64.1–65.7 | 0.52 | 0.44–0.60 |
| Medicaid | 206 | 36.8 | 31.7–42.2 | 5401 | 9.5 | 9.1–9.9 | 3.89 | 3.35–4.52 |
| Other | 67 | 10.7 | 8.0–14.0 | 2422 | 4.4 | 4.1–4.7 | 2.42 | 1.82–3.22 |
| Uninsured | 77 | 18.8 | 14.7–23.8 | 10,712 | 20.7 | 20.1–21.2 | 0.91 | 0.71–1.16 |
| ≥65 years | ||||||||
| Private | 59 | 49.1 | 39.5–58.7 | 6279 | 51.4 | 50.2–52.7 | 0.95 | 0.78–1.16 |
| Medicaid | 28 | 14.9 | 10.0–21.5 | 1355 | 7.5 | 7.0–8.1 | 1.98 | 1.34–2.91 |
| Other | 48 | 36.1 | 26.9–46.3 | 5191 | 39.9 | 38.8–41.1 | 0.90 | 0.69–1.19 |
| Uninsured | – | 134 | 1.0 | 0.8–1.2 | – | |||
| Employment category | ||||||||
| ≥18 years | ||||||||
| Employed | 176 | 32.2 | 27.1–37.8 | 35,413 | 61.1 | 60.5–61.7 | 0.53 | 0.45–0.62 |
| Student | – | 1591 | 3.0 | 2.8–3.3 | – | |||
| Retired | 100 | 13.9 | 12.2–15.9 | 11,292 | 15.9 | 15.5–16.4 | 0.88 | 0.77–0.99 |
| Disabled | 275 | 35.6 | 31.7–39.7 | 4020 | 5.8 | 5.5–6.1 | 6.14 | 5.41–6.97 |
| Unemployed | 85 | 13.8 | 10.7–17.7 | 8261 | 14.1 | 13.7–14.5 | 0.98 | 0.76–1.27 |
| 18–64 years | ||||||||
| Employed | 163 | 37.3 | 31.1–43.9 | 33,445 | 70.8 | 70.2–71.4 | 0.53 | 0.44–0.63 |
| Student | – | 1583 | 3.6 | 3.4–3.9 | – | |||
| Retired | – | 1562 | 3.3 | 3.1–3.5 | – | |||
| Disabled | 249 | 39.5 | 34.8–44.4 | 3276 | 6.0 | 5.6–6.3 | 6.63 | 5.78–7.60 |
| Unemployed | 76 | 15.4 | 11.7–20.1 | 7741 | 16.2 | 15.8–16.7 | 0.95 | 0.72–1.25 |
| ≥65 years | ||||||||
| Employed | – | 1968 | 16.1 | 15.2–17.0 | – | |||
| Student | – | – | – | |||||
| Retired | 87 | 68.2 | 59.5–75.9 | 9730 | 74.7 | 73.7–75.8 | 0.91 | 0.81–1.03 |
| Disabled | 26 | 15.2 | 9.8–22.7 | 744 | 5.1 | 4.6–5.6 | 2.98 | 1.96–4.55 |
| Unemployed | – | 520 | 4.0 | 3.6–4.5 | – | |||
Notes: ‘No epilepsy’ indicates persons providing no history of ever having been diagnosed with epilepsy or seizure disorder. ‘Private’ includes persons having commercial health insurance with or without Medicare insurance. ‘Other’ includes persons with Medicare insurance only.
“–” the estimate is unreliable because the relative standard error is >30%. Adjusted for sex, age, and race/ethnicity.
Among all adults with active epilepsy, 50% saw both a primary care physician and a neurologist or epilepsy specialist in the 12 months preceding the survey; 36% saw only a primary care physician; 8% saw only a neurologist or epilepsy specialist; and 6% saw neither.
At the time of data collection for this study, prior to the 2014 expansions in health-care coverage under the Affordable Care Act [14], the system of health insurance in the United States was more closely tied to employment. We examined the impact of active epilepsy on employment status as well as insurance status (Table 1). Among all adults with active epilepsy, only 32% reported being employed, while 36% reported being disabled. Compared to adults with no history of epilepsy, those with active epilepsy were significantly less likely to be employed (RR = 0.53 [0.45–0.62]) and much more likely to report being disabled (RR = 6.14 [5.41–6.97]). Among persons aged 18–64 years, adults with active epilepsy were much less likely to report being employed (RR = 0.53 [0.44–0.63]) and much more likely to report being disabled (RR = 6.63 [5.78–7.60]) than adults with no history of epilepsy. Similar relationships occurred among persons aged ≥ 65 years for being disabled (RR= 2.98 [1.96–4.55]), although only the latter was statistically significant. Among persons aged ≥65 years, 68% of those with active epilepsy and 75% of those without epilepsy described themselves as retired.
