Summary
Simvastatin induced rhabdomyolysis with renal failure is a well reported clinical entity with hyperkalemia recognized as a life threatening risk. The risk of delayed hypercalcemia during the recovery of renal function is not well appreciated as this varies in severity and can be caused by multiple mechanisms. We present a patient with high dose simvastatin induced rhabdomyolysis leading to late onset of severe hypercalcemia due to calcium phosphate deposition in muscles diagnosed by distinctive bone scintigraphy.
A 60-year-old Asian male was admitted to the hospital for profound weakness one week following the initiation of simvastatin 80 mg daily post myocardial infarction. His clinical course was complicated by contrast nephropathy. One week later, he developed progressive weakness in all his extremities and inability to raise his head and eat. Simvastatin was discontinued at this point. CPK elevation to greater than 425,000 U was found, consistent with rhabdomyolysis. He became oliguric requiring hemodialysis. Muscle biopsy showed severe muscle necrosis and type 2 fiber atrophy. One month later, he developed hypercalcemia with suppressed intact PTH and 1, 25(OH) D levels. Whole body bone scintigraphy showed calcium phosphate deposition throughout his musculature. His calcium levels normalized in 1 week on hemodialysis.
This patient’s experience illustrates the marked risk of delayed severe hypercalcemia from rhabdomyolysis due to dissolution of myocellular calcium phosphate deposits. It also provides an opportunity to review the different mechanisms of hypercalcemia especially in statin induced rhabdomyolysis. Recognition of this phenomenon is critical for appropriate follow up and treatment of such patients.
Keywords: bone scintigraphy, statins, rhabdomyolysis, myocellular calcium phosphate deposits, hypercalcemia
Introduction
Rhabdomyolysis is a syndrome of severe muscle cell damage with release of myoglobin and other toxic intracellular contents leading to acute renal failure with marked changes in calcium and phosphate (1, 2). Simvastatin is the most common statin medication associated with rhabdomyolysis (3). Statin induced rhabdomyolysis occurs in 0.3–13.5 cases per 1,000,000 statin prescriptions (4). In the recovery diuretic phase of acute renal failure, delayed hypercalcemia may occur by multiple mechanisms including secondary hyperparathyroidism and elevated 1, 25(OH) D levels. Myocellular calcium phosphate complexes begin to dissolve and result in marked hypercalcemia which has been reported in the past (10, 11).
Case report
A 60-year-old Asian male with past medical history of diabetes mellitus and hypertension was admitted to the hospital for profound weakness one week following the initiation of simvastatin 80 mg daily due to coronary artery disease requiring stent placement. His post cardiac catheterization period was complicated with contrast induced nephropathy.
One week later, he developed progressively worsening weakness in proximal upper and lower extremity, inability to raise his head and dysphagia. Simvastatin was discontinued at this point.
He became oliguric and emergent hemodialysis was initiated. Electromyograpy showed diffuse myopathy. Left shoulder MRI showed increased T2 signal involving almost all muscles of the shoulder, consistent with myositis.
Right leg muscle biopsy showed findings consistent with statin-associated myofiber lesions, severe myonecrosis, partial deficiency of myophosphorylase and myofiber type 2 atrophy (with no foci of calcium deposits). Electron microscopy revealed mitochondrial degeneration.
His muscle weakness improved but he remained oliguric and hemodialysis was continued.
One month later, he developed nausea, vomiting and abdominal pain. His calcium level was 13.7 mg/dL peaking at 14.1 mg/dL. Creatinine was 2.5 mg/dL. EKG was unremarkable for any acute changes. There was no history of any vitamin D or calcium supplements intake. Evaluation for humoral hypercalcemia of malignancy, sarcoidosis, and thyroid abnormality was negative. He had no history of exposure to tuberculosis.
