Supplemental Digital Content is available in the text.
Abstract
Problem
Engaging basic scientists in community-based translational research is challenging but has great potential for improving health.
Approach
In 2009, The Rockefeller University Center for Clinical and Translational Science partnered with Clinical Directors Network, a practice-based research network (PBRN), to create a community-engaged research navigation (CEnR-Nav) program to foster research pairing basic science and community-driven scientific aims. The program is led by an academic navigator and a PBRN navigator. Through meetings and joint activities, the program facilitates basic science–community partnerships and the development and conduct of joint research protocols.
Outcomes
From 2009–2014, 39 investigators pursued 44 preliminary projects through the CEnR-Nav program; 25 of those became 23 approved protocols and 2 substudies. They involved clinical scholar trainees, early-career physician–scientists, faculty, students, postdoctoral fellows, and others. Nineteen (of 25; 76%) identified community partners, of which 9 (47%) named them as coinvestigators. Nine (of 25; 36%) included T3–T4 translational aims. Seven (of 25; 28%) secured external funding, 11 (of 25; 44%) disseminated results through presentations or publications, and 5 (71%) of 7 projects publishing results included a community partner as a coauthor. Of projects with long-term navigator participation, 9 (of 19; 47%) incorporated T3–T4 aims and 7 (of 19; 37%) secured external funding.
Next Steps
The CEnR-Nav program provides a model for successfully engaging basic scientists with communities to advance and accelerate translational science. This model's durability and generalizability have not been determined, but it achieves valuable short-term goals and facilitates scientifically meaningful community–academic partnerships.
Problem
Basic science research and community-engaged translational research are commonly viewed as polar opposites on the translational spectrum and are rarely integrated in a single project. As leaders of The Rockefeller University Center for Clinical and Translational Science (RU-CCTS), which is supported by a Clinical and Translational Science Award (CTSA) from the National Institutes of Health, we sought to identify opportunities to align rigorous basic science investigation with research that broadly engages communities, community clinicians, patients, and other stakeholders. We hypothesized that such integration would be synergistic, resulting in projects with broader goals and participation, whose results could have a greater impact and likelihood of dissemination and implementation. We therefore sought to develop a process to facilitate the collaboration of researchers with expertise in basic mechanistic science with individuals representing the aims and health priorities of communities to develop joint projects that integrate basic science (T0) aims with early translational (T1–T2) aims and community, clinical, or public health (T3–T4) aims.1 To this end, the Community Engagement Core of the RU-CCTS partnered with Clinical Directors Network (a primary care practice-based research network [PBRN] designated by the Agency for Healthcare Research and Quality as a Center of Excellence [P30] in Primary Care Practice-Based Research and Learning) to develop a multidisciplinary supportive framework and process we have termed community-engaged research navigation (CEnR-Nav). CEnR-Nav uses expert intermediary CEnR navigators (navigators) to explicitly “reach in” to basic scientists and “reach out” to community clinicians, patients, and other collaborators, to foster the development of interdisciplinary research teams and to facilitate the conduct of research projects that address both scientific and community health aims. In this article, we describe our approach, the projects, and interim outcomes from this initiative and present recommendations for broader application of our approach.
Approach
This work was reviewed by the Institutional Review Board of The Rockefeller University and deemed exempt from board review.
Overview of the CEnR-Nav process
CEnR-Nav is an interdisciplinary framework consisting of a series of collaborative participatory meetings or consultations facilitated by expert navigators that progress through a set of conceptual and operational stages of project development (see Figure 1).
Figure 1.