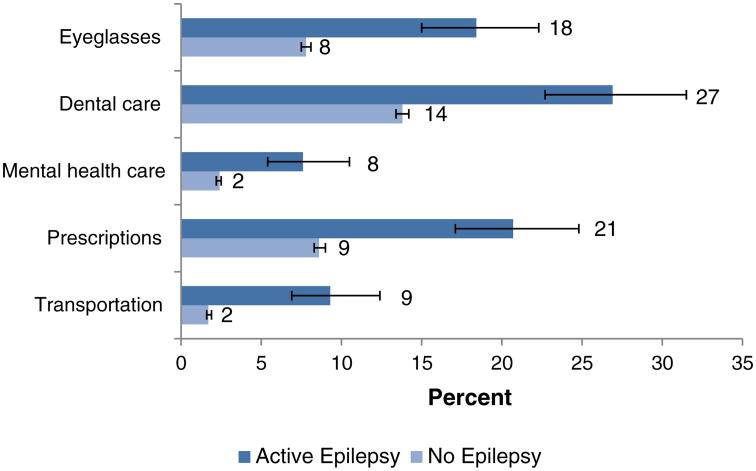
Potential consequences of inadequate health insurance coverage and reduced income are shown in Fig. 1. Compared to adults with no history of epilepsy, adults with active epilepsy much more likely reported an inability to afford the cost of eyeglasses (18% among those with active epilepsy vs. 8% of those without, RR = 2.36 [1.92–2.90]), dental care (27% vs. 14%, RR = 1.98 [1.69–2.33]), mental health care (8% vs. 2%, RR = 3.23 [2.29–4.54]), and prescription medication (21% vs. 9%, RR = 2.40 [1.99–2.89]) when these items or services were needed during the preceding year. Nine percent of those with active epilepsy could not obtain health care because they had no transportation, compared to only 2% of those without epilepsy (RR = 5.28 [3.93-7.09]).
Fig. 1.
Barriers to health care among adults with active epilepsy and without epilepsy. Notes: ‘No epilepsy’ indicates persons providing no history of ever having been diagnosed with epilepsy or seizure disorder.‘Eyeglasses’, ‘Dental care’, ‘Mental health care’, and ‘Medication’ refer to the percentage of respondents indicating an inability to afford these types of care when needed during the past year. ‘Transportation’ indicates an inability to obtain transportation to a site providing health care during the past year. Numbers indicate percentage point estimates. Error markers indicate 95% confidence limits.
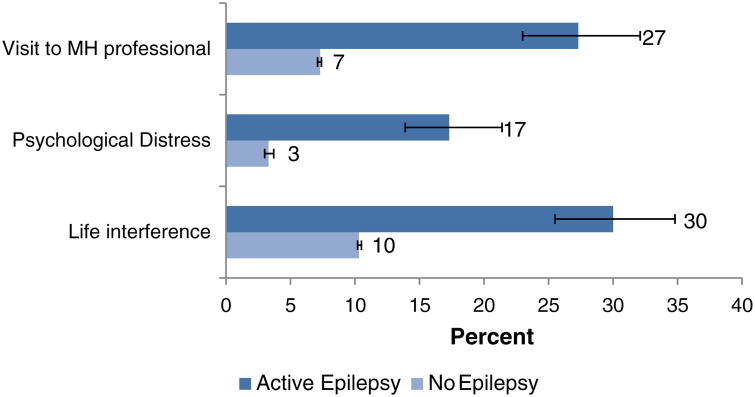
Twenty-seven percent (27%) of those with active epilepsy versus 7% of those with no history of epilepsy reported visits for services from a mental health-care provider in the past year (RR = 3.62 [3.06–4.29]) (Fig. 2). Serious psychological distress during the past 30 days was present in 17% of those with active epilepsy, compared to 3% of those without epilepsy (RR = 5.09 [4.07–6.37]). Distress in the past 30 days from any of the K6 items sufficient to interfere with ‘life or activities’ to a degree described as “‘a lot’ or ‘some’” was present in 30% of those with epilepsy, compared to 10% of those without (RR = 2.86 [2.44–3.34]).
Fig. 2.
