Abstract
Nutritional status may be a modifiable factor in the progression of dementia. We examined the association of nutritional status and rate of cognitive and functional decline in a U.S. population-based sample. Study design was an observational longitudinal study with annual follow-ups up to 6 years of 292 persons with dementia (72% Alzheimer’s disease, 56% female) in Cache County, UT using the Mini-Mental State Exam (MMSE), Clinical Dementia Rating Sum of Boxes (CDR-sb), and modified Mini Nutritional Assessment (mMNA). mMNA scores declined by approximately 0.50 points/year, suggesting increasing risk for malnutrition. Lower mMNA score predicted faster rate of decline on the MMSE at earlier follow-up times, but slower decline at later follow-up times, whereas higher mMNA scores had the opposite pattern (mMNA by time β = 0.22, p = 0.017; mMNA by time2 β = −0.04, p = 0.04). Lower mMNA score was associated with greater impairment on the CDR-sb over the course of dementia (β = 0.35, p < 0.001). Assessment of malnutrition may be useful in predicting rates of progression in dementia and may provide a target for clinical intervention.
Keywords: Cognitive symptoms, dementia, disease progression, malnutrition, nutrition assessment
INTRODUCTION
Although the number of people affected by dementia is growing exponentially, viable treatment options are scarce; therefore, many researchers have investigated the role of sociodemographic and environmental factors in the risk of developing dementia and influencing its progression after onset. Diet and nutritional factors have been studied extensively as modifiable risk factors for dementia and cognitive decline in late life [1–4]. Malnutrition, characterized by insufficient caloric intake, weight loss, deterioration of muscle mass, and poor appetite, is a frequent problem among older adults [5] and has been associated with more severe symptoms of dementia [6, 7]. Previous research indicates that specific aspects of nutritional status such as diet, weight, and body mass index (BMI) across the lifespan are associated with risk for dementia and its severity after onset. In particular, higher midlife BMI is associated with a 75% increase in risk for the development of Alzheimer’s disease (AD) or dementia [8, 9]; in later years, weight loss has been associated with dementia onset [10] and the development of severe dementia [11, 12]. Diets high in fruits and vegetables, whole grains, nuts, fish, and legumes reportedly decrease risk for cognitive impairment and development of AD [2, 13], though null findings have also been reported [14, 15].
In older adults with dementia, nutritional status has been found to predict cognitive, functional, and neuropsychiatric symptoms [6, 7, 12, 16, 17]. Using the multi-dimensional measure of malnutrition, the Mini-Nutritional Assessment (MNA) [18], many investigators have reported significant associations between degree of malnutrition and dementia outcomes. A large multi-center French study reported that malnourished AD patients declined more rapidly on the Mini-Mental State Exam (MMSE) in a two-year follow-up [7]. Malara and colleagues [16], with the Italian National Association of Third Age Structures (Calabria) Study, found a significant correlation between the MNA and MMSE in patients with dementia at both baseline and 6-month follow-up. Several cross-sectional studies have found significant associations between poor nutritional status and behavioral disturbances [6, 12], worse cognitive status [12], and more impaired functioning in adult daily living activities [6, 12]. While these studies have demonstrated an association between nutritional status and dementia symptoms, very few have examined longitudinal associations beyond one or two years, and the majority of the studies have been conducted in clinical samples. The current study examines the association of nutritional status and the progression of dementia in cognitive and functional domains in a population-based sample of persons with incident dementia from Cache County (Utah).
MATERIALS AND METHODS
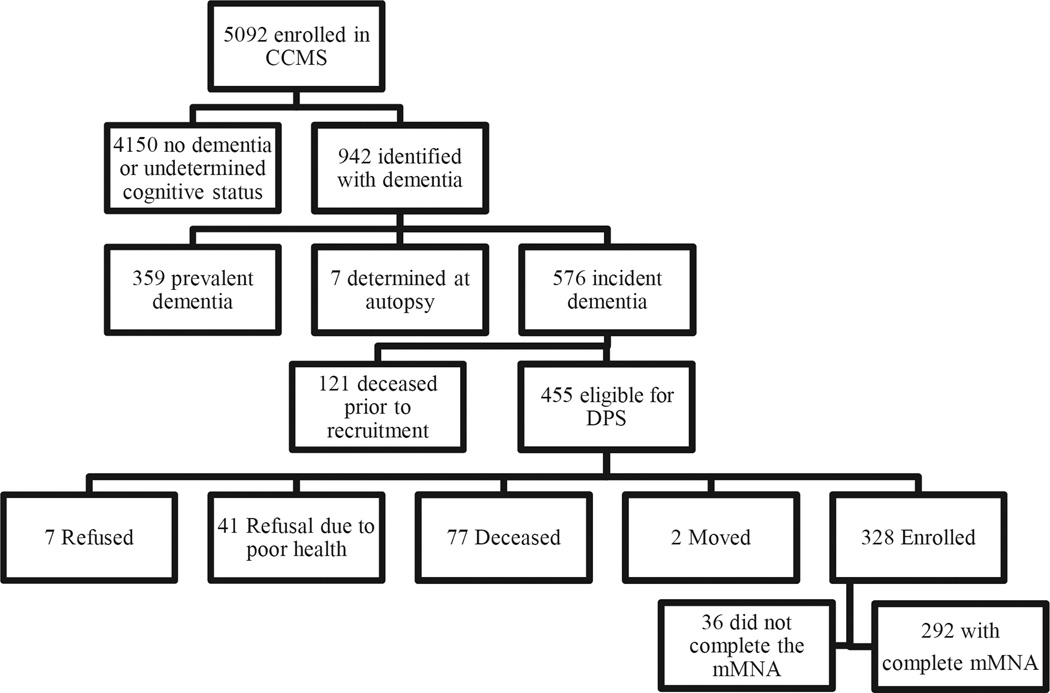