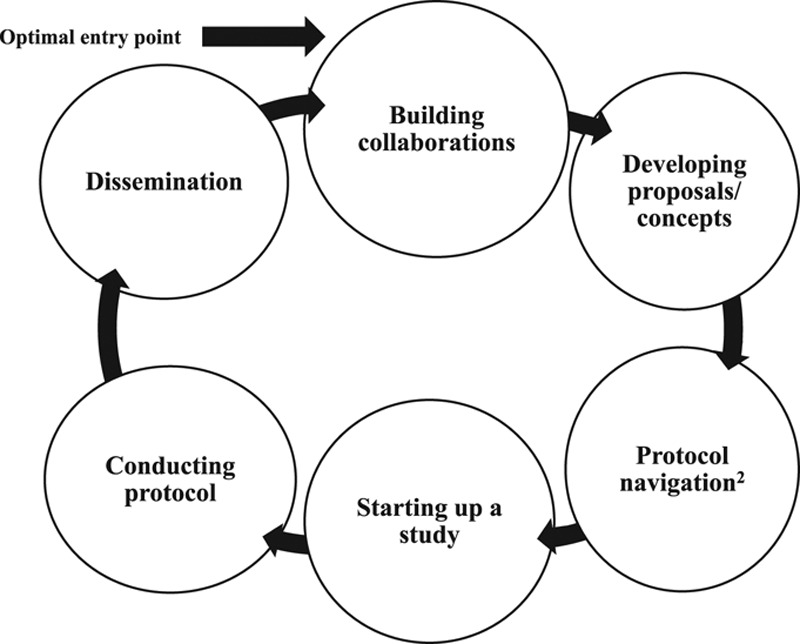
The stages and associated activities of the community-engaged research navigation (CEnR-Nav) process. (1) Building collaborations: meeting to identify the right community research partners; engaging with stakeholders to understand research priorities, concerns, and training needs; and developing research agreements or memoranda of understanding. (2) Developing proposals/concepts: articulating scientific and community aim(s); incorporating community-oriented research design; and reviewing ethical considerations of agency and human protections. (3) Protocol navigation2: refining protocol design for institutional review board and scientific review. (4) Starting up a study: developing data use agreements and data sharing tools; coordinating multisite approvals; and training study staff. (5) Conducting protocol: monitoring operations and informed consent; holding team meetings; engaging in scientific and operational problem solving; and facilitating communication. (6) Dissemination: developing a dissemination plan; and reaching community and academic stakeholders.
Optimally, an investigator or other stakeholder enters the CEnR-Nav process while the research concept is still being formulated. Under the guidance of the navigators, the basic science investigator and other stakeholders then move sequentially through the stages of building a partnership, aligning aims, jointly developing protocols and funding applications, conducting the study, analyzing and disseminating the results, and preparing applications for additional funding to sustain the partnership into subsequent projects.
In practice, requests for CEnR-Nav originate from several mechanisms. In the case of “bottom-up” requests, academic investigators or other stakeholders at any project stage seek to foster a new partnership, develop a new concept, enhance a project already under way, or engage stakeholders from a target population to enhance the design or conduct of their study. In the case of “top-down” requests, the RU-CCTS Action Committee for Community-Engaged Research (ACCER), which consists of RU-CCTS leadership, navigators, Community Engagement Core staff, faculty, scientific liaisons, and the director of the partnering PBRN, proactively reviews the research programs of investigators on the Rockefeller campus along with the interests of patients and clinicians at Community Health Centers or advocacy groups that are potential partners to identify research projects for which the goals of all stakeholders might be aligned. In the case of “middle-out” requests, the navigator, participant recruitment staff, institutional review board, and/or the research protocol navigation2 staff monitor other projects to identify those that might be enhanced by community engagement and recommend to the investigator that she or he enter the CEnR-Nav process.
The number and duration of the CEnR-Nav meetings for each project depend on the complexity of the project; projects are categorized as brief (1–3 meetings), moderate (4–10 meetings), or extended (> 10 meetings). For extended projects, the navigator often becomes a collaborator on the project to assist the partners in developing, practicing, and refining the skills needed for successful team science and participatory community-engaged research.
