Abstract
Background and Objectives
Ultrasound-guided regional anesthesia facilitates an approach to sensitive targets such as nerve clusters without contact or inadvertent puncture. We compared accuracy of needle placement with a novel passive magnetic ultrasound needle guidance technology (NGT) versus conventional ultrasound (CU) with echogenic needles.
Methods
Sixteen anesthesiologists and 19 residents performed a series of 16 needle insertion tasks each, 8 using NGT (n = 280) and 8 using CU (n = 280), in high-fidelity porcine phantoms. Tasks were stratified based on aiming to contact (target-contact) or place in close proximity with (target-proximity) targets, needle gauge (no. 18/no. 22), and in-plane (IP) or out-of-plane (OOP) approach. Distance to the target, task completion by aim, number of passes, and number of tasks completed on the first pass were reported.
Results
Needle guidance technology significantly improved distance, task completion, number of passes, and completion on the first pass compared with CU for both IP and OOP approaches (P ≤ 0.001). Average NGT distance to target was lower by 57.1% overall (n = 560, 1.5 ± 2.4 vs 3.5 ± 3.7 mm), 38.5% IP (n = 140, 1.6 ± 2.6 vs 2.6 ± 2.8 mm), and 68.2% OOP (n = 140, 1.4 ± 2.2 vs 4.4 ± 4.3 mm) (all P ≤ 0.01). Subgroup analyses revealed accuracy gains were largest among target-proximity tasks performed by residents and for OOP approaches. Needle guidance technology improved first-pass completion from 214 (76.4%) per 280 to 249 (88.9%) per 280, a significant improvement of 16.4% (P = 0.001).
Conclusions
Passive magnetic NGT can improve accuracy of needle procedures, particularly among OOP procedures requiring close approach to sensitive targets, such as nerve blocks in anesthesiology practice.
Ultrasound-guided needle placement has become an increasingly necessary skill in anesthesiology practice since it was first reported in 1989.1 Ultrasound provides a real-time visual aid that can decrease reliance on tactile sensations and improve accurate placement of the needle tip relative to adjacent nerves and adjacent anatomy.2–4 Because of the foreshortened perspective of the needle in out-of-plane (OOP) approaches, often complicated by acoustic artifacts from overlapping sonoanatomy in neuraxial approaches, achieving optimal needle insertion in ultrasound-guided regional anesthesia (UGRA) remains challenging.5–8 Moreover, placing the needle near complex anatomical targets has a high “learning curve,” potentially fostering reliance on suboptimal in-plane (IP) approaches.7,8 This study investigated a novel passive magnetic ultrasound needle guidance technology (NGT) designed to enhance needle visualization in both IP and OOP approaches.
Technological advances such as echogenic needles have improved UGRA outcomes, including accuracy, needling time, block onset, local anesthetic volumes, block duration, and efficacy (defined as reduced supplemental analgesia and rescue block).2–4 However, enhanced visibility with echogenic needles is of limited use in OOP needle procedures where needle visibility is limited. Needle guidance technologies have been developed that can further improve outcomes, both IP and OOP, through enhanced needle visualization and improved techniques, for example, reduced needle misalignment relative to the beam (beam misalignment), unintentional probe movement, and poor ergonomics of the hand, neck, and trunk.9 Robot-assisted guidance with reconstruction software10 and instrument-tracking solutions (such as needle-mounted optical stereoimaging) have been proposed as possible aids in UGRA.11 While both of these technologies can improve needle visualization, robotic and needle-mounted devices suffer from low portability and greater bulk, potentially altering needle technique, in particular adversely contributing to operator fatigue, gaze shifting, awkward hand positioning, and nonergonomic trunk and neck angle.7,10,12–14 Thus, new needle visualization solutions are needed to further improve needle procedure accuracy with minimal disruption in technique.
Passive magnetic NGT provides real-time beam-independent visualization of the needle on the ultrasound interface during UGRA. It has minimal bulk and a nonintrusive ergonomic profile due to its use of small magnetic components already integrated into the base of some commercial needles.7,12 Therefore, we hypothesized that passive magnetic NGT would help experienced anesthesiologists and residents achieve superior accuracy versus conventional ultrasound (CU) guidance with echogenic needles.
METHODS
The Western Institutional Review Board reviewed this research and determined that testing on ex vivo porcine tissue phantoms did not constitute human subjects research. Accuracy of tasks performed with a novel passive magnetic tracking NGT was compared with those performed with CU guidance with echogenic needles. Sixteen anesthesiologists with at least 2 years of UGRA experience and 19 anesthesiology residents were recruited from hospitals in the United States to perform needle insertion tasks in high-fidelity porcine phantoms. The number of tasks performed was based on a sample size calculation requiring at least 496 tasks based on the primary outcome measure of distance of the needle tip relative to the target (in millimeters) for NGT versus CU at a significance level of 0.025 at 90%. Sample size calculations were performed using PASS version 11 (NCSS Statistical Software, LLC, Kaysville, Utah) based on assumptions from initial feasibility tests performed by the authors and existing literature on passive magnetic tracking prototypes.7 The sample size was approximated upward to 560.
Needle tasks were performed using a Venue 50 Ultrasound prototype with L12n-SC transducer (GE Healthcare, Wauwatosa, Wisconsin). The prototype transducer and software were equipped with sensor and reconstruction software components capable of detecting passive magnetic signals from the needle-mounted magnet. The same system and transducer were used for all tasks, with and without the NGT functionality enabled.
Each participant performed 8 paired NGT and CU tasks (a series of 16 total needle tasks per participant). Each set of paired tasks was performed under fixed conditions of needle size, plane of approach, and target type, as follows: no. 18 or no. 22 Birmingham Wire Gauge (BWG) needle, IP or OOP approach, and target-proximity task (aiming to place the needle tip into close proximity with the target without contacting it, as in clinical nerve block) or target-contact task (aiming to place the needle tip into contact with the central target, as in clinical vascular puncture). Target-contact tasks were completed with either no. 18 or no. 22 BWG echogenic Pinpoint GT Needle Guidance Technology With Safety Introducer Needles (C. R. Bard, Inc, Salt Lake City, Utah). Target-proximity tasks were completed with etched echogenic Vasculae Sono Cannula Needles with injection lines and guidewires in no. 18 and no. 22 BWG (Pajunk, Geisingen, Germany). Tasks were performed in a prospective fixed order alternating between CU and NGT for each paired set of conditions until all 16 tasks were completed.
Participants were provided with 5 to 10 minutes of instructional video and an introduction to the NGT system before starting tasks. The device was precalibrated by the researchers for basic parameters (focus, depth, and gain), and participants performed tasks in their preferred ergonomic approach without intervention from the researchers.
