Abstract
Leukemia stem cells (LSCs) account for the development of drug resistance and increased recurrence rate in acute myeloid leukemia (AML) patients. Targeted drug delivery to leukemia stem cells remains a major challenge in AML chemotherapy. Overexpressed interleukin-3 receptor alpha chain, CD123, on the surface of leukemia stem cells was reported to be a potential target in AML treatment. Here, we designed and developed an antibody drug conjugate (CD123-CPT) by integrating anti-CD123 antibody with a chemotherapeutic agent, Camptothecin (CPT), via a disulfide linker. The linker is biodegradable in the presence of Glutathione (GSH, an endogenous component in cells), which leads to release of CPT. Anti-CD123 antibody conjugates showed significant higher cellular uptake in CD123-overexpressed tumor cells. More importantly, CD123-CPT demonstrated potent inhibitory effects on CD123-overexpressed tumor cells. Consequently, these results provide a promising targeted chemotherapeutical strategy for AML treatment.
Keywords: CD123, Camptothecin, Acute myeloid leukemia (AML), Leukemia stem cells (LSCs), Antibody drug conjugates (ADCs)
1. Introduction
Acute myeloid leukemia (AML) is an aggressive malignancy of the myeloid line of blood cells. AML accounts for 15–20% of leukemia in children with a relapse rate of 20–40%.1 The origin of the mature leukemic blasts that cause AML can be traced to leukemia stem cells (LSCs),2 which are distinct from mature leukemic blasts and are more resistant to conventional chemotherapeutic regimens.3,4 The propagation of these LSCs causes increased recurrence rate in patients with acute myeloid leukemia.5 In order to improve drug response and survival time for AML patients, a new therapeutic approach is urgently needed, which would be able to specifically eradicate leukemia stem cells.
Antibody drug conjugates (ADCs)6–8 facilitate the internalization of their payload by taking advantage of the specificity of antibodies. Currently, a few ADCs such as Kadcyla and Adcetris have been approved by Food and Drug Administration (FDA) and widely used for the treatment of cancers.9 Antibodies have demonstrated effective targeted therapeutic effects on many kinds of cancer types and their mechanism of action on targeted cells enables high efficiency with reduced toxicity in delivering anti-cancer agents.9,10 ADCs not only enhance the specificity to targeted cell population, but also improve pharmaceutical properties of chemotherapy drugs. Camptothecin, for example, is a potent anti-cancer agent, but possesses low water solubility and is formidable to be administrated to patients, which severely limited its therapeutic applications.11,12
Interleukin-3 receptor alpha chain, CD123, has been reported as a marker of quiescent leukemia stem cells (LSCs).13,14 Recent studies identified significant overexpression of CD123 in a large proportion (40–93%) of patients with AML.15 Moreover, clinical data indicates that high CD123 expression in AML is associated with poor prognosis and reduced survival rate.13 Monoclonal antibodies targeting CD123 have demonstrated efficacy against AML LSCs in xenotransplantation animal models.12 Currently, CSL362, a humanized anti-CD123 monoclonal antibody is in Phase I clinical trial for AML treatment. In addition, A.E. Frankel et al. fused anti-CD123 monoclonal antibody to a truncated diphtheria toxin and produced SL-401.16 Clinical results from a phase 1–2 study of SL-401 displayed potential therapeutic benefits for AML patients, demonstrating its potential in inducing growth arrest and apoptosis in AML blasts and LSCs by inhibiting protein synthesis and interfering with IL-3 signal transduction pathways.16–20 Encouraged by these results, we hypothesize that anti-CD123 antibody drug conjugates can be a promising strategy in delivering chemotherapeutical drugs in order to eliminate LSCs and treat AML.