Mental health-care needs and services among adults with active epilepsy and without epilepsy. Notes: ‘No epilepsy’ indicates persons providing no history of ever having been diagnosed with epilepsy or seizure disorder. ‘Visit to MH professional’ indicates the proportion of persons with visits for professional mental health services within the past year. ‘Psychological distress’ indicates the proportion of persons scoring 13 points or more on the Kessler 6 psychological distress scale, reflecting symptoms during the past 30 days. ‘Life interference’ indicates the proportion of persons reporting that any symptoms measured by K6 were sufficient to interfere with life or activities “a lot” or “some” in the past 30 days. Numbers indicate percentage point estimates. Error markers indicate 95% confidence limits.
3.2. Comparisons of persons with active epilepsy and with orwithout recent seizures
Fifty-eight percent [53.1%–63.3%] of those with active epilepsy reported having one or more seizures during the year preceding their interview. Among adults with active epilepsy, we compared health insurance coverage, employment status, and access to selected health services between those who reported having seizures during the past year and those who did not.
Table 2 compares the respective distributions of health insurance and employment categories. Adults with active epilepsy reporting seizures in the past year were more likely than those reporting no seizures to report being disabled (44% vs. 34%), although this finding was not statistically significant after adjustment (RR = 1.28 [0.99–1.66]). Health insurance coverage and other employment status did not differ significantly by level of seizure control.
Table 2.
Insurance and employment status among adults with active epilepsy by seizure status: NHIS ages 18 years and older.
| Recent seizures | No recent seizures | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n | % | 95% CI | n | % | 95% CI | RR | 95% CI | |
| Insurance type | ||||||||
| Private | 108 | 34.6 | 28.7–41.1 | 108 | 41.3 | 34.0–49.1 | 0.84 | 0.64–1.09 |
| Medicaid | 153 | 29.4 | 24.5–34.8 | 81 | 36.6 | 29.1–44.9 | 0.80 | 0.61–1.05 |
| Other | 61 | 16.1 | 11.8–21.6 | 54 | 16.6 | 12.1–22.2 | 0.97 | 0.64–1.48 |
| Uninsured | 62 | 19.9 | 15.1–25.8 | – | – | |||
| Employment | ||||||||
| Employed | 91 | 27.1 | 21.3–33.7 | 85 | 36.2 | 29.0–44.0 | 0.75 | 0.55–1.02 |
| Student | – | – | – | |||||
| Retired | 39 | 12.8 | 9.7–16.8 | 61 | 15.5 | 12.1–19.7 | 0.83 | 0.62–1.10 |
| Disabled | 186 | 43.7 | 38.6–49.0 | 89 | 34.1 | 27.2–41.9 | 1.28 | 0.99–1.66 |
| Unemployed | 62 | 13.4 | 9.9–17.9 | 23 | 11.1 | 6.3–18.8 | 1.20 | 0.64–2.27 |
Notes: ‘Recent seizures’ refers to persons with active epilepsy who report ≥1 seizure during the year prior to interview; ‘No recent seizures’ refers to persons with active epilepsy who report no seizures during the year prior to interview.“–” the estimate is unreliable because the relative standard error is ≥30%. Adjusted for sex, age, and race/ethnicity.
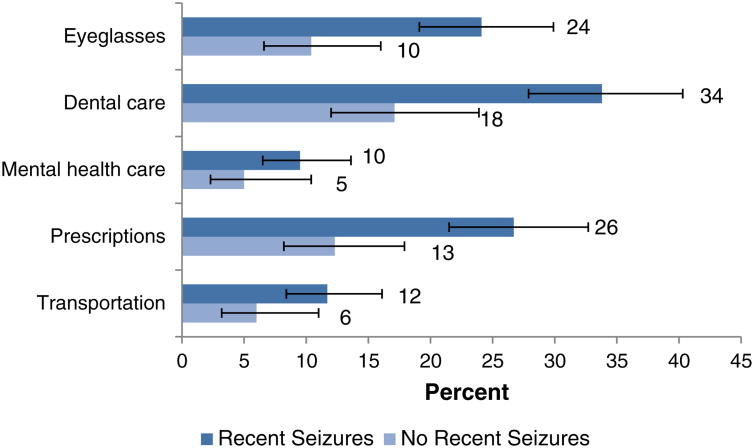
We examined potential consequences of inadequate health insurance and income barriers to health care among adults with active epilepsy by level of seizure control (Fig. 3). Those who reported recent seizures were much more likely than those without recent seizures to report being unable to afford eyeglasses (24% vs. 10%, RR = 2.34 [1.39–3.93]), dental care (34% vs. 18%, RR = 1.91 [1.27–2.88]), and prescription medication (26% vs. 13%, RR = 2.08 [1.30–3.32]).