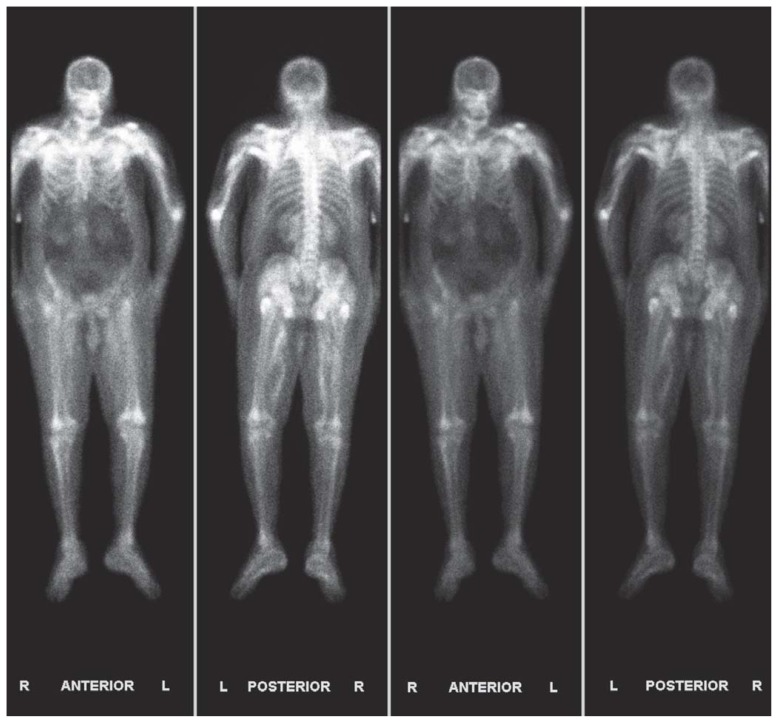
Two doses of calcitonin and one dose of zoledronic acid were given. Whole body bone scintigraphy showed diffuse uptake of technetium-99m MDP in soft tissues and muscles of the chest wall, shoulders, proximal upper and lower extremities, pelvis and hips (Figure 1). Hemodialysis was continued and normalization of his calcium to 8.2 mg/dL was achieved eight days later.
Figure 1.
Whole body bone scintigraphy showed diffuse uptake of technetium-99m MDP in soft tissues and muscles of the chest wall, shoulders, proximal upper and lower extremities, pelvis and hips.
Discussion
Calcium is a cation which is tightly regulated under normal conditions by Na+/Ca+2 exchanger and Ca+2 ATPase pump. In muscle fibers, calcium is stored primarily in sarcoplasmic reticulum.
Intracellular ionized calcium concentration is 10,000 times lower than the extracellular ionized calcium concentration (1, 2). Derangement of calcium metabolism is frequently associated with rhabdomyolysis and especially when induced by statins.
Statins are the preferred and most prescribed drugs for treatment of hypercholesterolemia and thereby primary and secondary prevention of atherosclerotic cardiovascular disease. Our patient was of Asian descent and had contrast induced nephropathy which is proposed risk factors for statin induced muscle injury (1, 2). Simvastatin is the most common statin medication associated with rhabdomyolysis (3).
Hypocalcaemia during the oliguric phase of acute renal failure is due to multiple underlying mechanisms: inhibition of kidney 1, alpha hydroxylase which results in impaired production of 1,25(OH)2D (2);hyperphosphatemia leading to precipitation of extensive calcium phosphate deposits in damaged muscles (5) and skeletal resistance to parathyroid hormone (5, 6).