In this study, we developed a new type of anti-CD123 antibody drug conjugates (CD123-CPT) in order to achieve targeted drug delivery of Camptothecin (CPT, a potent chemotherapy agent) to CD123-overexpressed cells. Figure 1 illustrates the design of CD123-CPT and its mechanism of action. CD123-CPT ADCs were constructed by conjugating anti-CD123 antibody with CPT via a disulfide linker. Flow cytometry analysis showed that anti-CD123 antibody facilitated specific target to CD123-overexpressed cells, and subsequently resulted in enhanced cellular uptake of its cargo. After internalization, the disulfide linker of CD123-CPT was cleaved by Glutathione (GSH), which resulted in release of CPT, and thus inhibited tumor cell proliferation and accomplished the therapeutic effects.
Figure 1.
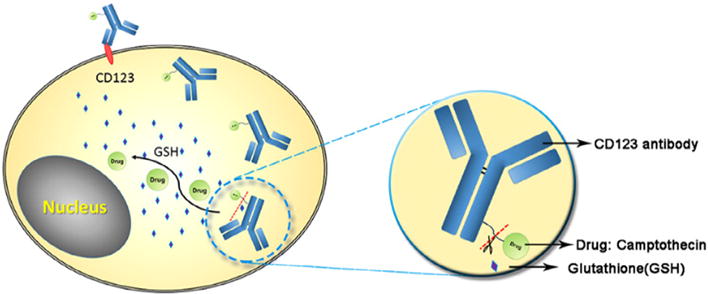
Illustration of anti-CD123 antibody drug conjugates. Anti-CD123 antibody facilitates specific binding with CD123 on the surface of CD123-overexpressed cells and cellular uptake of ADCs. After internalization, the disulfide linker in CD123-CPT is degraded by GSH and results in release of CPT. Then, CPT inhibits tumor cell proliferation and accomplishes the therapeutic effects.
2. Results and discussion
2.1. Design and synthesis of conjugates
Scheme 1 shows a synthetic route to CD123-CPT and CD123-IR conjugates. First, we installed a pyridyl disulfide reactive group on CPT through an esterification reaction to afford intermediate 1. Meanwhile, anti-CD123 antibodies were reacted with Traut’s reagent to produce intermediate 2. Lastly, intermediates 1 and 2 were conjugated via a disulfide linker at room temperature to afford CD123-CPT. In order to quantify cellular uptake, anti-CD123 antibody was conjugated with a NIR probe, IR-780 to afford (CD123-IR) conjugates. The reaction mixtures were dialyzed in PBS to remove excess Traut’s reagents and intermediates, and were used for the further assays.
Scheme 1.
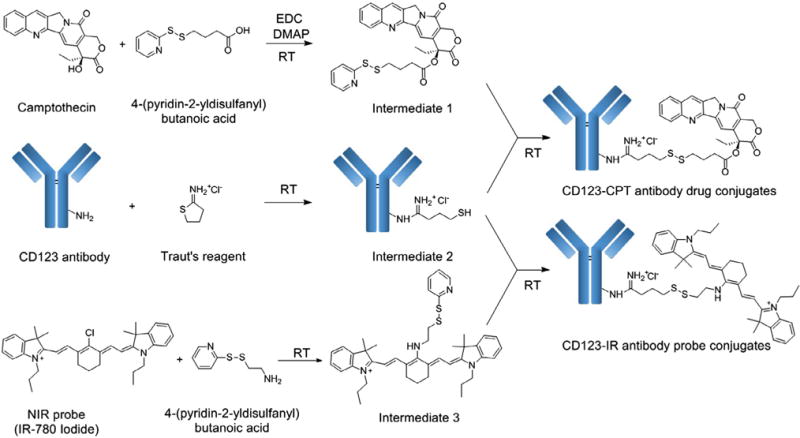
A synthetic route to CD123-CPT antibody drug conjugates and CD123-IR antibody probe conjugates.