Fig. 3.
Barriers to health care among adults with epilepsy, with and without seizures during the preceding year. Notes: All persons with active epilepsy aged ≥18 years. ‘Recent seizures’ refers to persons with active epilepsy who report ≥1 seizure during the year prior to interview; ‘No recent seizures’ refers to persons with active epilepsy who report no seizures during the year prior to interview. Numbers indicate point estimates. Error markers indicate 95% confidence limits.
Thirty-nine percent (39% [95% CI: 32–46]) of those experiencing seizures in the past year had not seen a neurologist, compared to 46% (95% CI: 39–54) of those whose seizures were reportedly controlled. Those with and without recent seizures did not differ statistically in reported psychological distress and use of mental health services.
4. Discussion
4.1. Interpretation
An important finding of this study is the low proportion of adults with active epilepsy – only 42% – who reported complete freedom from seizures during the year before interview. This finding may be considered in light of other studies indicating that with adequate care, at least two-thirds of patients with a current diagnosis of epilepsy can achieve complete seizure remission [15,16]. Also important is our finding that 38% of those who are not seizure-free have not seen a neurologist or epilepsy specialist in the past year, consistent with previous state estimates [7,17]. Together, these findings indicate a large treatment gap.
Other findings of our study add dimensions to this treatment gap. A consistent picture emerges, revealing very high rates of disability or unemployment among adults with epilepsy, consistent with substantially lower incomes, a finding in the previous analysis [8]. Adults with active epilepsy are somewhat (but not statistically significantly) less likely to be uninsured than those without epilepsy. However, adults with active epilepsy (especially those of working age, 18–64 years old) are less likely to have private health insurance and more likely instead to be covered by Medicaid. While benefits such as medications and specialty care access vary among private health insurance policies, such benefits appear more restricted on the whole with public insurance such as Medicaid and may be unaffordable to many who lack health insurance [18–20]. These findings deserve further analysis in future studies, but taking them into account, it is perhaps not surprising that a substantial proportion of adults with active epilepsy report being unable to afford important elements of health care, including medication. This might explain previous findings related to medication nonadherence among adults with active epilepsy [7], problems that are amplified among those whose seizures are not in remission. Our findings also provide a more comprehensive assessment of gaps and barriers in other critical areas of health and daily functioning for adults with epilepsy — including challenges with dental and vision care. However, for some adults with epilepsy, addressing other basic needs in the context of social and economic disadvantages (e.g., employment, meeting other household caregivers' needs) may take precedence over health-care needs, underscoring the necessity for expanded supportive services, programs, and policies to reduce health inequities [21]. The 2010 Affordable Care Act created an optional Medicaid State Plan benefit for states to establish Health Homes to coordinate care for people with Medicaid who have chronic conditions such as epilepsy. Health homes include a broad range of mandated services, including comprehensive care management, care coordination, patient and family support, and referral to community and social support services. States can target health home enrollment by condition [7]. Future studies can examine whether health homes improve outcomes in Medicaid recipients with epilepsy.
Among the comorbidities of epilepsy, mental health disorders are important. Our analysis confirms earlier findings [22] that psychological distress is substantially more prevalent among adults with active epilepsy, making their greater need for mental health services evident. The findings from this survey indicate not only that adults with active epilepsy use more mental health services than adults without epilepsy but also that significant numbers are unable to afford or find access to sufficient services. Another study among community-dwelling adults with epilepsy in California found a large gap between perceived mental health need and provision of mental health care [23]. The degree to which mental health care needs exceed available services deserves further study.
Providing additional training about epilepsy, its comorbidities, psychosocial challenges, and appropriate care to primary care providers might assist them in providing more comprehensive care to their patients with epilepsy. Additional research is also needed to assess access to appropriate specialized care for other common comorbidities of epilepsy, the ways adults with epilepsy prioritize and manage their health problems, and the effects of distance from patients' residences to epilepsy and other specialized care centers. Other studies have identified disparities in access to health care and related problems among people with other chronic diseases [24–26]. Thus, such problems are not unique to people with epilepsy, although further research is needed to elucidate the extent to which people with epilepsy may be disproportionately disadvantaged, for example, with transportation barriers, functional neurological impairments, and stigma. Such research could lead to more specific policy recommendations. Noting, however, that people with chronic disorders spend most of their time in community settings (e.g., homes, worksites, schools, senior centers, faith-based organizations) versus physicians' offices, ensuring that these individuals have access to chronic disease self-management support is also critical in helping individuals, such as those with epilepsy, manage their condition [27,28].