Participants were diagnosed with dementia via the Cache County Study on Memory in Aging (CCSMA) and, together with their caregivers, were followed in the Cache County Dementia Progression Study (DPS). The recruitment and dementia ascertainment procedures have been described elsewhere [19]. Briefly, the CCMSA is a population-based, longitudinal study located in Cache County, Utah. In 1995, the CCSMA enrolled 5,092 older residents of Cache County. Through an intensive screening and assessment process conducted over four triennial waves, the study identified 942 total participants with dementia (see Fig. 1). Of the 576 with incident dementia (identified within approximately 3 years of onset), 328 participants and their caregivers were enrolled in the DPS, a longitudinal, population-based study of the determinants of the rate of progression in dementia [20]. Thus, the DPS had an 87% enrollment rate when excluding those deceased at recruitment. Home visits with care-recipient and caregiver dyads occurred approximately every 6 months for up to 7.7 years. All methods complied with the ethical and legal standards of the Institutional Review Boards at Utah State University and the Johns Hopkins University.
Fig. 1.
Enrollment flow-chart. Identification of persons with dementia in the Cache County Study on Memory in Aging (CCSMA) and subsequent recruitment for the Dementia Progression Study (DPS). Only participants with incident (onset within approximately 3 years) dementia were recruited for DPS. Of those who enrolled in DPS, 292 completed the mMNA at least once and were included in the present analyses.
Each DPS visit with the subject (person with dementia) and his/her caregiver was conducted by a research nurse and trained neuropsychological technician. Visits consisted of neuropsychological testing, clinical interview in which measures of dementia severity were completed, and a nurse’s health interview and physical examination [20]. Among the measures, the MMSE and Clinical Dementia Rating Sum of Boxes (CDR-sb; see below) were used to assess cognitive status and severity of dementia, respectively. The nurse’s health interview and examination included a general health inventory, medication use, activities of daily living, neuropsychiatric symptoms, physical and cognitive activities, and nutritional intake (administered on alternating visits). Directly measured were blood pressure, height and weight to determine BMI, and neurological status. Based on the health inventory, medication usage and the medical and neurological exam, the nurse completed the General Medical Health Rating (GMHR), an indicator of overall health status [21].
Measures of cognition, function, and nutritional status
Mini-mental state exam
The MMSE [22] is an 11-item measure of global cognitive function with documented reliability and validity. The test assesses orientation to place and time, memory, attention, ability to follow verbal instructions and produce written language, and visuospatial skills with a maximum score of 30. As reported previously, an adjusted score was calculated for persons whose performance was adversely affected by sensory-motor impairments, up to a maximum of 10% of the total items affected [20].
Clinical dementia rating scale-sum of boxes
The CDR [23] is a numeric rating scale used to estimate the severity of cognitive and functional impairments of dementia across six domains: memory, orientation, judgment & problem solving, community affairs, home & hobbies, and personal care. Based on a semi-structured interview of clinical symptoms administered to the caregiver and participant, the nurse rated each domain on a scale ranging from 0 = no impairment, 0.5 = questionable impairment, 1 = mild impairment,2 = moderate impairment, 3 = severe impairment, 4 = profound impairment, and 5 = terminal status [23]. The CDR-Sum of Boxes (CDR-sb) was calculated by adding the domain scores together to create a total score ranging from 0 to 30. With approximately 94% accuracy in diagnosing dementia stages, the CDR-sb allows for greater precision in representing dementia severity than the Global Score method [24].