2.2. Characterization and drug release response of conjugates
Structures of intermediates were confirmed by 1H NMR, 13C NMR and mass spectrometry (Figs. S1 and S2). CD123-CPT and CD123-IR conjugates were characterized using MALDI-TOF-MS. Figure 2 shows that molecular weights of anti-CD123 antibody, CD123-IR, and CD123-CPT are 150,784 Da, 152,087 Da and 152,675 Da, respectively. These results suggested that an average of 3.2 CPT per antibody were present. Similarly, the number of IR-780 was determined as 1.8. In order to examine the cleavage of disulfide linker on CD123-CPT conjugate, we incubated CD123-CPT with GSH, the most abundant thiol compound in cells,21,22 since previous studies reported that GSH was able to react with the disulfide linker.23 After 4 h incubation, the corresponding molecular peak found in the mass spectrum (Fig. S3A) indicated that CPT was successfully released from CD123-CPT conjugates owing to the disulfide linker cleavage. Thus, we proposed the potential mechanism of drug release as shown in Figure S3B.
Figure 2.
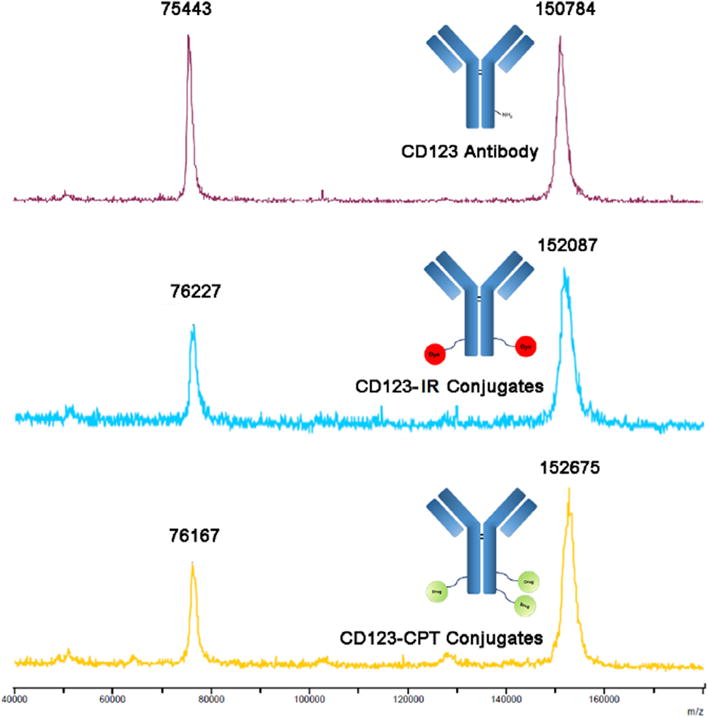
MALDI-TOF mass spectrum of CD123 antibody, CD123-IR conjugates and CD123-CPT conjugates.
2.3. Cellular uptake of conjugates
In order to quantify cellular uptake of conjugates, we incubated CD123-IR with THP-1 cells (a human acute myeloid leukemia cell line) with high-level of CD12324 and Hep3B cells (a human hepatoma cell line) with low-level of CD123. Figure 3 depicted the uptake of CD123-IR in THP-1 and Hep3B cell lines, respectively. Based on the fluorescence-activated cell sorting (FACS) analysis, at both concentrations of 5 and 25 nM, fluorescence intensity in THP-1 cells was over two-fold higher than that in Hep3B cells, which revealed significant uptake of CD123-IR in THP-1 cells as compared to Hep3B cells. These results demonstrated that the interleukin-3 receptor alpha chain (CD123) promoted the uptake of anti-CD123 antibody conjugates.
Figure 3.