Ultrasound-Guided Regional Anesthesia Simulation Phantom and Targets
Fresh porcine shoulder tissues were used to construct high-fidelity ex vivo UGRA simulation phantoms with approximate dimensions of 15 cm (width) × 20 cm (height) × 10 cm (depth). Two target types were constructed based on the type of task (target-proximity or target-contact tasks). Targets were embedded at a depth of 2.5 cm using a minimally disruptive incision on the dependent surface of the model. Prepared models were stored under refrigerated conditions for up to 2 days, warmed to ambient temperature, and stabilized using acrylic panels on a work surface to ensure normal tissue elasticity before use. Target depth was confirmed prior to each task to limit possible bias due to tissue movement.
Two types of targets were constructed for target-proximity and target-contact tasks. Targets were constructed to approximate in vivo sonoanatomic characteristics of neurovascular anatomy, characterized by heterogeneous diffuse hypoechoic and hyperechoic regions. The model was adapted from high-fidelity phantoms reported in anesthesiology training.15 The fidelity of the phantom models is demonstrated against representative clinical images acquired from the ultrasound system outside the present study (Fig. 1). The targets used in target-proximity tasks were constructed of noncollapsible cylindrical oil-filled capsules approximately 1.5-cm length × 0.5-cm diameter (Fig. 1C). Capsules were selected for their sensitivity to needle puncture (indicating a failed task due to unintentional contact) and similar appearance to hypoechoic arterial structures.15,16 Targets used in target-contact tasks were constructed of a flat metallic “nail head” anchor with 2.0-mm-diameter circular surface (appearing as a short, flat hyperechoic region in profile view on ultrasound). Target-contact targets were embedded in the center of a semiabsorbent, moderately echoic membrane with concentric markings at 1-mm intervals. The metallic anchor was selected based on its sonographic resemblance to the heterogeneous, hyperechoic appearance of nerve sonoanatomy (particularly relatively small distal nerves; Fig. 1E, top left).16
FIGURE 1.
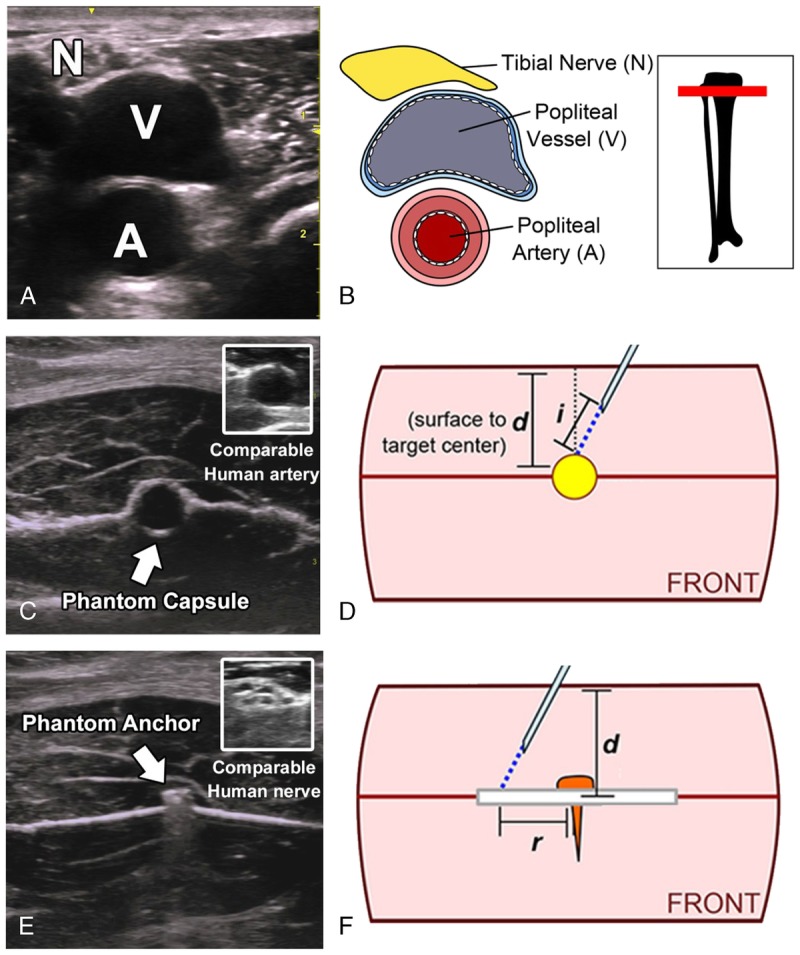
Fidelity of phantom targets is demonstrated by side-by-side comparison to human neurovascular sonoanatomy. A and B, Image and diagram of typical human neurovascular ultrasound showing characteristic diffuse hyperechoic neuroanatomy and hypoechoic vasculature with hyperechoic regions in the margins and deep acoustic shadow. C, Targets for target-proximity tasks were constructed of oil-filled capsules resembling human hypoechogenic arterial sonoanatomy (C, top right: similar sonoanatomic characteristics of defined circular boundaries and inner hypoechoic regions in representative human median nerve). D, Accuracy for target-proximity tasks was assessed by distance in millimeters (displacement distance, i) of a guidewire extended from the needle tip into contact with the target. E, Targets for target-contact tasks were constructed of metallic “nail head” anchors resembling small regions of diffuse hyperechoic nervous sonoanatomy (E, top right: similar sonoanatomic characteristics of diffuse hyperechoic regions in representative human median nerve). F, Accuracy for target-contact tasks was assessed by distance in millimeters (r) from target center to the needle puncture in the adjacent membrane.
Interface Characteristics
The NGT prototype on the portable Venue 50 system has several interface features, most notably a real-time graphical overlay on the ultrasound interface capable of indicating needle trajectory and tip location relative to the target. Needle guidance technology calculates the needle position by modeling the passive magnetic signals from the small needle-mounted magnetic elements as the needle travels across the beam. The NGT interface shows the needle segment in the beam as a solid green line and calculates the position of segments posterior or anterior to the beam, which are shown as dotted green lines. The calculated trajectory appears as a single line extending from the needle tip on the interface, as shown in Figure 2. The yellow target identifies the crossover point between the needle and beam, assisting in OOP needle advancement (Fig. 3). The study used the base configuration, with additional features (such as needle-bending detection) enabled.
FIGURE 2.