4.2. Strengths and limitations
This study is more broadly representative of the U.S. population than studies limited to more specific localities [6,7,29]. Its findings serve to complement the findings of these more specific studies, although this study focuses only on adults. The NHIS is a large-scale survey, yielding relatively many sampled cases of active epilepsy (n = 644) on which to base estimates. Although this large sample provides good precision of estimates for the larger groups we analyzed, the precision of estimates for smaller groups is reduced, including the adjusted estimates of relative risk.
The sources of NHIS data are self-reports which, compared to clinical sources, are likely to yield an increased proportion of misclassified cases. Self-reports may yield inaccuracies in other relevant variables as well. An indication of the magnitude of case misclassification is provided by a validation study of the subset of questions used to identify active epilepsy in the NHIS, which found a sensitivity of 81%, a specificity of 99%, and a positive predictive value of 74% using abstracted information from medical records as a reference standard [30]. The inclusion of some persons who do not have true epilepsy among the reported cases of epilepsy in our study is likely to bias some of our results. This bias would serve to underestimate differences comparing cases with noncases. Accordingly, our comparative estimates are likely to be conservative.
4.3. Conclusion
Epilepsy is a chronic condition often requiring lifelong medical care. As it is frequently difficult to manage, specialty care is important. The consequences of poorly managed epilepsy are severe: Recurrent seizures may result in loss of gainful employment or inability to work, reduced income, reduced opportunities in education, decreased social participation, limitations in mobility with loss of driving privileges, other decrements of quality of life, and an increased risk of injury, disability, and death [1]. The extent to which recent health-care reforms in the United States, under the Affordable Care Act, may improve access to quality care for epilepsy and mitigate some of the findings remains to be seen. Continuing surveillance is warranted to assess these outcomes.
Footnotes
Disclaimer: The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest: The authors have no conflicts of interest to report.
References
- 1.Institute of Medicine (IOM) Epilepsy across the spectrum: promoting health and understanding. Washington DC: The National Academies Press; 2012. p. 537. [PubMed] [Google Scholar]
- 2.Department of Health and Human Services (DHHS) Healthy People 2020: disability and health. [Internet] Available at: http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=9.
- 3.Burneo JG, Jette N, Theodore W, Begley C, Parko K, Thurman DJ, et al. Disparities in epilepsy: report of a systematic review by the North American Commission of the International League Against Epilepsy. Epilepsia. 2009;50:2285–95. doi: 10.1111/j.1528-1167.2009.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher RS, Vickrey BG, Gibson P, Hermann B, Penovich P, Scherer A, et al. The impact of epilepsy from the patient's perspective. I: Descriptions and subjective perceptions. Epilepsy Res. 2000;41:39–51. doi: 10.1016/s0920-1211(00)00126-1. [DOI] [PubMed] [Google Scholar]
- 5.Fisher RS, Vickrey BG, Gibson P, Hermann B, Penovich P, Scherer A, et al. The impact of epilepsy from the patient's perspective. II: Views about therapy and health care. Epilepsy Res. 2000;41:53–61. doi: 10.1016/s0920-1211(00)00128-5. [DOI] [PubMed] [Google Scholar]
- 6.Begley CE, Basu R, Reynolds T, Lairson DR, Dubinsky S, Newmark M, et al. Sociodemographic disparities in epilepsy care: results from the Houston/New York City health care use and outcomes study. Epilepsia. 2009;50:1040–50. doi: 10.1111/j.1528-1167.2008.01898.x. [DOI] [PubMed] [Google Scholar]
- 7.Kobau R, Zahran H, Thurman DJ, Zack MM, Henry TR, Schachter SC, et al. Epilepsy surveillance among adults – 19 states, Behavioral Risk Factor Surveillance System, 2005. MMWR. 2008;57(No.SS-6):1–20. published erratum in: MMWR 2008; 57(32): 876. [PubMed] [Google Scholar]
- 8.Kobau R, Luo YH, PhD Zack MM, Helmers S, Thurman DJ. Epilepsy in adults and access to care – United States, 2010. MMWR. 2012;61(45):909–13. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) About the National Health Interview Survey. [Internet] Available at: http://www.cdc.gov/nchs/nhis/about_nhis.htm#sample_design.