CEnR-Nav expands the multidisciplinary model of mentored research protocol navigation, which we have previously reported on,2 and incorporates the principles of community engagement, team science, and community-engaged participatory research.3 Often, CEnR-Nav participants have not previously engaged in transdisciplinary collaborations, and so a series of CEnR-Nav meetings may form the first introduction to the principles of community engagement for a basic science investigator and the first introduction to scientific project development (including hypothesis-generating clinical research, involving the design of a clinical protocol and human subject protections and regulations) for the community partners. Thus, CEnR-Nav functions as a critical bridge to facilitate communication and explicitly translate principles between the clinical, scientific, public health, and lay community cultures to foster the development of sustainable partnerships.
Leadership, personnel, and support in the CEnR-Nav process
The CEnR-Nav program is led by two navigators (0.20 full-time equivalents each) who work closely with the CTSA principal investigator (B.S.C.). The academic navigator (R.G.K.), who serves as the codirector of the Community Engagement Core, is a translational research-trained physician with expertise in human subject protections, participant advocacy, patient engagement, and scientific and ethical review of research projects; she has eight years of experience fostering community-engaged research among basic scientists at the RU-CCTS. The PBRN navigator (J.N.T.) serves both as codirector of the Community Engagement Core and as president/CEO of Clinical Directors Network. He is a PhD-trained epidemiologist with extensive experience partnering with Community Health Centers and academic health centers to conduct community-engaged, comparative effectiveness, and health disparities research. The RU-CCTS and Clinical Directors Network entered into a memorandum of agreement for this codirector to (1) provide representation on RU-CCTS and CTSA committees; (2) mentor and teach epidemiology, research design, and community-based comparative effectiveness research to clinical scholars master’s degree program students and postdoctoral fellows; and (3) provide CEnR-Nav services to faculty and trainees. The third Community Engagement Core member is the community engagement specialist (1.0 full-time equivalent) (A.L.-J.) who has an MPH and is trained in health disparities research, public-health-based research, and evaluation; she has 10 years of experience building collaborations among diverse stakeholder groups.
Oversight of CEnR-Nav is provided by ACCER, which is a subcommittee of and reports to the RU-CCTS governance committee. ACCER provides guidance on community engagement programming, the identification of scientific faculty for the evaluation of partnership and funding opportunities, and targeted assistance in developing and facilitating individual research collaborations. For the complete CEnR-Nav organizational chart, see Supplemental Digital Appendix 1 at http://links.lww.com/ACADMED/A349.
Funding, services, pilot grant opportunities, and scientific and institutional review board review for CEnR-Nav programs are supported by the CTSA grant, The Rockefeller University, and targeted philanthropic gifts. In addition to CEnR-Nav consultations, investigators and community partners receive assistance in protocol submission,2 biostatistics, and medical informatics from the RU-CCTS. Investigators are also eligible to compete annually for RU-CCTS pilot award funding, some of which is specifically designated for community-engaged research. The PBRN staff, who are supported by funding from the National Institutes of Health, the Centers for Disease Control and Prevention, the Patient-Centered Outcomes Research Institute, and the Agency for Healthcare Research and Quality P30 program, provide research assistance and data management support during the partnership devel opment phase. The Rockefeller University Institutional Review Board reviews protocols involving Rockefeller investigators, and Clinical Directors Network has agreements in place to act as the institutional review board of record for the Community Health Centers in the PBRN. Both institutions have agreements in place to use single-institutional-review-board review platforms.
Evaluation of the CEnR-Nav process
We reviewed meeting notes, minutes, navigators’ notes, and project protocols to track the process and progress of CEnR-Nav teams and projects. We analyzed for (1) the scientific and community engagement content, (2) stakeholder engagement, (3) the community engagement content of public health impact statements, (4) the research hypothesis and objectives, (5) the target populations, and (6) the protocol-specific aims. Each protocol aim was assigned a location along the translational continuum using the definitions proposed in the Institute of Medicine report on the CTSA program.1 Finally, we collected presentations, publications, and internal and external funding award data from RU-CCTS metric-tracking sources and public records. We provide descriptive data for those projects begun from 2009 to 2014.