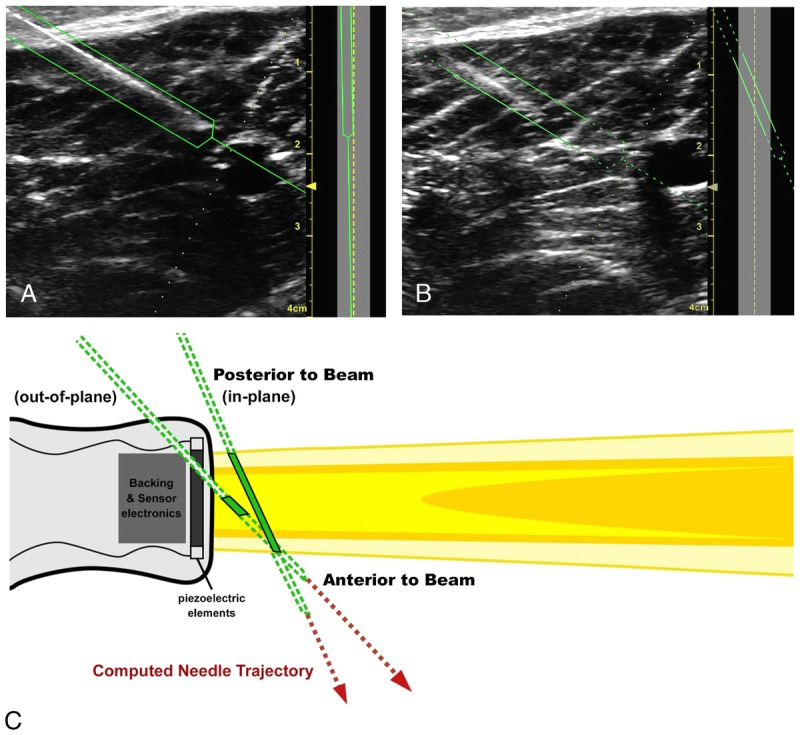
Photographs of the of the NGT interface showing correct needle angle relative to the beam for IP approach (A) and correction based on the NGT solid-to-dotted green lines, indicating that the operator should alter needle position relative to the beam. Needle guidance technology can help anesthesiologists recognize common technique issues that occur when the angle is inadvertently altered by ergonomic issues, operator fatigue, or inadvertent movement of the transducer (B). The needle segment in the beam is represented by a solid green line, and calculated positions of anterior and posterior segments are represented by dotted green lines. The NGT interface aids in visualizing needle position relative to the beam cross section (A, B: left). The corresponding appearance of the needle IP and OOP relative to the linear array probe is demonstrated in the diagram (C).
FIGURE 3.
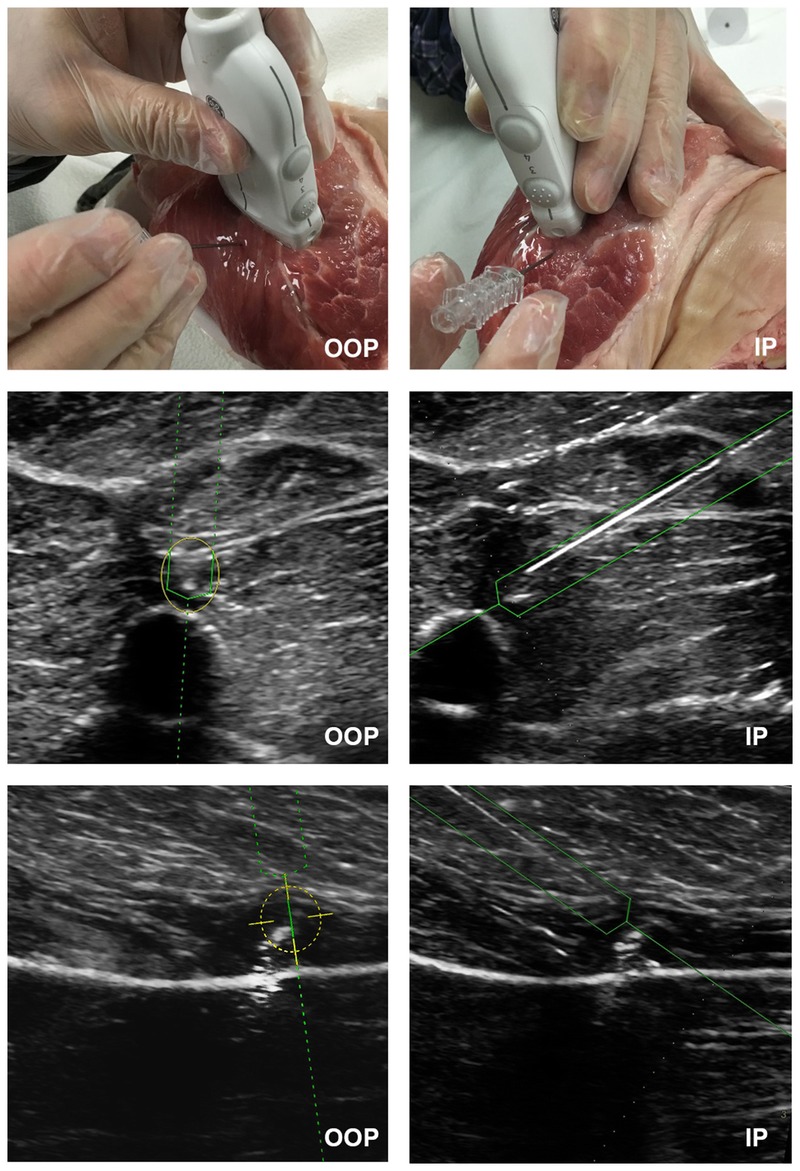
The passive magnetic NGT interface display is shown for IP and OOP target-proximity tasks (middle) and target-contact tasks (bottom). This image shows the ability of NGT to assist in real-time localization of the needle tip as the needle passes through the beam (solid green lines). Needle guidance technology is also able to calculate the position of needle segments anterior and posterior to the beam (dotted green lines). For OOP approaches, the yellow target appears at the projected intersection with the target.
Assessments
For target-proximity tasks, participants were asked to advance the needle tip into close proximity (as close as possible) without contacting the target, and accuracy was assessed as the displacement distance (in millimeters) from the needle tip to the target. This distance was measured by advancing a guidewire into contact with the target after final needle placement. The needle and guidewire were then removed, and the length of guidewire extension beyond the needle tip was measured and recorded as accuracy (displacement distance, in millimeters). For complete lateral misses with no discrete value for displacement distance (guidewire advanced to maximum without target contact) and those making unintentional contact with the target, distance was imputed based on twice the average SD of existent displacement distances for the analysis. In addition to distance from the target, we also recorded whether the target was contacted or punctured during the task (termed unintentional contact). Target-proximity tasks were considered successfully completed when it did not result in lateral miss or unintentional contact.
For target-contact tasks, participants were asked to advance the needle into direct contact with the central metallic target or until palpable puncture of the adjacent membrane. Accuracy (distance to target, in millimeters) was assessed based on the lateral distance from the target center to the puncture in the adjacent membrane. If the needle contacted the target center, it was assigned a distance value of 0 mm. Target-contact tasks that contacted the central target were considered successfully completed.
Each time a participant inserted and advanced the needle was considered 1 pass. Participants were allowed to remove and reposition the needle as many times as necessary until satisfied with final placement, without intervention from the researchers. The total number of passes and the number of tasks completed in only 1 pass (completion on the first pass) were recorded.
All tasks were performed by residents and anesthesiologists not affiliated with the device manufacturer, GE Healthcare. Tasks were performed according to a prospective schedule known to participants and researchers, and accuracy measurements were recorded immediately following final needle placement for each task.