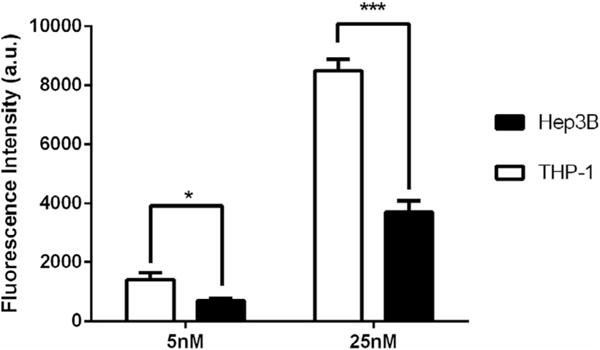
Cellular uptake of CD123-IR in THP-1 and Hep3B cell lines (*, p < 0.05; **, p < 0.01; ***, p < 0.001; t test, double-tailed).
In order to further differentiate whether the CD123 antibody probe conjugates (CD123-IR) were internalized by the cells or bound to cell surface, we did a 3D stack confocal laser scanning experiment. Figure 4 demonstrated images collected by confocal microscope, where nuclei of cells were stained by HOECHST 33342 and marked as blue, and IR-780 probe was marked as red. Based on the 3D stack images, there was no red signal seen at either the top (Fig. 4a) or the bottom layer (Fig. 4b) of the cell. However, obvious signals were detected in the middle layer of transverse plane (Fig. 4c), which indicated that CD123-IR antibody probe conjugates were internalized by the cells.
Figure 4.
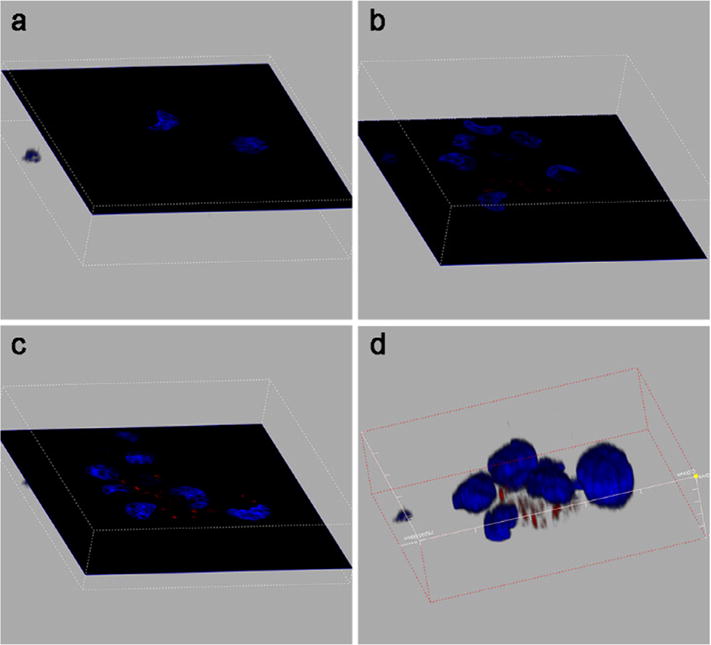
3D stack confocal laser scanning images. Nuclei of THP-1 cells were stained by HOECHST 33342 and marked as blue. IR-780 probe was marked as red. The top (a), bottom (b), middle (c) layers of the cell transverse planes and 3D stack (d) images.
2.4. Cytotoxicity assay
In order to evaluate cytotoxic activity of CD123-CPT, we treated THP-1 and Hep3B cells with CD123-CPT conjugates, as well as anti-CD123 antibody, free CPT, intermediate 1 at equivalent dose as control groups. As determined by cell viability curves (Fig. 5 and Fig. S4A–C), the IC50 value of free CPT against THP-1 and Hep3B cells was 0.666 μM and 7.765 μM, respectively, while in the case of CD123-CPT conjugates, the IC50 value was 0.306 μM and 7.308 μM. According to the results of cytotoxicity studies, CD123-CPT conjugates induced over 90% of THP-1 cell death at the dose of 2.8 μM, while only caused less than 30% of Hep3B cell death at the same dose. By comparing the IC50 of CD123-CPT antibody drug conjugates and CPT free drugs in each cell lines, we can see that the CD123-CPT conjugates significantly reduced the IC50 against THP-1 cells from 0.666 μM to 0.306 μM as compared with free CPT; while CD123-CPT conjugates and free CPT did not show significant difference in potency on Hep3B cells, which are 7.308 μM and 7.765 μM, respectively. Hence, CD123-CPT conjugate demonstrated superior cytotoxicity towards THP-1 cells in comparison with Hep3B cells.