Modified mini-nutritional assessment score
Nutritional status was assessed using the MNA [18], adapted for the current study. The MNA is a well-established and widely used 18-item, multidimensional measure of nutritional status in older adults. These items are categorized into four domains to quantify global nutritional status: anthropometric assessment (four items capturing BMI, weight loss, and arm and calf circumferences), medical assessment (six items related to mobility, medication use, lifestyle, and psychiatric symptoms), short dietary assessment (eight items about type (e.g., protein, fruits, and vegetables), frequency, and mode of food and fluid intake), and subjective assessment (two items related to self-view of nutritional and health status). With a total of 30 points possible, the MNA uses the following validated cut-offs to indicate malnourishment, risk for malnutrition, and well-nourished: less than 17, 17–23.5, and 24–30, respectively. These cut-offs were created based on validation studies against clinical status as judged by physicians using a comprehensive evaluation of anthropomorphic, dietary, and biological markers of malnutrition [18]. For the current study, the MNA score was determined from data gathered from the health, nutrition, daily functional status and physical examinations in the DPS. The modified version of the MNA (mMNA) used here excludes the subjective view of nutritional and health status due to questionable reliability and validity of responses from participants with dementia. Psychiatric items were excluded in order to avoid redundancy with the cognitive and dementia outcomes. Furthermore, presence of skin ulcers (medical rubric) and calf-circumference (anthropomorphic rubric) were not available. New threshold values for the modified scale (now 22 pt maximum) can be calculated using percentage equivalent scores for the original cut-off scores as: ≤12.5=malnourished, 13–17.5 = at risk for malnutrition, >17.5 = well-nourished.
Data analysis
To examine change in nutritional status over the course of dementia, we ran a linear mixed effects model where mMNA score was the outcome and time (in years) and other risk factors for nutritional status were examined as predictors. To examine the association between mMNA score with cognitive and functional decline in dementia, we ran separate linear mixed effects models using the MMSE and CDR-sb as outcomes. In all models, mMNA was treated as a continuous variable due to scarcity of individuals that scored in the “malnourished” category at latter time points. Maximum Likelihood (ML) estimation was used in all linear mixed models to allow for missing data and maximum inclusion of participant data over time. However, because of the small number of participants who completed longer follow-ups (e.g., 16 participants had follow-ups of six or more years and only six participants had follow-ups of seven or more years), we restricted the current analyses to follow-ups of up to six years duration. In addition to mMNA score (time varying), we also tested the following covariates: gender, dementia type (AD, vascular dementia, other dementia), age of dementia onset, presence of the apolipoprotein (APOE) E4 allele, education, and dementia duration at baseline. Linear mixed models with random slopes and intercepts estimated the trajectories of best fit by adding covariates sequentially and comparing nested models using negative-2 log likelihood (-2LL) values for the chi-square test of independence. Linear and quadratic terms for time were also tested for significance in each model using this method. Data were analyzed using SPSS version 21.
RESULTS
Sample characteristics
Of the persons diagnosed with dementia during the CCSMA, 328 were enrolled in the DPS with 292 having completed the mMNA at least once over the course of the study. Those with follow-up mMNA scores (N= 189) had mean (SD) visit number and follow-up times of 3.22 (1.16) visits and 3.01 (1.45) years, respectively. The mean time interval between visits ranged from 1.09 to 1.40 years. Table 1 displays baseline characteristics of those included in the mMNA analyses while Table 2 indicates the number of participants at follow-up years from baseline. The most common reason for lack of follow-up was due to participant death across all visits (13–28%). Other reasons for drop-outs included refusals (1–2%), or break-off (1–3%).The majority of the sample was female (56%) and had AD (72%). Dementia severity at baseline was generally mild as indicated by the baseline CDR and MMSE. The majority was also residing at home and rated in ‘good health’ on their GMHR. Mean (SD) mMNA score at baseline was 16.54 (2.96), which is in range of “at risk for malnutrition.” Persons missing the mMNA and excluded from the present analyses were more likely to be female, live with professional assistance in either a residential care facility or nursing home, have longer dementia duration at baseline, and worse cognitive and functional ability. Table 1 shows the differences in baseline characteristics between persons with and without mMNA scores.
Table 1.
| mMNA (n = 292) | No mMNA (n = 36) | Chi2 or t value | |
|---|---|---|---|
| Female N (%) | 164 (56.2) | 26 (72.2) | 3.39 |
| Dementia Type N (%) | 0.70 | ||
| AD | 210 (71.9) | 26 (72.2) | |
| Vascular dementia | 36 (12.3) | 3 (8.3) | |
| Other dementia | 46 (15.8) | 7 (19.4) | |
| Baseline Place of residence N (%) | 15.09** | ||
| Home/Outpatient | 206 (70.5) | 14 (38.9) | |
| Assisted Living | 56 (19.2) | 10 (27.8) | |
| Nursing Home | 29 (9.9) | 10 (27.8) | |
| Missing | 1 (0.3) | 2 (5.6) | |
| Baseline GMHR a N (%) | 2.13 | ||
| Fair-Poor | 58 (19.9) | 6 (16.7) | |
| Good | 192 (65.8) | 26 (72.2) | |
| Excellent | 41 (14.0) | 2 (5.6) | |
| Missing | 1 (0.3) | 2 (5.6) | |
| Baseline mMNA M (SD) | 16.54 (2.96) | NA | NA |
| Education M (SD) | 13.3 (3.0) | 13.3 (2.6) | −0.03 |
| Onset Age M (SD) | 82.2 (6.0) | 84.2 (6.0) | −1.96* |
| Baseline Dementia duration M (SD) | 3.5 (1.9) | 4.4(1.8) | −2.7** |
| Baseline MMSEa M (SD) | 20.5 (6.8) | 17.2 (7.9) | 2.5* |
| Baseline CDR-sba M (SD) | 6.9 (4.8) | 9.3 (6.2) | −2.2a* |
| Baseline mMNA M (SD) | 16.54 (2.96) | NA | NA |
p ≤ 0.05;
p ≤ 0.01.
mMNA, modified Mini-Nutritional Assesment; GMHR, General Medical Health Rating; MMSE Mini-Mental State Exam; CDR-sb, Clinical Dementia Rating-Sum of Boxes.
corrected for Levene’s Test for Equality of Variances.