Statistical Analysis
Data analyses were conducted in Microsoft R Open version 3.2.3 and the dplyr package (Microsoft Corporation, Redmond, Washington). The primary effects of interest (distance from needle tip to the target, successful completion of needle tasks by type [target-proximity or target-contact], number of passes, and completion on the first pass) were tested using a nested split-plot (mixed-design) analysis of variance (ANOVA) test with parameters estimated through restricted maximum likelihood (REML) in the nlme package for R. If NGT versus CU comparisons were found significant by ANOVA testing, a posteriori testing was performed for NGT versus CU subgroups via paired t tests and adjusted to control family-wise error rate using Holm's method. Results are presented descriptively alongside percentage differences calculated as |(NGT − CU) / CU)| × 100. P values were considered statistically significant at P < 0.05.
RESULTS
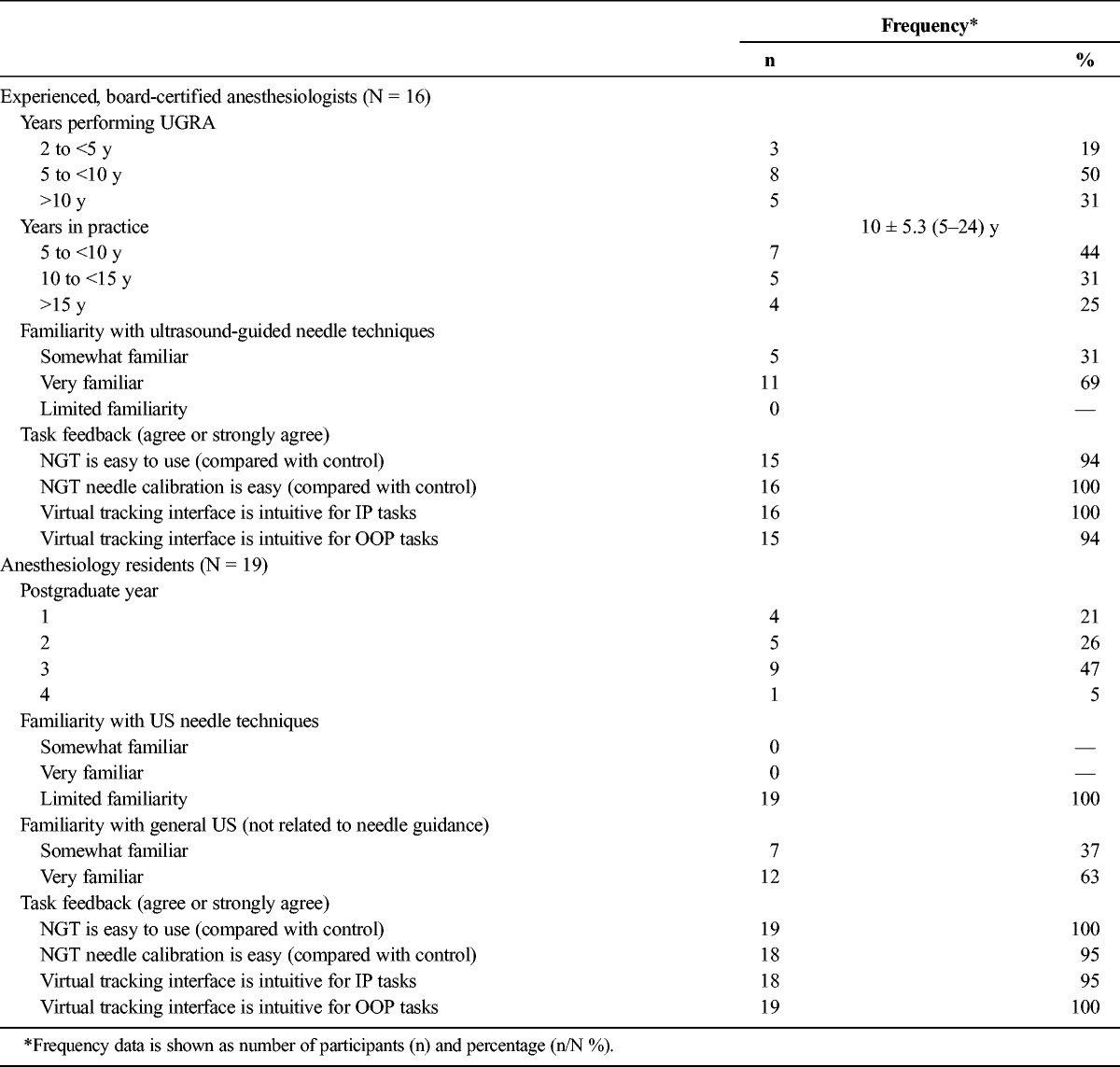
Sixteen anesthesiologists each with more than 2 years' UGRA experience and 19 residents with limited familiarity with needle-guided ultrasound performed a series of 16 paired needle insertion tasks each, 8 tasks using NGT (total n = 280) and 8 tasks using CU (total n = 280). Each participant completed all allocated needle tasks and provided information about their experience (including years in practice for anesthesiologists and postgraduate year for residents) and how easy they felt the NGT device was to use (Table 1). Notably, no significant differences were observed based on needle size (no. 18 or no. 22 BWG); thus, needle size subgroups are not shown.
TABLE 1.
Participating User Characteristics and Task Feedback Survey Results by Experience Level (Anesthesiologist or Resident)
Needle Tip to Target Distance
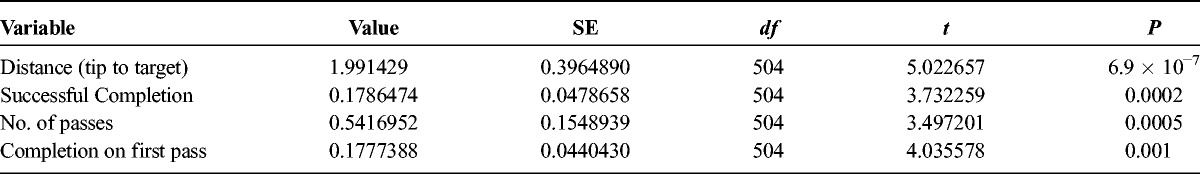
Needle tip to target distance was reported overall for NGT (total n = 280) and CU (total n = 280) tasks. Overall, NGT tasks came closer to targets by 2.0 mm on average (57.1% improvement) compared with CU (1.5 ± 2.4 mm vs 3.5 ± 3.7 mm, respectively; Table 2). Furthermore, this effect was statistically significant in a full-effects ANOVA model accounting for multiple variables tested (t = 5.02, P < 6.9 × 10−7; complete ANOVA results are detailed in Table 3).
TABLE 2.