Figure 5.
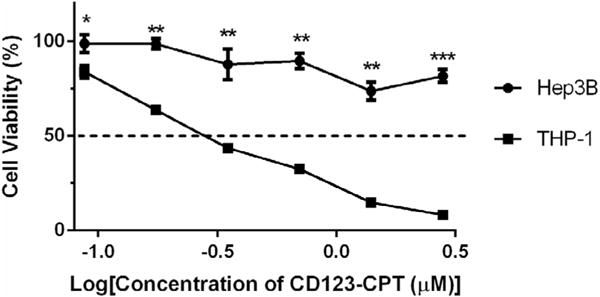
Cell viability of Hep3B and THP-1 cell lines after being treated with CD123-CPT conjugates (*, p < 0.05; **, p < 0.01; ***, p < 0.001; t test, double-tailed).
3. Conclusion
In summary, a new anti-CD123 antibody drug conjugate (CD123-CPT) was designed and synthesized by integrating anti-CD123 antibody with Camptothecin (CPT). Anti-CD123 antibody exhibited dramatic increase of cellular uptake in CD123 overexpressed cells. Consistent with our design, GSH was capable of cleaving the disulfide bond in CD123-CPT and releasing CPT. Most importantly, CD123-CPT showed potent cytotoxicity in THP-1 cells with an IC50 of 0.306 μM. Therefore, the development of anti-CD123 antibody drug conjugates provides a promising chemotherapeutical strategy for efficiently targeting and eliminating leukemia stem cells in AML treatment.
4. Experimental procedure
4.1. Materials
Camptothecin was purchased from Medkoo Biosciences (NC, USA). Traut’s reagent was purchased from ACROS ORGANICS (NJ, USA). CD123 antibody was given as a gift from Harlan Bioproduct for Science. N,N-Dimethylpyridin-4-amine (DMAP) was purchased from Alfa Aesar (MA, USA). N-Ethyl-N′-(3-dimethylaminopropyl)-carbodiimide (EDC) was purchased from Ark Pharm, Inc. (IL, USA). Other chemical agents were purchased from Sigma–Aldrich (MO, USA). RPMI-1640 Medium was purchased from American Type Culture Collection (ATCC, VA, USA). Eagle’s Minimum Essential Medium (EMEM) was purchased from Corning Incorporated (NY, USA).
4.2. Synthesis of CD123 antibody drug conjugates (CD123-CPT)
Conjugates were prepared with a previously reported conjugation method.25 Firstly, Camptothecin (CPT) and 4-(pyridin-2-yldisulfanyl)butanoic acid (molar ratio 1:1) were dissolved in anhydrous THF. Then 4-dimethylaminopyridine (DMAP, 0.30 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodi imide hydrochloride (EDC, 0.30 mmol) were added. The resulting mixture was stirred for 7 h at room temperature. The solution was then filtered, washed with DCM, extracted with saturated NaHCO3, brine, dried over Na2SO4, and concentrated in vacuo. The residue was purified by flash chromatography using eluent EtOAc/Hexanes (5:1). Product fractions were collected to give intermediate 1 as white powder. The structure was confirmed by 1H, 13C NMR on Bruker 400 MHz spectrometers with TMS as internal standard, and ESI-MS on a LTQ Orbitrap mass spectrometer. Secondly, Traut’s Reagent (10-fold molar excess) was added to CD123 Antibody solution (3.57 mg/ml in PBS, pH 7.4) to obtain intermediate 2. Lastly, the intermediate 1 (2 mg/mL in DMSO) and intermediate 2 were mixed at 7.5:1 (molar ratio) and incubated for 1 h at RT. Unconjugated intermediate 1 was removed by dialysis using Slide-A-Lyzer membrane (MW cutoff, 3500) against 1L of PBS overnight at RT and then further purified with Zeba™ Spin desalting column (40 K MWCO) to obtain CD123-CPT.