Prior to the widespread application of the intact PTH assay to renal failure, the hypercalcemia of the diuretic phase following rhabdomyolysis was thought to be the result of the persisting increase in PTH in reaction to the marked hyperphosphatemia during the oliguric phase. The finding of suppressed iPTH during the diuretic phase in this patient is consistent with flux of calcium back into the systemic circulation as serum phosphate levels are cleared by diuresis (5). Hypercalcemia due to elevated 1, 25-(OH) 2D levels (7, 8) and secondary hyperparathyroidism (9)has been reported. Calcium deposition in injured muscles is marked in patients with acute renal failure induced by rhabdomyolysis than those induced by other causes (8).Immobilization induces bone calcium resorption which in combination with impaired renal excretion in rhabdomyolysis with renal failure can also contribute to hypercalcemia late in the course. Serial techitium-99m MDP scans show intake of calcium by the injured muscle cells during hypocalcaemia followed by release of calcium from those cells during the recovery phase (10). There has been histological evidence of active calcium phosphate deposits dissolution on muscle biopsy (11). Hypercalcemia occurring after immobilization with normal to low PTH levels and low 1,25(OH)2D levels in patients with renal failure (12) has been demonstrated.
So far the exact mechanism of statin induced rhabdomyolysis is not clear. It appears to be multifactorial. Deficiencies of synthetic products of the HMG (3-hydroxy-3-methylglutaryl) CoA reductase pathway have been implicated as possible mechanisms. Abnormal membrane integrity secondary to cholesterol deficiency, abnormal mitochondrial respiratory function secondary to coenzyme Q10 deficiency and abnormality in cell signaling cascades involving Rho, Ras and Rap-1a, apoptosis secondary to prenylated protein deficiency are the proposed mechanisms (13, 14).
Simvastatin has been shown to increasing cytosolic calcium by direct diffusion through sarcolemma and impairing membrane integrity due to prenylated protein deficiency which is a class effect. In addition, it induced altered mitochondrial function and thereby mitochondrial calcium efflux through the Na+/Ca2+ exchanger or permeability transient pore which leads to large calcium release from sarcoplasmic reticulum (13, 14). This along with defective oxidative metabolism in the setting of rhabdomyolysis leads to Ca+2 ATPase pump dysfunction due to ATP depletion.
Our patient had Type 2 fiber atrophy with mitochondrial degeneration on muscle biopsy. Also the type of muscle fiber affected is based on its metabolic nature. Type 1 muscle fibers are predominantly oxidative in nature where as Type 2 fibers are glycolytic in nature. Early involvement of mitochondria in selective glycolytic muscle fiber necrosis following inhibition of the enzyme HMG-CoA reductase has been shown in rats (15).
Pathogenesis of delayed hypercalcemia in our patient could be due to calcium mobilization from the muscles and interstitium along with immobilization due to weakness. Appropriately suppressed intact PTH and 1, 25(OH) 2D rule out secondary hyperparathyroidism and elevated 1, 25(OH) 2D as the etiology of delayed hypercalcemia.
Conclusion
Myocellular calcium phosphate deposits seen on whole body bone scintigraphy along with suppressed intact PTH and 1,25-(OH)2D levels indicate that the hypercalcemia was likely the result of dissolution of calcium phosphate complexes in injured muscles or their interstitium. The patient’s profound weakness associated with myopathy likely resulted in a component of immobilization hypercalcemia as well. Moreover, our patient was still on hemodialysis. It is important for health care providers to understand this particular mechanism of delayed severe hypercalcemia in order to treat such patients in an appropriate and timely manner.
Summary points
Delayed hypercalcemia during the recovery phase of renal function is not always due to secondary hyperparathyroidism and elevated 1,25(OH)2D levels.
Mobilization and dissolution of calcium phosphate deposits in the injured muscle sites lead to delayed hypercalcemia as illustrated in our patient.
Bone scintigraphy is the imaging of choice to demonstrate the calcium phosphate deposits in the body.
Recognition of this mechanism is important for appropriate follow up and treatment of hypercalcemia to prevent life threatening complications.