Accuracy Based on Distance From the Needle Tip to the Target
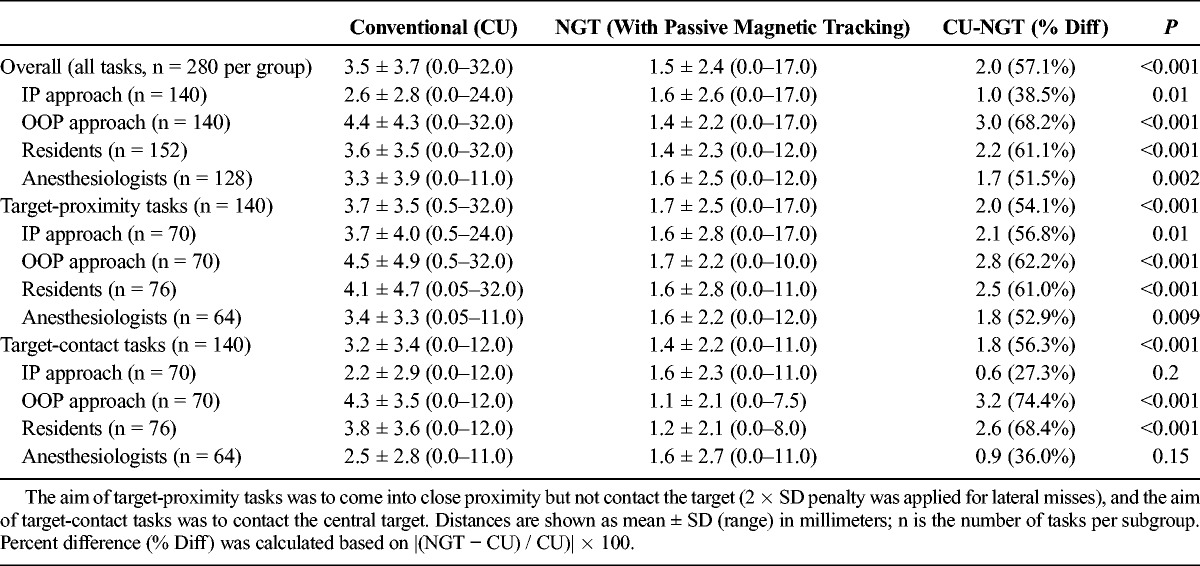
TABLE 3.
Statistical Results of Nested Split-Plot (Mixed-Design) ANOVA for NGT Versus CU Overall (Considering All 560 Tasks) for Each End Point
Needle Tip to Target Distance by Experience Subgroups
Based on the significance of overall findings, distance results were further stratified by whether the participant was a resident (n = 152 NGT tasks, n = 152 CU tasks) or an anesthesiologist (n = 128 NGT tasks, n = 128 CU tasks). Among residents, NGT tasks came closer to targets by 2.2 mm on average (61.1% improvement) compared with CU (1.4 ± 2.3 mm vs 3.6 ± 3.5 mm, respectively; P < 0.001; Table 2). Comparatively, NGT tasks performed by anesthesiologists came closer to targets by 1.7 mm on average (51.5% improvement) compared with CU (1.6 ± 2.5 mm vs 3.3 ± 3.9 mm; P = 0.002; Table 2).
Needle Tip to Target Distance by Plane-of-Approach Subgroups
Distance results were also stratified by approaches performed IP (n = 140 NGT tasks, n = 140 CU tasks) or OOP (n = 140 NGT tasks, n = 140 CU tasks). For tasks performed IP, NGT tasks came closer to targets by 1.0 mm on average (38.5% improvement) compared with CU (1.6 ± 2.6 vs 2.6 ± 2.8, P = 0.01; Table 2). Comparatively, for tasks performed OOP, NGT tasks came closer to targets by 3.0 mm on average (68.2% improvement) compared with CU (1.4 ± 2.2 vs 4.4 ± 4.3; P < 0.001; Table 2).
Needle Tip to Target Distance by Task Type
Among target-contact tasks designed to model vascular puncture procedures (n = 140 NGT, n = 140 CU tasks), NGT tasks came closer to the target by 1.8 mm on average (56.3% improvement) compared with CU (1.4 ± 2.2 mm vs 3.2 ± 3.4 mm, P = 0.001; Table 2). Furthermore, NGT significantly reduced distance among target-contact tasks performed OOP by 3.2 mm (74.4% improvement) and for target-contact tasks among residents by 2.6 mm (68.4% improvement) compared with CU (both P < 0.001; Table 2).
Among target-proximity tasks designed to model nerve block procedures (n = 140 NGT, n = 140 CU tasks), NGT tasks came closer to the target by 2.0 mm on average (74.1% improvement) compared with CU (3.7 ± 3.5 mm vs 1.7 ± 2.5 mm, P < 0.001; Table 2). Furthermore, NGT significantly reduced the distance from the tip to the target among target-proximity tasks in both anesthesiology and resident subgroups (P < 0.001) and among IP and OOP approach subgroups (P ≤ 0.01), as shown in Table 2. A histogram of results stratified by task type (target-proximity or target-contact) was generated to show actual distance distributions (Fig. 4). Notably, unintentional contacts in target-proximity tasks were less frequent with NGT than CU.
FIGURE 4.
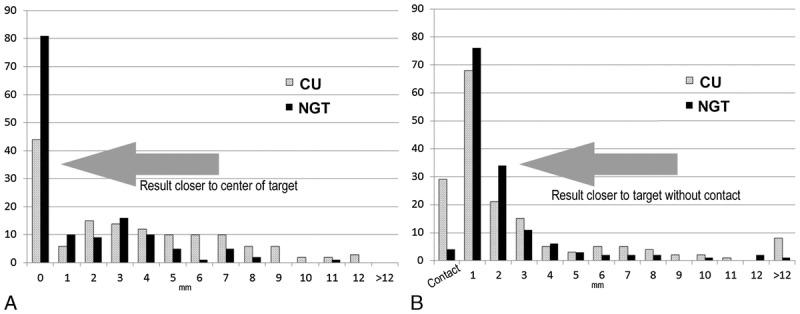
Histograms of accuracy based on the distance (in millimeters) relative to the intended target by task type for (A) target-contact tasks and (B) target-proximity tasks, showing frequency as number n of tasks (y axis) where the needle tip was placed at a distance relative to the intended target in 1-mm increments (x axis). More NGT tasks (black bars) achieved distances closer to targets compared with CU tasks (gray bars). Furthermore, the number of unintentional contacts (B, leftmost bars) and wide misses (>12 mm, rightmost bars) were lower among NGT tasks for both target-proximity and target-contact tasks.
Successful Completion of Needle Tasks
Tasks that contacted the central target (target-contact tasks) or came into close proximity without contact (target-proximity tasks) were considered successfully completed. Across all NGT (n = 280) and CU (n = 280) tasks, 55.4% (155/280) of CU tasks and 77.9% (218/280) of NGT tasks were successfully completed, demonstrating a 33.8% improvement in NGT tasks compared with CU (P < 0.001; Table 4). Furthermore, this effect was found statistically significant in a full-effects ANOVA statistical model accounting for multiple variables tested (t = 3.73, P = 0.0002; ANOVA results are detailed in Table 3). Notably, the nature of phantom targets in this study (intended to measure deviation in placement with high precision) is not based on tolerance success in any specific clinical procedure.