4.3. Synthesis of CD123 antibody probe conjugates (CD123-IR)
CD123 antibody probe conjugates were synthesized as fluorescent probes for in vitro cellular uptake studies. IR-780 dye was synthesized according to previously reported method.26 Then IR-780 dye was further modified with pyridyl disulfide reactive group. IR-780 (51.0 mg) and 4-(pyridin-2-yldisulfanyl)butanoic acid (30.0 mg) were dissolved in acetonitrile. Then DIEA (27.0 mg) was added and the resulting mixture was refluxed for 2 h. The resulting mixture was condensed under vacuum. And intermediate 3 was purified to afford as a blue solid. The remaining procedures were similar with the synthesis of CD123-CPT.
4.4. Characterization of antibody drug/probe conjugates
The CD123 Antibody Drug/Probe Conjugates were characterized by MALDI-TOF mass spectrometry (Ultraflextreme, Bruker) using sinapinic acid as the matrix and protein II (Bruker) & bovine serum albumin as the standard. The ratio of antibody to drug/probe was calculated based on their molecular weight.
4.5. Drug release from CD123 antibody drug conjugates
The drug release response by Glutathione (GSH) reduction of the disulfide linker was monitored using mass spectrometer.23 50 μL of the CD123-CPT (11.8 μM) was incubated with 97.5 μL of GSH (18.15 mM in DPBS) at the final concentration of CD123-CPT and GSH is 4 μM and 12 mM. Four hours later, the MW of the mixture was measured with ESI-MS (LTQ Orbitrap, Thermo Fisher Scientific, USA).
4.6. Cellular uptake of CD123 antibody probe conjugates (CD123-IR)
Human acute monocytic leukemia THP-1 cell line (ATCC) was cultured at 37 °C with an air atmosphere of 5% CO2 in RPMI-1640 Medium supplemented with 10% heat inactivated FBS. Human hepatocellular carcinoma Hep3B cell line (ATCC) was cultured at 37 °C with an air atmosphere of 5% CO2 in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% heat inactivated FBS.
Cellular uptake of CD123 antibody probe conjugates (CD123-IR) was examined on Hep3B and THP-1 cell lines by FACS. Cells were seeded in a 6-well plate at density of 4 × 105 cells/well. After overnight incubation at 37 °C, CD123-IR antibody probe conjugates were added to Hep3B and THP-1 cells, at the final concentrations of 5 nM and 25 nM, respectively. Two hours later, medium was removed and cells were collected. After thorough washing for twice, cells were re-suspended in PBS and the fluorescence intensity was determined on a BD LSR II flow cytometer (BD Biosciences, San Jose, CA).
A 3D stack confocal laser scanning study was conducted on THP-1 cell line under Multiphoton Laser Scanning Confocal microscope (FV1000 MPE, Olympus). CD123-IR antibody probe conjugates were added to THP-1 cells at the concentrations of 25 nM. After two hours’ incubation, HOECHST 33342 was added into the medium to stain the nuclei of the cells. A 3D stack confocal laser scanning was then conducted on the treated THP-1 cells.
4.7. Cytotoxicity of CD123 antibody drug conjugates
Cytotoxicity of CD123 antibody drug conjugates, anti-CD123 antibody, CPT free drug and intermediate 1 were examined on Hep3B and THP-1 cell lines by MTT assays as previously reported.27,28 Cytotoxicity assays were performed in triplicate and cell viability of each treated groups was normalized by untreated cells.