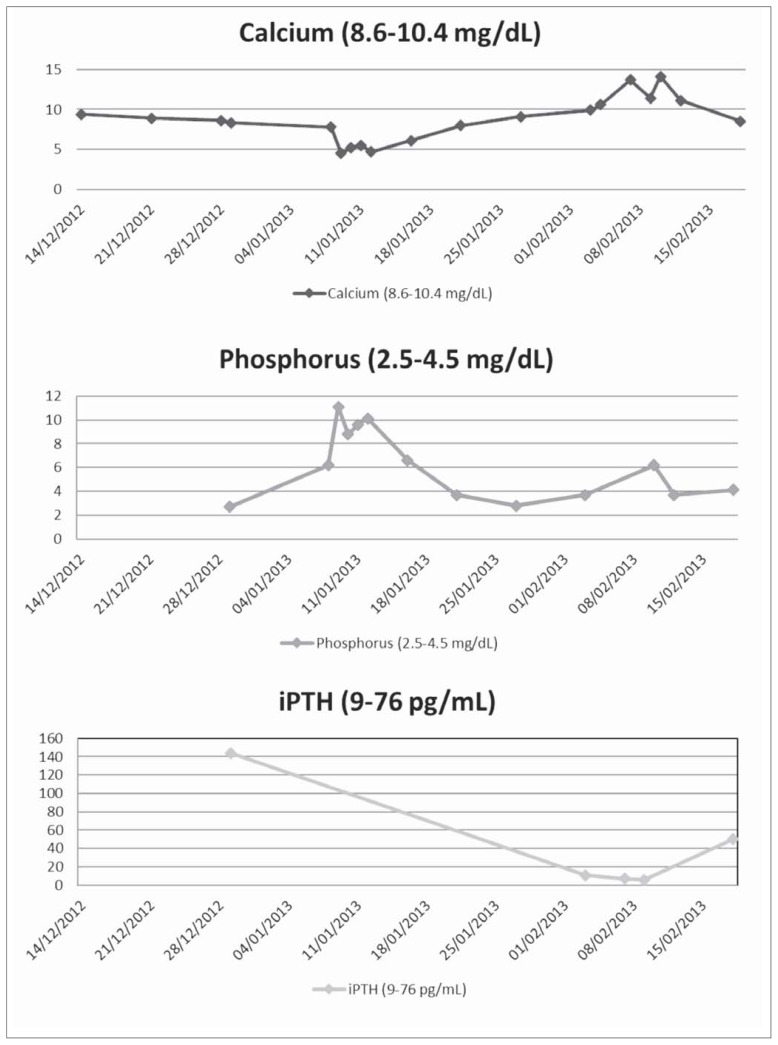
Figure 2.
Graphical representation of Calcium, Phosphorus and intact PTH during the course of illness.
Table 1.
Laboratory data.
| Normal range | on admission | 1 week later | |
|---|---|---|---|
| BUN | 6–23 mg/dl | 47 | 47 |
| Creatinine | 0.5–1.2 mg/dl | 2.4 | 2.1 |
| Potassium | 3.5–5.5 mEq/L | 6.2 | 6.5 |
| CPK | 25–210 U/L | 153 | 426,270 |
| Calcium | 8.6–10.4 mg/dL | 8.6 | 5.5 |
| Phosphorus | 2.5–4.5 mg/dL | 2.7 | 7.6 |
| Intact PTH | 9–76 pg/mL | 144 | --- |
| 25(OH)D | 25–80 ng/mL | 20 | --- |
| Ionized Ca | 4.6–5.3 mg/dL | --- | 3.0 |
| AST | 10–40 U/L | --- | 4489 |
| ALT | 7–56 U/L | --- | 667 |
| Urine myoglobin | Negative | --- | Positive |
Table 2.
Labs 1 month later.
| Normal Range | 1 month later | |
|---|---|---|
| Calcium | 8.6–10.4 mg/dL | 13.7 to 14.1 |
| Phosphorus | 2.5–4.5 mg/dL | 2.6 |
| Intact PTH | 9–76 pg/mL | 7 |
| 1,25(OH)2D | 18–64 pg/mL | <8 |
| Ionized Calcium | 4.6–5.3 mg/dL | 7.3 |
| Alkaline Phosphatase | 25–100 U/L | 261 |
| TSH | 0.35–5.5 mIU/L | 6.89 |
| Free T4 | 0.9–1.8 ng/dL | 1.38 |
Footnotes
Financial disclosure and conflict of interest
None to disclose by any of the Authors.