TABLE 4.
Number of Successfully Completed Needle Tasks Based on Placement Relative to Intended Target
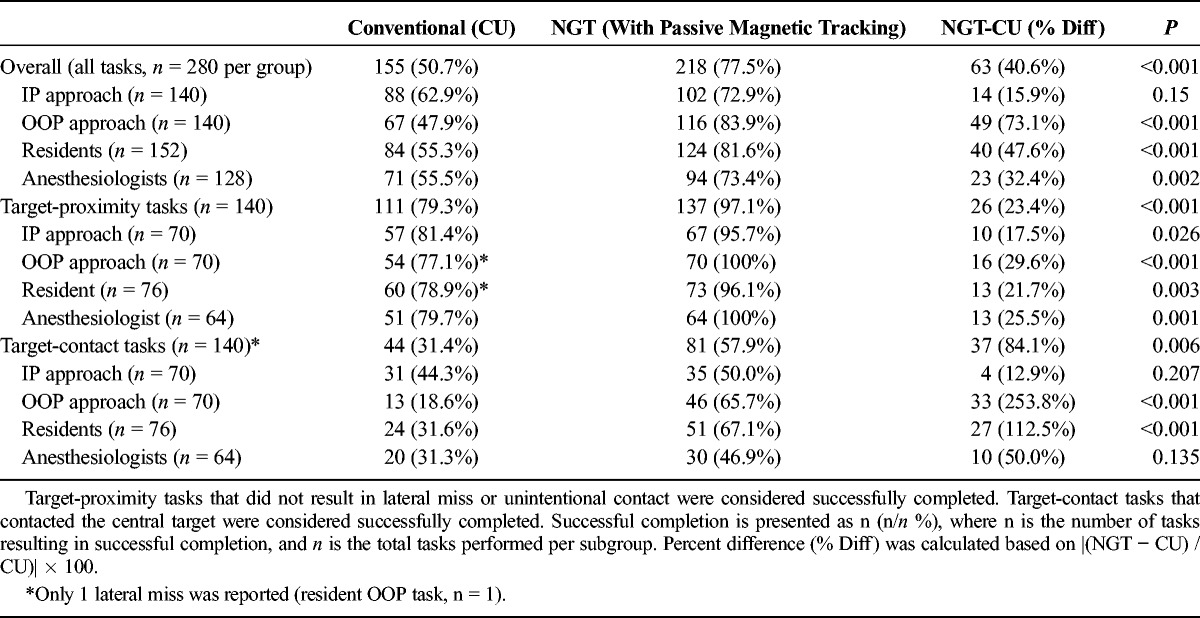
Successful completion results were further stratified by subgroup based on whether tasks were performed by residents (n = 152 NGT, n = 152 CU tasks) or anesthesiologists (n = 128 NGT, n = 128 CU tasks). Needle guidance technology increased successful completion among residents by 47.6% (124/152 NGT vs 84/152 CU tasks completed successfully; P < 0.001; Table 4) and, to a lesser degree, among anesthesiologists by 32.4% (94/128 NGT vs 71/128 CU tasks completed successfully; P = 0.002; Table 4).
Successful completion results were also stratified based on whether the approach was performed IP (n = 140 NGT, n = 140 CU tasks) or OOP (n = 140 NGT, n = 140 CU tasks). Needle guidance technology increased successful completion among IP approach tasks by 15.9% (102/140 NGT vs 88/140 tasks CU tasks completed successfully; Table 4), but this increase was not statistically significant (P = 0.15). Comparatively, NGT increased successful completion among OOP approach tasks by 73.1% compared with CU (67/128 NGT vs 116/128 CU tasks, respectively; P < 0.001; Table 4).
Compared with CU, NGT significantly improved successful completion by 23.4% for target-proximity tasks (111/140 vs 137/140, respectively; P < 0.001) and by 84.1% for target-contact tasks (44/140 vs 81/140, respectively; P < 0.001). When target-contact and target-proximity task subgroups were further stratified by plane of approach and experience level, improvements using NGT were observed in all subgroups for task-proximity tasks (P ≤ 0.02) and in the resident and OOP approach subgroups for target-contact tasks (P < 0.001; Table 4).
Number of Passes and Tasks Completion on First Pass
Across all NGT (n = 280) and CU (n = 280) tasks, the number of tasks performed on the first pass was also higher for NGT compared with CU by 25% (249/280 NGT vs 215/280 CU tasks performed on first pass; t = 4.04; P = 0.001; Tables 3 and 5). Furthermore, the mean number of passes was significantly lower for NGT, falling from 1.6 ± 1.7 (range, 1–19) with CU to 1.2 ± 0.8 (range, 1–10) with NGT (t = 3.49; P = 0.0005; Tables 3 and 6). When stratified by task type (target-contact or target-proximity), plane of approach (IP or OOP), and experience (anesthesiologist or resident), NGT performed better in all subgroups. These findings were statistically significant among resident, OOP approach, and target-proximity task subgroups (P = 0.003, P = 0.001, and P < 0.001, respectively; Table 5).
TABLE 5.
Number of Tasks Completed on the First Pass
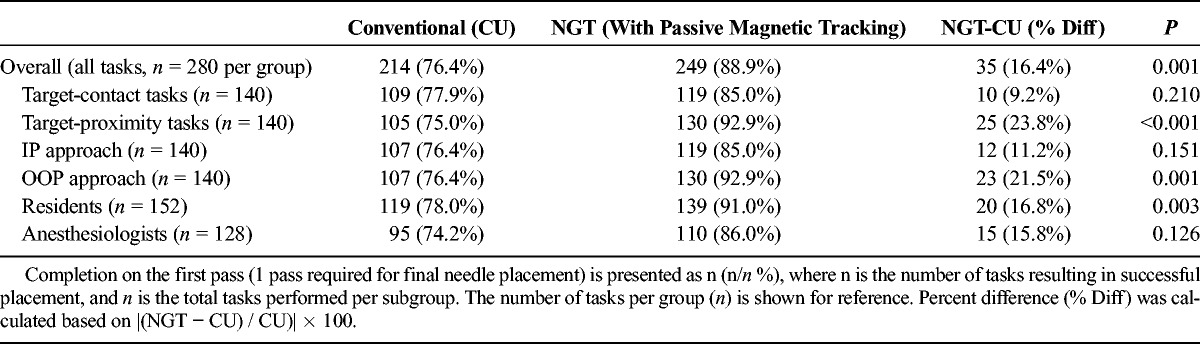
TABLE 6.