Table 2.
Follow-up Summary of Cache County Dementia Progression Participants with completed mMNAa scoresb by year
| Years | Participants |
|---|---|
| Baseline | 257 |
| 1–2 | 156 |
| 2–3 | 99 |
| 3–4 | 69 |
| 4–5 | 38 |
| 5–6 | 24 |
mMNA: modified Mini-Nutritional Status, 22-pt max where higher scores indicate better nutritional status.
35 participants were missing mMNA scores at baseline, but contributed to subsequent time-points and were included in the linear mixed models.
mMNA trajectory
The trajectory of mMNA scores declined significantly over time by approximately half a point for each year of observation. Those who were older at the time of dementia onset as well as those with longer disease duration at baseline had significantly worse nutritional status, while males scored approximately 1 point higher on the mMNA. Additionally, those in the “other” category of dementia (non-AD and nonvascular dementia) had a 2-pt lower mMNA score compared to an AD diagnosis. Linear mixed model results are shown in Table 3.
Table 3.
Linear Mixed Effects Model: mMNAa modeled as a function of demographic and clinical variables related to dementia in the Cache County Dementia Progression Study
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Estimate | St. Error | df | t | Sig. | Lower | Upper | |
| Intercept | 29.44 | 2.45 | 297.33 | 12.00 | <0.001 | 24.61 | 34.26 |
| Time | −0.58 | 0.08 | 105.41 | −7.26 | <0.001 | −0.73 | −0.42 |
| Dementia Typeb | |||||||
| Other | −2.02 | 0.43 | 294.08 | −4.69 | <0.001 | −2.87 | −1.17 |
| Vascular | −0.17 | 0.47 | 257.40 | −0.36 | 0.716 | −1.09 | 0.75 |
| Males | 1.14 | 0.32 | 278.50 | 3.56 | <0.001 | 0.51 | 1.77 |
| Onset Age | −0.15 | 0.03 | 298.33 | −5.23 | <0.001 | −0.20 | −0.09 |
| Baseline Dementia Duration | −0.32 | 0.09 | 281.48 | −3.55 | <0.001 | −0.49 | −0.14 |
Note that the presented analysis is the most parsimonious model. Findings remained robust in sensitivity analyses with the inclusion of non-significant variables: education (β = 0.06, p= 0.271) and absence of APOE (β = 0.04, p= 0.901).
mMNA, modified Mini-Nutritional Assessment, 22-pt max where higher scores indicate better nutritional status.
Reference category: AD.
MMSE trajectory
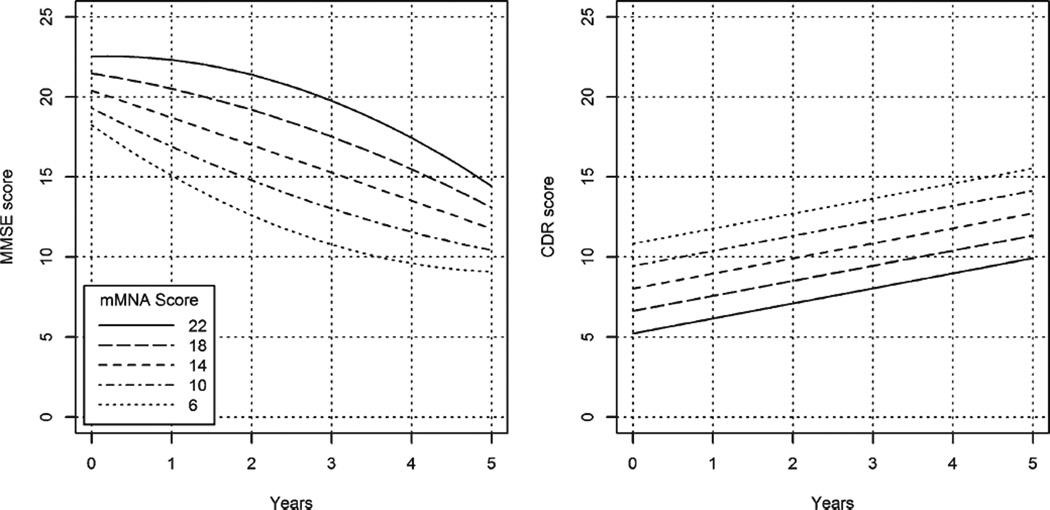
In modeling the MMSE trajectory, a quadratic time (time2) term was statistically significant (as previously demonstrated in this cohort [20, 25]), indicating a nonlinear rate of decline in MMSE. Additionally, there was a significant interaction between time and time2 with mMNA score. MMNA score was associated with the rate of MMSE decline over time such that lower mMNA scores predicted more rapid decline during the first several years of follow-up before slowing whereas higher mMNA scores were associated with slower decline during the first several years of follow-up before accelerating (see Fig. 2, which displays the association of mMNA score and rate of MMSE decline for selected values of the mMNA). Results remained significant after the inclusion of significant covariates (dementia duration at baseline and presence of the APOE E4). Table 4 displays the model results. As an illustration of effect sizes, Table 5 shows estimated values of the MMSE by selected mMNA score at years of follow-up. Both the figure and table illustrate the complex relationship between mMNA and MMSE scores over time. For example, in persons with an mMNA score of 22, the difference in estimated MMSE score differs by 0.23 between baseline and year 1 and 0.93 between years 1 and 2, but gradually increases to 3.02 between years 4 and 5, thus showing an acceleration in the rate of decline in MMSE over time. Contrast this pattern with those who score 6 on the mMNA where the difference in estimated MMSE score differs by 3.14 between baseline and year 1 and 2.48 between years 1 and 2, but gradually decreases to 0.52 between years 4 and 5, thus showing a deceleration in the rate of decline in MMSE over time.