References
- 1.Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90–100. doi: 10.1016/j.ejim.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Graziani G, Calvetta A, Cucchiari D, et al. Life-threatening hypercalcemia in patients with rhabdomyolysis-induced oliguric acute renal failure. J Nephrol. 2011;24:128–31. doi: 10.5301/jn.2010.5794. [DOI] [PubMed] [Google Scholar]
- 3.Mendes P, Robles PG, Mathur S. Statin induced rhabdomyolysis, A comprehensive review of case reports. Physiotherapy Canada. 2014 Spring;66(2):124–132. doi: 10.3138/ptc.2012-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson MH, Clark JA, Glass LM, et al. Statin safety: an appraisal from the adverse event reporting system. Am J Cardiol. 2006;97(8A):32C–43C. doi: 10.1016/j.amjcard.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Shrestha SM, Berry JL, Davies M, et al. Biphasic hypercalcemia in severe rhabdomyolysis: serial analysis of PTH and vitamin D metabolites. A case report and literature review. Am J Kidney Dis. 2004;43(3):e31–35. doi: 10.1053/j.ajkd.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 6.Sperling LS, Tumlin JA. Case report: delayed hypercalcemia after rhabdomyolysis-induced acute renal failure. Am J Med Sci. 1996;311(4):186–8. doi: 10.1097/00000441-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Llach F, Felsenfeld AJ, Haussler MR. The pathophysiology of altered calcium metabolism in rhabdomyolysis-induced acute renal failure. N Engl J Med. 1981;305:117–23. doi: 10.1056/NEJM198107163050301. [DOI] [PubMed] [Google Scholar]
- 8.Akmal M, Bishop JE, Telfer N, Norman AW, Massry SG. Hypocalcemia and hypercalcemia in patients with rhabdomyolysis with and without acute renal failure. J Clin Endocrinol Metab. 1986;63:137–42. doi: 10.1210/jcem-63-1-137. [DOI] [PubMed] [Google Scholar]
- 9.Tavill AS, Evanson JM, Baker SB, Hewitt V. Idiopathic paroxysmal myoglobinuria with acute renal failure and hypercalcemia. N Engl J Med. 1964;271:283–7. doi: 10.1056/NEJM196408062710603. [DOI] [PubMed] [Google Scholar]
- 10.Lane JT, Boudreau RJ, Kinlaw WB. Disappearance of muscular calcium deposits during resolution of prolonged rhabdomyolysis-induced hypercalcemia. Am J Med. 1990;89:523–5. doi: 10.1016/0002-9343(90)90385-q. [DOI] [PubMed] [Google Scholar]
- 11.Hadjis T, Grieff M, Lockhat D, Kaye M. Calcium metabolism in acute renal failure due to rhabdomyolysis. Clin Nephrol. 1993;39:22–7. [PubMed] [Google Scholar]
- 12.Prince RL, Eilman JA, Simpson RW. Hypercalcemia in association with renal failure: The role of immobilization. Aust NZ Med. 1983;13:8–10. doi: 10.1111/j.1445-5994.1983.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 13.Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006 May;119(5):400–9. doi: 10.1016/j.amjmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Sirvent P, Mercier J, Vassort G, Lacampagne A. Simvastatin triggers mitochondria-induced Ca. Biochem Biophys Res Commun. 2005;329:1067–1075. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 15.Westwood FR, Bigley A, Randall K, Marsden AM, Scott RC. Statin-induced muscle necrosis in the rat: distribution, development, and fibre selectivity. Toxicol Pathol. 2005;33(2):246–257. doi: 10.1080/01926230590908213. [DOI] [PubMed] [Google Scholar]