Number of Passes Required to Complete Tasks
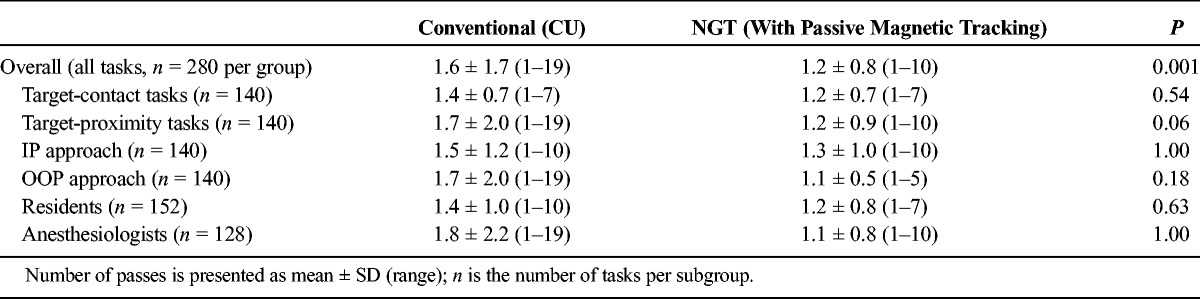
DISCUSSION
Passive magnetic tracking NGT can improve the accuracy of ultrasound-guided needle procedures. In this study, NGT enabled anesthesiologists and residents to position the needle tip closer to intended targets while reducing the number of passes and incidence of unintentional contact or puncture of the target. This suggests that NGT can benefit UGRA procedures done in anesthesiology practice, where coming into close proximity without damaging targets is critical.
Ultrasound guidance remains the standard for UGRA and is consistently reported to improve accuracy compared with unassisted procedures performed without imaging.17,18 Phantom studies have been used to assess accuracy (or average error based on distance to intended target) of other ultrasound needle guidance technologies, such as robot-assisted ultrasound needle guidance with reported accuracy of 2.54 mm19 and stereovision guidance with accuracy of 3.27 mm.11 In the present study, the accuracy of NGT was 1.5 mm on average, 2.0 mm (57.1%) closer than the CU control studies and notably closer values reported in prior phantom studies of other technologies.11,19 Participants also reported that NGT was easy to use (Table 1). Cumulatively, the improvements in accuracy and ease of use suggest the clinical utility of NGT.
We hypothesized that the significant improvement in accuracy using NGT was related to its ability to enable real-time self-correction of needle position and technique compared with CU alone. Improper needling technique has been identified by the American Society of Regional Anesthesia and Pain Medicine as a central cause of block failure and nerve injury.2 Issues in technique are most common in single-operator UGRA, where quality-compromising behaviors can be both more prevalent and more difficult to detect.5,9,20 While tactile feedback may be sufficient to enable self-correction of improper technique in procedures such as vascular access, arterial line contact, lumbar puncture, and biopsy,2,21,22 imaging can assist anesthesiologists performing UGRA procedures that lack tactile feedback associated with vessel puncture. In addition, NGT's small form factor minimally alters natural operator movements and does not add appreciable bulk or weight that can contribute to problematic technique. Based on these findings, NGT may be able to facilitate iterative self-evaluation and correction of otherwise imperceptible variations in technique during needle procedures.
The benefits of NGT were most pronounced among tasks performed in the OOP approach, which reflects the ability of NGT to assist in estimating the position of the needle tip in the foreshortened perspective imposed by OOP approach. While echogenic needles are helpful in IP procedures, technologies such as NGT are needed to improve OOP accuracy. Particularly in OOP approach tasks, we observed that users frequently self-corrected insertion angle and beam alignment iteratively based on the NGT guidance, potentially helping users identify not only the needle position but also problematic aspects of their own technique (Fig. 5). This is supported by prior observations that ultrasound guidance systems can reduce time to proficiency among trainees by enabling identification and rapid self-correction issues common in OOP approach technique, such incorrect needle progression speed, hand position, and insertion angle.20,23 Needle guidance technology thus offers a potential solution to enable better accuracy in OOP approaches.
FIGURE 5.
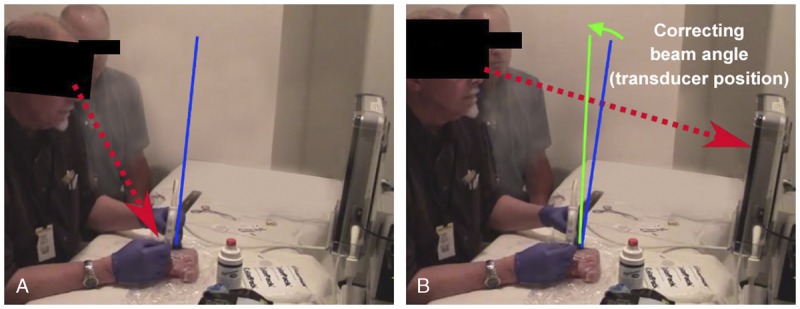
The NGT operator is shown shifting gaze between the needle insertion and US interface, correcting beam misalignment by adjusting the angle of the linear array probe by approximately 8 degrees based on alignment information visualized on the NGT interface.
Some NGT users in the study still missed the target by a wide margin, as shown in the histogram (Fig. 4). Wide misses were less common in NGT than CU and occurred primarily among residents. We observed that wide misses were centrally due to bending of the needle, which can be readily avoided using bending detection features on the device in clinical practice (which were not enabled for this study). Because NGT uses signals from passive magnets mounted in the needle base, torsional forces on the needle and resultant bending can interfere with needle tip localization on the interface. This is perceptible on the ultrasound interface as slight misalignment of NGT lines and the underlying hyperechoic needle, which is correctable by alleviating needle bending (such as using a lighter touch and less pressure on the needle). Because the hyperechoic needle remains visible, such curvature can be readily detectable by trained anesthesiologists and would be immediately recognized by the system's computations, which warn the user of needle bending. Thus, while wide misses are still possible with NGT, use of the bending detection feature and cognizance of the underlying sonographic image are likely to further improve clinical accuracy in practice.
Assessing the true clinical benefits of new needle guidance systems is challenging. Most phantom studies of needle guidance systems do not distinguish between tasks that come into contact or close proximity with targets, and some evidence indicates that conventional target-contact test methods may disproportionately favor needle guidance systems.2 For this reason, we also utilized tasks that aim to place the needle tip in close proximity to the target, a technique translatable to nerve block procedures that must avoid inadvertent intraepineural contact. While accuracy is important in clinical procedures, it is important to note that local anesthetic volumes, neurotoxicity, and metabolic effects of underlying health conditions and surgical-related insults also contribute to clinical efficacy.2 Clinical measures such as motor responses (such as patellar snap in femoral block), block character (duration and time), rescue block, and a myriad of other surrogate end points can be measured only in humans.17,23 Because of accuracy gains observed with NGT, comprehensive exploration of NGT in clinical settings is merited.
Our study has some limitations, including lack of blinding and fixed-order task performance, possibly introducing bias due to fatigue in later tasks (although minimal as all participants completed in approximately 10 minutes). Further randomized controlled trials will be needed to confirm clinical outcomes and workflow in humans rather than porcine tissue models.17,24 We also note that overfitting of statistical results is possible, even with appropriate statistical methods for subgroup testing, and should be interpreted conservatively.