Fig. 2.
Nutritional Status is associated with cognitive (MMSEa) decline and functional (CDR-sbb) status. The left-most figure shows the association between mMNA and rate of cognitive decline (MMSE) for selected values of the mMNA (range of 6 through 22). The figure on the right shows the association between mMNA values and dementia severity (CDR-sb). Note, there is no differential rate of change in dementia severity by mMNA scores. aMMSE, Mini-Mental State Exam, 30-pt max where higher scores indicate better cognitive status. bCDR-sb, Clinical Dementia Rating-Sum of Boxes, 30-pt max where higher scores indicate more severe dementia.
Table 4.
Linear Mixed Effects Models: MMSEa and CDR-sba trajectories modeled as a function of mMNAa and covariates
| MMSE | CDR-sb | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95 % CI | 95 % CI | |||||||||||||
| Estimate | St. Error | df | t | Sig. | Lower | Upper | Estimate | St. Error | df | t | Sig. | Lower | Upper | |
| Intercept | 20.61 | 2.01 | 471.19 | 10.26 | <0.001 | 16.66 | 24.56 | 9.36 | 1.04 | 618.94 | 9.02 | <0.001 | 7.32 | 11.40 |
| Time | −4.81 | 1.60 | 459.76 | −3.00 | 0.003 | −7.96 | −1.66 | 0.94 | 0.11 | 140.22 | 8.93 | <0.001 | 0.73 | 1.15 |
| Time2 | 0.58 | 0.35 | 382.91 | 1.66 | 0.098 | −0.11 | 1.27 | |||||||
| mMNA | 0.27 | 0.10 | 452.11 | 2.57 | 0.011 | 0.06 | 0.48 | −0.35 | 0.05 | 615.39 | −6.85 | <0.001 | −0.45 | −0.25 |
| Time*mMNA | 0.22 | 0.09 | 440.08 | 2.40 | 0.017 | 0.04 | 0.41 | |||||||
| Time2*mMNA | −0.04 | 0.02 | 371.35 | −2.06 | 0.040 | −0.08 | −0.002 | |||||||
| No APOE E4b | 1.36 | 0.71 | 277.07 | 1.93 | 0.054 | −0.03 | 2.75 | |||||||
| Baseline Dementia Duration |
−1.53 | 0.19 | 281.02 | −8.17 | <0.001 | −1.90 | −1.16 | 1.02 | 0.12 | 277.46 | 8.17 | <0.001 | 0.77 | 1.26 |
| Dementia Typec | ||||||||||||||
| Vascular | −2.13 | 0.71 | 259.87 | −3.01 | 0.003 | −3.52 | −0.74 | |||||||
| Other | −0.43 | 0.66 | 286.05 | −0.66 | 0.51 | −1.72 | 0.86 | |||||||
Note that the presented analyses are the most parsimonious models. The strength of significant variables remained in sensitivity analyses of the MMSE with the inclusion of non-significant variables: male gender (β = −0.10, p = 0.894), onset age (β = −0.06, p = 0.374), education( β = −0.18, p= 0.140) and dementia type (Compared to AD, other dementias : β = 1.62, p = 0.117; vascular dementia : β = 1.28, p = 0.231). Findings also remained robust in the sensitivity analyses of the CDR-sb when including non-significant covariates: male gender (β = −0.70, p= 0.162), onset age (β= −0.01, p= 0.768), education (β = 0.04, p = 0.582) and absence of APOE E4 (β= −0.32, p = 0.515).
mMNA, modified Mini-Nutritional Assessment, 22-pt max where higher scores indicate better nutritional status. MMSE, Mini-Mental State Exam, 30-pt max where higher scores indicate better cognitive status. CDR-sb, Clinical Dementia Rating-Sum of Boxes, 30-pt max where higher scores indicate worse functional status.