For Hep3B cell line, cells were grown in 96-well plates 24 h prior to treatment at a density of 1 × 104 cells/well. After incubation with CD123-CPT conjugates, anti-CD123 antibody, free CPT or intermediate 1 for 4 h, drug was removed and replaced with medium. Then the cells were further incubated at 37 °C for 44 h. MTT was added to each well and the cells were then incubated for 4 h at 37 °C. Then the medium was removed, and 150 μL of dimethyl sulfoxide (DMSO) was added. After shaking for 10 min, the absorbance at a wavelength of 570 nm was measured on a SpectraMax M5 microplate reader.27
For THP-1 cell line, cells were grown in 96-well plates 24 h prior to treatment at a density of 3 × 104 cells/well. After incubation with CD123-CPT conjugates, anti-CD123 antibody, free CPT or intermediate 1 for 4 h, drug was removed by centrifuge at 1000 rpm for 5 min, and cells were washed with PBS and re-suspended with medium. Then the cells were further incubated at 37 °C for 44 h. MTT was added to each well and the cells were then incubated for 4 h at 37 °C. Subsequently, 10% SDS solution was added to each well and incubated at 37 °C for overnight. The absorbance at a wavelength of 570 nm was measured on a SpectraMax M5 microplate reader.28
Supplementary Material
Acknowledgments
This work was supported by the start-up fund from the College of Pharmacy at The Ohio State University, United States. We acknowledge Dr. John C. Byrd for providing THP-1 cells and helpful discussion.
Abbreviations
- AML
acute myeloid leukemia
- LSCs
leukemia stem cells
- ADCs
antibody drug conjugates
- CPT
Camptothecin
- GSH
Glutathione
Footnotes
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmc.2016.09.043.
References and notes
- 1.Zwaan CM, Kolb EA, Reinhardt D, Abrahamsson J, Adachi S, Aplenc R, De Bont ES, De Moerloose B, Dworzak M, Gibson BE, Hasle H, Leverger G, Locatelli F, Ragu C, Ribeiro RC, Rizzari C, Rubnitz JE, Smith OP, Sung L, Tomizawa D, van den Heuvel-Eibrink MM, Creutzig U, Hasle H. J Clin Oncol. 2015;33:2949. doi: 10.1200/JCO.2015.62.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, Ashton JM, Pei S, Grose V, O’Dwyer KM, Liesveld JL, Brookes PS, Becker MW, Liesveld JL. Cell Stem Cell. 2013;12:329. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker MW, Jordan CT. Blood Rev. 2011;25:75. doi: 10.1016/j.blre.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Konopleva MY, Jordan CT. J Clin Oncol. 2011;29:591. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Chng WJ. World J Stem Cells. 2014;6:473. doi: 10.4252/wjsc.v6.i4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng L, Chen X. Bioconjug Chem. 2015;26:2169. doi: 10.1021/acs.bioconjchem.5b00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qasba PK. Bioconjug Chem. 2015;26:2170. doi: 10.1021/acs.bioconjchem.5b00173. [DOI] [PubMed] [Google Scholar]
- 8.Merten H, Brandl F, Plückthun A, Zangemeister-Wittke U. Bioconjug Chem. 2015;26:2176. doi: 10.1021/acs.bioconjchem.5b00260. [DOI] [PubMed] [Google Scholar]
- 9.Hamann PR, Hinman LM, Beyer CF, Lindh D, Upeslacis J, Flowers DA, Bernstein I. Bioconjug Chem. 2002;13:40. doi: 10.1021/bc0100206. [DOI] [PubMed] [Google Scholar]
- 10.Naujokat C. Immunotherapy. 2014;6:290. doi: 10.2217/imt.14.4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Lu W, Tang K, Wang H, Liu Y, Bao B, Fang Y, Zhang X. Bioconjug Chem. 2016;27:1267. doi: 10.1021/acs.bioconjchem.6b00099. [DOI] [PubMed] [Google Scholar]