Outcomes
Descriptive data
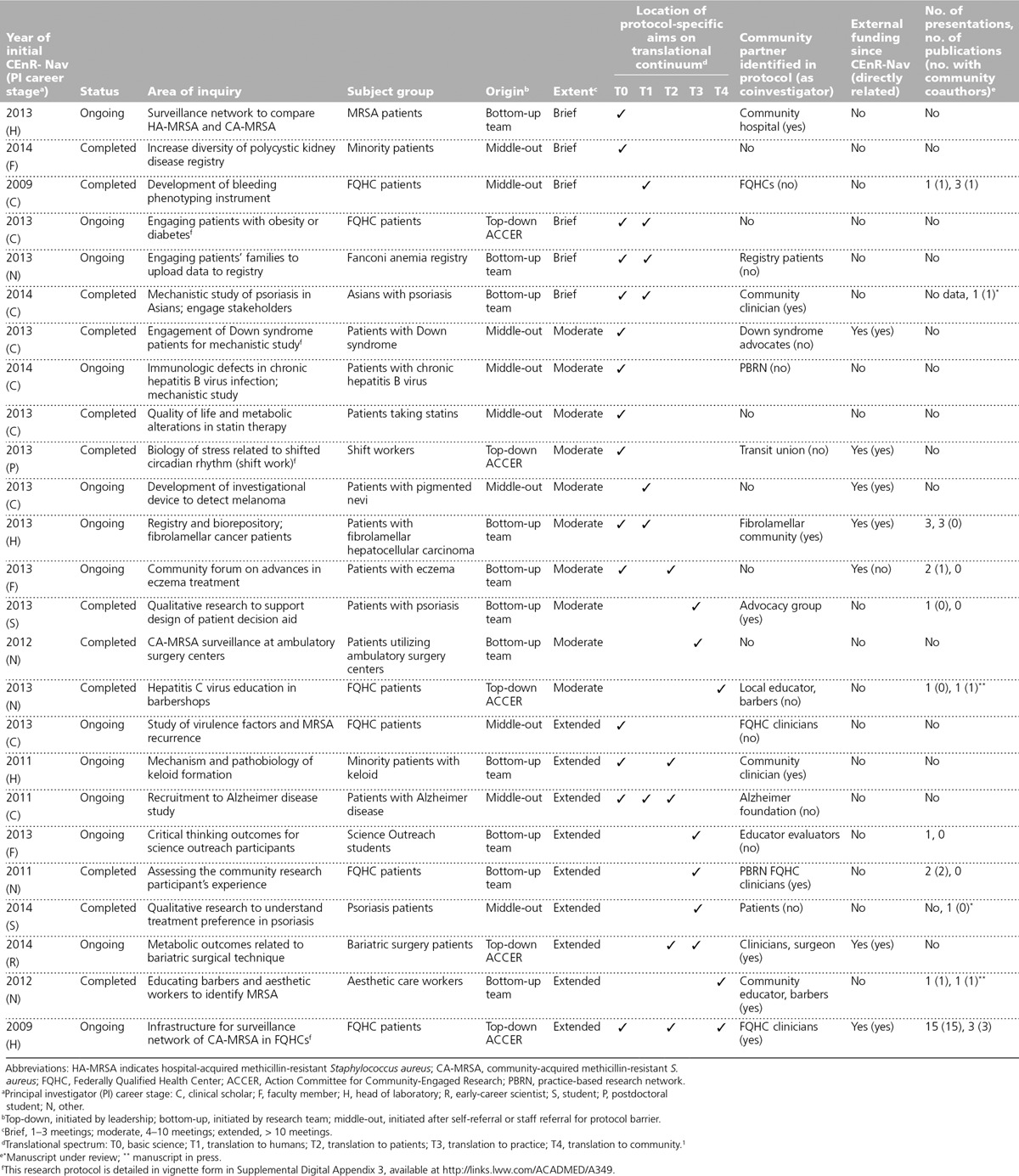
From 2009 to 2014, we provided CEnR-Nav services to 44 unique collaborative projects involving 39 individual principal investigators (15 PhDs, 10 MDs, 11 MD/PhDs, 2 students, and 1 MS/genetic counselor). These principal investigators were clinical scholar trainees and early-career physician–scientists (15), faculty (12), students or postdoctoral fellows (6), and other (6). Twenty-five projects involving 23 investigators developed into 23 institutional-review-board-approved clinical and translational protocols and 2 substudies. Characteristics and outcomes of these 25 projects are detailed in Appendix 1. (The 19 projects that did not lead to approved protocols are detailed in Supplemental Digital Appendix 2 available at http://links.lww.com/ACADMED/A349.)