Reference category: Presence of APOE E4 allele.
Reference category: AD.
Table 5.
| mMNA | Baseline | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 6 | 18.40 (1.20) | 15.26 (0.93) | 12.78 (1.01) | 10.95 (1.08) | 9.78 (1.35) | 9.26 (2.18) |
| 10 | 19.48 (0.83) | 17.07 (0.68) | 14.97 (0.75) | 13.19 (0.80) | 11.73 (0.98) | 10.59 (1.51) |
| 14 | 20.56 (0.55) | 18.87 (0.52) | 17.17 (0.57) | 15.44 (0.63) | 13.69 (0.74) | 11.92 (1.00) |
| 18 | 21.64 (0.51) | 20.68 (0.52) | 19.36 (0.58) | 17.68 (0.65) | 15.65 (0.77) | 13.26 (0.99) |
| 22 | 22.71 (0.76) | 22.48 (0.70) | 21.55 (0.76) | 19.93 (0.85) | 17.61 (1.04) | 14.59 (1.48) |
MMSE, Mini-Mental State Exam, 30-pt max where higher scores indicate better cognitive status.
mMNA, modified Mini-Nutritional Assessment, 22-pt max where higher scores indicate better nutritional status.
Using estimated mean MMSE scores calculated at the mean of covariates and absence of APOE E4 allele.
CDR-sb trajectory
Unlike the MMSE trajectory, a non-linear (time2) term was not significant in modeling the CDR-sb. With the inclusion of covariates, mMNA was associated with overall CDR-sb trajectory, such that each additional point on the mMNA was associated with a 0.35 lower score on the CDR-sb, indicating that better nutritional status is associated with less severe dementia across time. Significant covariates included dementia duration at baseline (p< 0.001) and type of dementia (p < 0.05). Table 4 displays model results and Fig. 2 illustrates mean differences in CDR-sb for selected mMNA values.
DISCUSSION
As the prevalence of dementia increases world-wide and in the absence of a cure, research on factors associated with the rate of progression of dementia is critically important. Previous research in clinic-based dementia samples had demonstrated that those who are malnourished or at risk for malnutrition display worse cognitive and functional outcomes compared to those who are well-nourished [12, 17]. The current study confirms and extends these findings in a population-based sample with longitudinal follow-up for up to 6 years. Nutritional status was associated with dementia severity as measured by the CDR-sb and the rate of cognitive decline as assessed by the MMSE in persons with dementia. Although at longer follow-up times, the rate of decline slowed in those with low mMNA scores and accelerated in those with high mMNA scores, over the short term, there was greater maintenance of MMSE scores for those with higher mMNA. Added to the differences in baseline MMSE scores, this finding suggests those with higher mMNA scores would likely experience higher overall cognitive abilities over the course of dementia than those with lower mMNA scores. Nonetheless, with the rate of MMSE change over time, the mean difference in MMSE was less at baseline and at later years of follow-up; the latter may reflect the possible differential drop out (mortality) of those with the poorest nutritional status or floor effects of measuring cognitive impairment in persons with severe dementia. In overall dementia severity, each point higher on the mMNA predicted an approximately 1/3 point better score on the CDR-sb. This translates to an approximate 6-point difference when comparing a person with a score of 6 to an individual with a score of 22. The current results imply that monitoring nutritional status and possibly intervening in those with poor nutritional status may improve both cognitive and overall dementia severity in persons with dementia.
While older adults in general are at greater risk for malnutrition and its consequences, individuals with dementia are especially vulnerable. Significant weight loss, an indicator of malnutrition, has been associated with greater dementia severity and mortality in persons with AD [26]. In fact, the risk for mortality increases exponentially as 8% or more body weight is lost [26]. Oral nutritional supplements have been shown to significantly increase body weight and BMI in nursing home residents with and without dementia [27]. Alternatively, personalized nutritional counseling without supplements was found to be beneficial for community-dwelling, cognitive-healthy older adults. This intervention led to maintenance and improvements in nutritional status (weight, BMI, MNA, and albumin levels) over two years in the group that received counseling compared to those in the control group [28]. The Health and Nutrition Promotion Program for Patients with Dementia (NutriAlz) researchers pioneered a similar dietary educational approach with community dwelling persons with mild-moderate AD and their caregivers [29]. While they found an improvement in nutritional status over 1 year compared to controls, they did not see associated group differences in independence (ADLs and IADLs), dementia severity (CDR), or cognitive function (MMSE); however, there were trends for the intervention group to have less change in measures of behavioral disturbances (NPI) and caregiver burden. It is possible that with longer follow-up, greater effects of the nutritional intervention may emerge. Our data suggest that the greatest effects of nutritional status may be early in the course of dementia, arguing for early monitoring and intervention.