In summary, this phantom study of the passive magnetic NGT system showed significant improvements in accuracy, number of passes, successful completion, and completion on the first pass among both experienced anesthesiologists and residents. While accuracy gains were observed in both target-proximity and target-contact tasks, the benefit was most pronounced among target-proximity tasks resembling nerve block. This is, in part, due to the ability of NGT to enhance operator visualization of the needle trajectory in real time and make necessary self-corrections. These findings indicate that passive magnetic NGT merits consideration as a tool to improve UGRA accuracy in clinical practice.
ACKNOWLEDGMENTS
The authors thank Dr Eunji Kang of the University of Michigan and Naomi Sato, JAMT, for their input on technology development and assessments.
Footnotes
Funding for this study was provided by GE Healthcare, Wauwatosa, WI, through the GE Healthcare Ultrasound Business operating in partnership with C. R. Bard, Inc. The Pinpoint GT needle guidance technology is used under license from C. R. Bard, Inc.
Initial findings of this study were presented as an abstract at the 2016 American Association of Physicists in Medicine annual conference, July 31–August 3, 2016, Washington, DC.
The authors who participated in the conduct of this research and drafted the manuscript are employees or contractors of GE Healthcare. The opinions and information presented herein are solely those of the authors. All practitioners performing needle tasks for this study were recruited from independent sites and not affiliated with GE Healthcare.
REFERENCES
- 1.Ting PL, Sivagnanaratnam V. Ultrasonographic study of the spread of local anaesthetic during axillary brachial plexus block. Br J Anaesth. 1989;63:326–329. [DOI] [PubMed] [Google Scholar]
- 2.Neal JM, Brull R, Chan VW, et al. The ASRA evidence-based assessment of ultrasound-guided regional anesthesia and pain medicine: executive summary. Reg Anesth Pain Med. 2010;35:S1–9. [DOI] [PubMed] [Google Scholar]
- 3.Koscielniak-Nielsen ZJ. Ultrasound-guided peripheral nerve blocks: what are the benefits? Acta Anaesthesiol Scand. 2008;52:727–737. [DOI] [PubMed] [Google Scholar]
- 4.Nowakowski P, Bieryło A, Duniec L, Kosson D, Łazowski T. The substantial impact of ultrasound-guided regional anaesthesia on the clinical practice of peripheral nerve blocks. Anaesthesiol Intensive Ther. 2013;45:223–229. [DOI] [PubMed] [Google Scholar]
- 5.Wegener JT, van Doorn CT, Eshuis JH, Hollmann MW, Preckel B, Stevens MF. Value of an electronic tutorial for image interpretation in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2013;38:44–49. [DOI] [PubMed] [Google Scholar]
- 6.Luyet C, Schüpfer G, Wipfli M, Greif R, Luginbühl M, Eichenberger U. Different learning curves for axillary brachial plexus block: ultrasound guidance versus nerve stimulation. Anesthesiol Res Pract. 2010;2010:309462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swenson JD, Klingler KR, Klinger K, Pace NL, Davis JJ, Loose EC. Evaluation of a new needle guidance system for ultrasound: results of a prospective, randomized, blinded study. Reg Anesth Pain Med. 2016;41:356–361. [DOI] [PubMed] [Google Scholar]
- 8.Marhofer P. Technique limitations and suggestions for a training concept. In: Ultrasound Guidance in Regional Anesthesia: Principles and Practical Implementation. 2nd ed London, UK: Oxford University Press; 2010. [Google Scholar]
- 9.Sites BD, Spence BC, Gallagher JD, Wiley CW, Bertrand ML, Blike GT. Characterizing novice behavior associated with learning ultrasound-guided peripheral regional anesthesia. Reg Anesth Pain Med. 2007;32:107–115. [DOI] [PubMed] [Google Scholar]
- 10.Atchabahian A, Hemmerling TM. Robotic anesthesia: how is it going to change our practice? Anesth Pain Med. 2014;4:e16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolka PJ, Foroughi P, Rendina M, Weiss CR, Hager GD, Boctor EM. Needle guidance using handheld stereo vision and projection for ultrasound-based interventions. Med Image Comput Comput Assist Interv. 2014;17:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gofeld M, Brown MN, Bollag L, Hanlon JG, Theodore BR. Magnetic positioning system and ultrasound guidance for lumbar zygapophysial radiofrequency neurotomy: a cadaver study. Reg Anesth Pain Med. 2014;39:61–66. [DOI] [PubMed] [Google Scholar]
- 13.Langford RA, Hockey B, Leslie K. Monitor position and the accuracy and speed of ultrasound-guided nerve blocks. Anaesthesia. 2009;64:845–849. [DOI] [PubMed] [Google Scholar]
- 14.Ajmal M, Power S, Smith T, Shorten GD. Ergonomic task analysis of ultrasound-guided femoral nerve block: a pilot study. J Clin Anesth. 2011;23:35–41. [DOI] [PubMed] [Google Scholar]
- 15.Souzdalnitski D, Lerman I, Halaszynski TM. How to improve needle visibility: training and phantom simulation. In: Narouze S, ed. Atlas of Ultrasound-Guided Procedures in Interventional Pain Management. New York, NY: Springer; 2011. [Google Scholar]
- 16.Ihnatsenka B, Boezaart AP. Ultrasound: basic understanding and learning the language. Int J Shoulder Surg. 2010;4:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelfand HJ, Ouanes JP, Lesley MR, et al. Analgesic efficacy of ultrasound-guided regional anesthesia: a meta-analysis. J Clin Anesth. 2011;23:90–96. [DOI] [PubMed] [Google Scholar]
- 18.Abrahams MS, Aziz MF, Fu RF, Horn JL. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2009;102:408–417. [DOI] [PubMed] [Google Scholar]
- 19.Boctor EM, Choti MA, Burdette EC, Webster RJ. Three-dimensional ultrasound-guided robotic needle placement: an experimental evaluation. Int J Med Robot. 2008;4:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrington MJ, Wong DM, Slater B, Ivanusic JJ, Ovens M. Ultrasound-guided regional anesthesia: how much practice do novices require before achieving competency in ultrasound needle visualization using a cadaver model. Reg Anesth Pain Med. 2012;37:334–339. [DOI] [PubMed] [Google Scholar]
- 21.Khati NJ, Gorodenker J, Hill MC. Ultrasound-guided biopsies of the abdomen. Ultrasound Q. 2011;27:255–268. [DOI] [PubMed] [Google Scholar]
- 22.McVicar J, Niazi AU, Murgatroyd H, Chin KJ, Chan VW. Novice performance of ultrasound-guided needling skills: effect of a needle guidance system. Reg Anesth Pain Med. 2015;40:150–153. [DOI] [PubMed] [Google Scholar]
- 23.Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2011;36:162–170. [DOI] [PubMed] [Google Scholar]
- 24.Apesteguía L, Pina LJ. Ultrasound-guided core-needle biopsy of breast lesions. Insights Imaging. 2011;2:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]