Nineteen of these 25 protocols (76%) identified community partners, of which 9 (47%) named them as coinvestigators; 9 protocols (36%) included a T3 or T4 translational aim. Clinical scholars were less likely than investigators at other career stages to incorporate T3 or T4 aims. All protocols secured at least one round of internal institutional pilot award funding. External funding was secured for 5 (26%) of the 19 projects that identified a community partner in the protocol and for 2 (33%) of the 6 projects that did not name a community partner. Of projects with long-term navigator participation, 9 (of 19; 47%) incorporated T3 or T4 aims and 7 (of 19; 37%) secured external funding. As of November 2015, 12 (48%) of the 25 projects have been completed, and 11 (44%) have disseminated their results through presentations or publications. Five (71%) of 7 projects with published or submitted manuscripts included at least one community coauthor (see Appendix 1).
Case studies
In Supplemental Digital Appendix 3, we describe in detail four projects with T0 or T1 aims, investigators at different career stages, and different initial goals (available at http://links.lww.com/ACADMED/A349). Two of the projects resulted in comparative effectiveness research trials incorporating mechanistic aims and earning support from the Patient-Centered Outcomes Research Institute and other external funding sources.
Next Steps
The 2013 Institute of Medicine report on the CTSA program identified five phases of translational science, depicted as a spectrum from T0 or basic/mechanistic science research to T4 or community/population health research.1 Community-engaged research offers a cross-cutting strategy to promote and accelerate the effective translation of research from discovery to practice. Because it has the potential to span the translational spectrum, it avoids both the delays in translation that are associated with research that is positioned narrowly on the spectrum4 and the tendency to focus community engagement research only on T3 or T4 aims.5
The Federation of American Societies for Experimental Biology produced a report in 2012 that offered recommendations to increase the engagement of basic scientists in translational research. These recommendations included the following: (1) Learn to define a health need with the same precision as a basic science hypothesis; (2) seek mentors and collaborators from different disciplines; and (3) seek funding to work in the translational space.6 The CEnR-Nav program addresses all three of these goals by catalyzing relationships between basic scientists and community clinicians at crucial points in protocol development, with the potential for research, clinical, and public health synergy. Further, the CEnR-Nav infrastructure and navigators nurture relationships with community partners as collaborators and coauthors and have demonstrated success at securing external funding.
Although The Rockefeller University is structured as a research institute, we believe that larger academic health centers can develop CEnR-Nav programs similar to ours. On the basis of our experience, we identified five factors that are important for the success of a CEnR-Nav program in facilitating engagement between basic scientists, community members, clinicians, and patient advocates. First, senior leadership must support and actively encourage collaborations with basic scientists. Second, the CEnR-Nav process itself, as a multistep iterative program that focuses on mentored partnership skills, tangible benefits for all partners, aligned aims, and aggressive identification of funding opportunities, is key to the program’s success. Third, the collective expertise of the navigators must span the full range of translational science from T0 to T4 so that they are able to reach in to basic scientists and reach out to clinicians and communities to connect cultures and foster partnerships. Fourth, funding from the institution (e.g., from the CTSA or university) is needed to support the navigators, the protocol development infrastructure, and pilot project funding and can act as a stepping stone to external funding.