We identified several factors associated with nutritional status in dementia. Persons with non-AD and non-vascular forms of dementia, females, and those with older onset ages and longer dementia duration may be especially at risk for declining nutritional status. For each additional year of dementia duration, there was an associated loss of 1/3-pt on the mMNA, and those with a non-AD and non-vascular dementia scored 2 points lower on the mMNA than those with AD. Previous research in older adults with and without dementia indicates that nutritional status declines with age and that women are more likely to be malnourished [12, 17, 30, 31]. While some have speculated that women may have worse nutritional status because they were older [12], others have suggested that this may reflect gender role differences with women having higher financial dependency and in an Indian sample, less purchasing power over food decisions [30]. Though differences between male and female caregiver coping have been documented [32], it is unclear how the adequacy of caregiving tasks (such as nutrition) compares between genders. Male caregivers often take on an unfamiliar role (with respect to traditional gender roles) when completing domestic tasks, such as cooking, for their female spouses with dementia [33], and male caregivers are less likely to utilize community or government support for caregiving activities [32], thus nutritional quality of meals may differ depending on the care-giver gender. Furthermore, Rullier et al. reported that the nutritional status of the person with dementia is positively associated with the nutritional status of the caregiver [34]. Nutritional monitoring and/or interventions should take place early at the identification of dementia with the hope of affecting dementia outcomes. In as much as the mMNA assesses nutritional status across a number of domains, identifying specific areas (e.g., nutrient intake, mobility, etc.) related to dementia outcomes is an important next step as is the development of and test of nutritional interventions.
The current sample was comprised of a relatively homogenous sample of Caucasian older Americans with low prevalence of health-risk behaviors such as drinking and smoking. While this may reduce the generalizability to more diverse populations, past research on factors influencing dementia outcomes with this cohort have been broadly consistent with the literature. A strength of the present study is the population-based sample that was followed for up to 6 years, with high participation rates at follow-up. This longitudinal design allowed us to conduct time-varying analyses to determine the specific roles of factors over time. Including all-cause dementia cases increased the relevance of this research, while including dementia type as a variable in statistical models enabled us to control for and examine differences between forms of dementia.
In summary, the present study offers evidence that nutritional status is an important factor associated with cognitive health and functional abilities over the course of dementia. The findings discussed here are based on significant associations and cannot confirm a cause and effect relationship between nutritional status and dementia progression, as it is also possible that declining nutritional status represents a characteristic feature of the advancing dementia syndrome. Further investigation is warranted, particularly with research designs that involve random assignment to nutrition intervention versus no-intervention control to examine subsequent effects on dementia progression. Understanding the specific aspects of nutritional status that potentially affect dementia progression and the efficacy of interventions targeting malnutrition may hold promise in reducing the degree of disability, costs and caregiver burden associated with dementia.
Acknowledgments
This study is supported by NIH grants: R01A G21136, R01AG11380, and the Johns Hopkins Alzheimer’s Disease Research Center P50AG005 146.
Footnotes
Presented in preliminary form at the 2013 Alzheimer’s Association International Conference, Boston, MA, USA.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0528r1).
REFERENCES
- 1.Alles B, Samieri C, Feart C, Jutand MA, Laurin D, Barberger-Gateau P. Dietary patterns: A novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev. 2012;25:207–222. doi: 10.1017/S0954422412000133. [DOI] [PubMed] [Google Scholar]
- 2.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wengreen HJ, Neilson C, Munger R, Corcoran C. Diet quality is associated with better cognitive test performance among aging men and women. J Nutr. 2009;139:1944–1949. doi: 10.3945/jn.109.106427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris MC. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71:1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- 5.Dorner B. Position of the American Dietetic Association: Individualized nutrition approaches for older adults in health care communities. J Am Diet Assoc. 2010;110:1549–1553. doi: 10.1016/j.jada.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Spaccavento S, Del Prete M, Craca A, Fiore P. Influence of nutritional status on cognitive, functional and neuropsychiatric deficits in Alzheimer’s disease. Arch Gerontol Geriatr. 2009;48:356–360. doi: 10.1016/j.archger.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Cortes F, Nourhashémi F, Guérin O, Cantet C, Gillette-Guyonnet S, Andrieu S, Ousset P, Vellas B REAL, Group FR. Prognosis of Alzheimer’s disease today: A two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008;4:22–29. doi: 10.1016/j.jalz.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 9.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesen-berry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling adults. J Am Geriatr Soc. 1996;44:1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 11.Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Dementia severity and weight loss: A comparison across eight cohorts. The 10/66 study. Alzheimers Dement. 2013;9:649–656. doi: 10.1016/j.jalz.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerin O, Soto ME, Brocker P, Robert PH, Benoit M, Vellas B REAL, FR, GROUP. Nutritional status assessment during Alzheimer’s disease: Results after one year (the REAL French Study Group) J Nutr Health Aging. 2005;9:81–84. [PubMed] [Google Scholar]