- 12.Hu M, Huang P, Wang Y, Su Y, Zhou L, Zhu X, Yan D. Bioconjug Chem. 2015;26:2497. doi: 10.1021/acs.bioconjchem.5b00513. [DOI] [PubMed] [Google Scholar]
- 13.Al-Hussaini M, Rettig MP, Ritchey JK, Karpova D, Uy GL, Eissenberg LG, Gao F, Eades WC, Bonvini E, Chichili GR, Moore PA, Johnson S, Collins L, Moore PA. Blood. 2016;127:122. doi: 10.1182/blood-2014-05-575704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belmonte M, Hoofd C, Weng AP, Giambra V. Curr Oncol. 2016;23:34. doi: 10.3747/co.23.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks CL, Cirrito TP, Hoberman K, Rowinsky E. Blood. 21. Vol. 120. 2021 L ST NW, Suite 900, Washington DC 20036 USA: Amer Soc Hematology; 2012. [Google Scholar]
- 16.Frankel AE, Ramage J, Kiser M, Alexander R, Kucera G, Miller MS. Protein Eng. 2000;13:575. doi: 10.1093/protein/13.8.575. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen JL, Wang SA, Huang X, González GMN, Brooks C, Rowinsky E, Levis M, Zhou J, Ciurea SO, Alatrash G, Cortes JE, Kantarjian HM, Andreeff M, Ravandi F, Konopleva M. Blood. 2013;122:359. [Google Scholar]
- 18.Frankel AE, Woo JH, Ahn C, Pemmaraju N, Medeiros BC, Carraway HE, Frankfurt O, Forman SJ, Yang XA, Konopleva M, Garnache-Ottou F, Angelot-Delettre F, Brooks C, Szarek M, Rowinsky E. Blood. 2014;124:385. doi: 10.1182/blood-2014-04-566737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frolova O, Benito J, Brooks C, Wang RY, Korchin B, Rowinsky EK, Cortes J, Kantarjian H, Andreeff M, Frankel AE, Konopleva M, Konopleva M. Br J Haematol. 2014;166:862. doi: 10.1111/bjh.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelot-Delettre F, Roggy A, Frankel AE, Lamarthee B, Seilles E, Biichle S, Royer B, Deconinck E, Rowinsky EK, Brooks C, Bardet V, Benet B, Bennani H, Benseddik Z, Debliquis A, Lusina D, Roussel M, Solly F, Ticchioni M, Saas P, Garnache-Ottou F, Bardet V. Haematologica. 2015;100:223. doi: 10.3324/haematol.2014.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachhawat AK, Thakur A, Kaur J, Zulkifli M. Biochim Biophys Acta. 2013;1830:3154. doi: 10.1016/j.bbagen.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Lushchak VI. J Amino Acids. 2012;736837 doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onyango JO, Chung MS, Eng CH, Klees LM, Langenbacher R, Yao L, An M. Angew Chem, Int Ed Engl. 2015;54:3658. doi: 10.1002/anie.201409770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Yin C, Feng L, Ma L, Wei Y, Sheng G. Oncol Rep. 2013;29:1923. doi: 10.3892/or.2013.2316. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Zheng YB, Li DD, Zhen YS. J Pharm Sci. 2014;103:1204. doi: 10.1002/jps.23893. [DOI] [PubMed] [Google Scholar]
- 26.Samanta A, Vendrell M, Das R, Chang YT. Chem Commun. 2010;7406 doi: 10.1039/c0cc02366c. [DOI] [PubMed] [Google Scholar]
- 27.Paranjpe PV, Chen Y, Kholodovych V, Welsh W, Stein S, Sinko PJ. J Control Release. 2004;100:275. doi: 10.1016/j.jconrel.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Papa V, Tazzari PL, Chiarini F, Cappellini A, Ricci F, Billi AM, Evangelisti C, Ottaviani E, Martinelli G, Testoni N, McCubrey JA, Martelli AM. Leukemia. 2008;22:147. doi: 10.1038/sj.leu.2404980. Supplementary Material. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