The fifth factor that can contribute to the success of a CEnR-Nav program is an established community-based partner with academic–community research experience and expertise. PBRNs in particular are well suited for this role, as are networks of PBRNs. Other entities, such as clinical research networks supported by the National Institutes of Health, Clinical Data Research Networks and Patient-Powered Research Networks supported by the Patient-Centered Outcomes Research Institute, Health Center-Controlled Networks supported by the Health Resources and Services Administration, and Prevention Research Centers supported by the Centers for Disease Control and Prevention, contain similar elements and goals. These entities can also serve as strong partner organizations with CTSAs given their shared commitment to research and experience in competing for National Institutes of Health funding. Senior leaders at these organizations are likely to have the requisite expertise and experience to serve as excellent navigators, providing them with the opportunity to participate in high-quality community-engaged research and a meaningful academic career that bridges the spectrum of translational science.
In conclusion, we anticipate that the rigorous, ongoing assessment of CEnR-Nav projects as they mature will provide insight into additional predictors of success, durability, and generalizability of partnerships, as well as new models for integrated full-spectrum translational research.
Acknowledgments: The authors would like to acknowledge the participation of the many scientists, community clinicians, community partners, patients, support staff, and other colleagues who by their participation in the community-engaged research navigation (CEnR-Nav) process enabled the development and refinement of this model. The authors also would like to thank Dr. J. Lloyd Michener for his thoughtful advice and encouragement.
Supplementary Material
Appendix 1.
Characteristics of Investigators and Research Protocols in the Community-Engaged Research Navigation (CEnR-Nav) Program at The Rockefeller University Center for Clinical and Translational Science and Clinical Directors Network, 2009 to 2014, With Outcomes Through 2015
Footnotes
Supplemental digital content for this article is available at http://links.lww.com/ACADMED/A349.
An AM Rounds blog post on this article is available at academicmedicineblog.org.
Funding/Support: This work was supported in part by grant #UL1 TR000043 (Barry S. Coller) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, and by a 2011 CTSA Community Engagement Administrative Supplement Award NIH-NCATS grant #UL1 TR000043-07S1 (Barry S. Coller/Jonathan N. Tobin), as well as by funding from the Agency for Healthcare Research and Quality grant #1 P30-HS-021667 (Jonathan N. Tobin) and from the Patient-Centered Outcomes Research Institute grant #CER-1402-10800 (Jonathan N. Tobin).
Other disclosures: None reported.
Ethical approval: The program evaluation work reported here was reviewed by The Rockefeller University Institutional Review Board and was found to not constitute human subjects research and, therefore, to be exempt from further review.
Previous presentations: Some aspects of the community-engaged research navigation model were presented at the 2015 Annual Conference of the Association for Clinical and Translational Research, April 16–18, Washington, DC, by Andrea Leinberger-Jabari, Rhonda G. Kost, Joel Correa da Rosa, Teresa H. Evering, Maija Neville-Williams, Peter R. Holt, Jonathan N. Tobin, and Barry S. Coller, as a poster entitled “Fostering collaborations among basic scientists and community-engaged researchers across the translational spectrum.”
References
- 1.The CTSA Program at NIH: Opportunities for Advancing Clinical and Translational Research. 2013Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 2.The Rockefeller University Navigation Program: A structured multidisciplinary protocol development and educational program to advance translational research. Clin Transl Sci. 2014;7:12–19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A logic model for community engagement within the Clinical and Translational Science Awards consortium: Can we measure what we model? Acad Med. 2013;88:1430–1436.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Practice-based research—“blue highways” on the NIH roadmap. JAMA. 2007;297:403–406.. [DOI] [PubMed] [Google Scholar]
- 5.Clinical and translational science awards and community engagement: Now is the time to mainstream prevention into the nation’s health research agenda. Am J Prev Med. 2009;37:464–467.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Federation of American Societies for Experimental Biology. Engaging Basic Scientists in Translational Research: Identifying Opportunities, Overcoming Obstacles. 2012. Bethesda, Md: Federation of American Societies for Experimental Biology; http://www.faseb.org/portals/2/PDFs/opa/TranslationalReportFINAL.pdf. Accessed March 3, 2016. [Google Scholar]