- 13.Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, O’Connor HM, Petersen RC. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29:413–423. doi: 10.1159/000305099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: The PATH Through Life study. Am J Geriatr Psychiatry. 2012;20:635–639. doi: 10.1097/JGP.0b013e31823032a9. [DOI] [PubMed] [Google Scholar]
- 15.Fe´art C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malara A, Sgro G, Caruso C, Ceravolo F, Curinga G, Renda GF, Spadea F, Garo M, Rispoli V. Relationship between cognitive impairment and nutritional assessment on functional status in Calabrian long-term-care. Clin Interv Aging. 2014;9:105–110. doi: 10.2147/CIA.S54611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vellas B, Lauque S, Gillette-Guyonnet S, Andrieu S, Cortes F, Nourhashemi F, Cantet C, Ousset PJ, Grand-jean H GROUPRF. Impact of nutritional status on the evolution of Alzheimer’s disease and on response to acetylcholinesterase inhibitor treatment. J Nutr Health Aging. 2005;9:75–80. [PubMed] [Google Scholar]
- 18.Guigoz Y, Vellas B, Garry PJ. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. In: Vellas BJ, Guigoz Y, Garry PJ, Albarede JL, editors. Facts and Research in Gerontology. New York: Springer Publishing Company; 1994. pp. 15–59. [Google Scholar]
- 19.Breitner JS, Wyse BW, Anthony JC, Welsh-Bohmer KA, Stefens DC, Norton MC, Khachaturian AA. APOE-ε4 count predicts age when prevalence of AD increases, then declines. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 20.Tschanz JT, Corcoran C, Schwartz S, Treiber KA, Green R, Norton MC, Mielke MM, Piercy K, Steinberg M, Rabins PV, Leoutsakos J, Welsh-Bohmer K, Breitner JC, Lyketsos CG. Progression of cogntive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: The Cache County Dementia Progression Study. Am J Geriatr Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyketsos CG, Galik E, Steele C, Steinberg M, Rosenblatt A, Warren A, Sheppard JM, Baker A, Brandt J. The General Medical Health Rating: A bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State” A practical method for grading the cogntive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 24.O’Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, Massman PJ, Hobson V, Cullum CM. Validation of the new interpretive guidelines for the clinical dementia rating scale sum of boxes score in the national Alzheimer’s coordinating center database. Arch Neurol. 2010;67:746–749. doi: 10.1001/archneurol.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tschanz JT, Piercy K, Corcoran CD, Fauth E, Norton MC, Rabins PV, Tschanz BT, Deberard MS, Snyder C, Smith C, Lee L, Lyketsos CG. Caregiver coping strategies predict cognitive and functional decline in dementia: The Cache County Dementia Progression Study. Am J Geriatr Psychiatry. 2013;21:57–66. doi: 10.1016/j.jagp.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: A longitudinal analysis. J Am Geriatr Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 27.Stange I, Bartram M, Liao Y, Poeschl K, Kolpatzik S, Uter W, Sieber CC, Stehle P, Volkert D. Effects of a low-volume, nutrient- and energy-dense oral nutritional suppliment on nutritional and functional status: A randomized, controlled trial in nursing home residents. J Am Med Dir Assoc. 2013;14:628.e621–628.e628. doi: 10.1016/j.jamda.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Nykanen I, Rissanen TH, Sulkava R, Hartikainen S. Effects of individual dietary counseling as part of a comprehensive geriatric assessment (CGA) on nutritional status: A population-based intervention study. J Nutr Health Aging. 2014;18:54–58. doi: 10.1007/s12603-013-0342-y. [DOI] [PubMed] [Google Scholar]
- 29.Salva A, Andrieu S, Fernandez E, Schiffrin EJ, Moulin J, Decarli B, Rojano-I-Luque X, Vellas B, Group N. Health and nutrition promotion program for patients with dementia (NutriAlz): Cluster randomized trial. J Nutr Health Aging. 2011;15:822–830. doi: 10.1007/s12603-011-0363-3. [DOI] [PubMed] [Google Scholar]
- 30.Agarwalla R, Saikia AM, Baruah R. Assessment of the nutritional status of the elderly and its correlates. J Family Community Med. 2015;22:39–43. doi: 10.4103/2230-8229.149588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roque M, Salva A, Vellas B. Malnutrition in community-dwelling adults with dementia (NutriAlz Trial) J Nutr Health Aging. 2013;17:295–299. doi: 10.1007/s12603-012-0401-9. [DOI] [PubMed] [Google Scholar]
- 32.Baker KL, Robertson N. Coping with caring for someone with dementia: Reviewing the literature about men. Aging Ment Health. 2008;12:413–422. doi: 10.1080/13607860802224250. [DOI] [PubMed] [Google Scholar]
- 33.Calasanti T, Bowen ME. Spousal caregiving and crossing gender boundaries: Maintaining gendered identities. J Aging Stud. 2006;20:253–263. [Google Scholar]
- 34.Rullier L, Lagarde A, Bouisson J, Bergua V, Barberger-Gateau P. Nutritional status of community-dwelling older people with dementia: Associations with individual and family caregivers’ characteristics. Int J Geriatr Psychiatry. 2013;28:580–588. doi: 10.1002/gps.3862. [DOI] [PubMed] [Google Scholar]