- 10.Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), National Health Interview Survey. Questionnaires, datasets, and related documentation 1997 to the present. [Internet] Available at: http://wwwcdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- 11.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184–9. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- 13.Research Triangle Institute. SUDAAN example manual, release 10.0. 1st. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 14.U.S. Department of Health and Human Services (HHS) Health Care. [Internet] doi: 10.3109/15360288.2015.1037530. Available at: http://www.hhs.gov/healthcare/index.html. [DOI] [PubMed]
- 15.Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346:140–4. doi: 10.1016/s0140-6736(95)91208-8. [DOI] [PubMed] [Google Scholar]
- 17.Kobau R, Gilliam F, Thurman DJ. Prevalence of self-reported epilepsy or seizure disorder, and its associations with self-reported depression and anxiety: results from the 2004 HealthStyles Survey. Epilepsia. 2006;47(11):1915–21. doi: 10.1111/j.1528-1167.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 18.Burns ME. Medicaid managed care and health care access for adult beneficiaries with disabilities. Health Serv Res. 2009;44:1521–41. doi: 10.1111/j.1475-6773.2009.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carper K, Machlin S. Statistical Brief #274. Rockville, MD: Agency for Healthcare Research and Quality; 2009. Variations in perceived need and access to specialist care among adults in the U.S. civilian noninstitutionalized population, 2007. [Google Scholar]
- 20.Coughlin TA, Long SK, Shen YC. Assessing access to care under Medicaid: evidence for the nation and thirteen states. Health Aff (Millwood) 2005;24:1073–83. doi: 10.1377/hlthaff.24.4.1073. [DOI] [PubMed] [Google Scholar]
- 21.Pincus T, Esther R, DeWalt DA, Callahan LF. Social conditions and self-management are more powerful determinants of health than access to care. Ann Intern Med. 1998;129:406–11. doi: 10.7326/0003-4819-129-5-199809010-00011. http://dx.doi.org/10.7326/0003-4819-129-5-199809010-00011. [DOI] [PubMed] [Google Scholar]
- 22.Kobau R, Cui W, Kadima N, Zack MM, Sajatovic M, Kaiboriboon K, et al. Tracking psychosocial health in adults with epilepsy — estimates from the 2010 National Health Interview Survey. Epilepsy Behav. 2014;41:66–73. doi: 10.1016/j.yebeh.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson AW, Kobau R, Park R, Grant D. Epilepsy care and mental health care for people with epilepsy: California Health Interview Survey. Prev Chronic Dis. 2012;9:110–40. doi: 10.5888/pcd9.110140. published erratum in: Prev Chronic Dis 2012;9:110140e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strine TW, Chapman DP, Flowers N. Psychological distress and impaired quality of life common among community-dwelling adults with lower gastrointestinal disorders. Dig Dis Sci. 2007;52:70–7. doi: 10.1007/s10620-006-9466-9. [DOI] [PubMed] [Google Scholar]
- 25.Gulley SP, Rasch EK, Chan L. The complex web of health: relationships among chronic conditions, disability, and health services. Public Health Rep. 2011;126:495–507. doi: 10.1177/003335491112600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hossain WA, Ehtesham MW, Salzman GA, Jenson R, Calkins CF. Healthcare access and disparities in chronic medical conditions in urban populations. South Med J. 2013;106:246–54. doi: 10.1097/SMJ.0b013e31828aef37. [DOI] [PubMed] [Google Scholar]
- 27.IOM (Institute of Medicine) Living well with chronic illness: a call for public health action. Washington, DC: The National Academies Press; 2012. [Google Scholar]
- 28.Brady TJ, Anderson LA, Kobau R. Chronic disease self-management support: public health perspectives. Front Public Health. 2014;2:234 h. doi: 10.3389/fpubh.2014.00234. http://dx.doi.org/10.3389/fpubh.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson PL, Chiprich J, Smith G, Dong B, Wannamaker BB, Kobau R, et al. Prevalence of self-reported epilepsy, health care access, and health behaviors among adults in South Carolina. Epilepsy Behav. 2008;13:529–34. doi: 10.1016/j.yebeh.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Brooks DR, Avetisyan R, Jarrett KM, Hanchate A, Shapiro GD, Pugh MJ, et al. Validation of self-reported epilepsy for purposes of community surveillance. Epilepsy Behav. 2012;23:57–63. doi: 10.1016/j.yebeh.2011.11.002. [DOI] [PubMed] [Google Scholar]