Abstract
Burkitt lymphoma (BL) is a malignant disease, which is frequently found in areas with holoendemic Plasmodium falciparum malaria. We have previously found that the VAR2CSA protein is present on malaria-infected erythrocytes and facilitates a highly specific binding to the placenta. OfCS is absent from other non-malignant tissues and thus VAR2CSA generally facilitates parasite sequestration and accumulation in pregnant women. In this study, we show that the specific receptor for VAR2CSA, the oncofetal chondroitin sulfate (ofCS), is likewise present in BL tissue and cell lines. We therefore explored whether ofCS in BL could act as anchor-site for VAR2CSA-expressing infected erythrocytes. In contrast to the placenta, we found no evidence of in vivo sequestering of infected erythrocytes in the BL tissue. Furthermore, we found VAR2CSA specific antibody titers in children with endemic BL to be lower than in control children from the same malaria endemic region. The abundant presence of ofCS in BL tissue and the absence of ofCS in non-malignant tissue, encouraged us to examine whether recombinant VAR2CSA could be used to target BL. We confirmed the binding of VAR2CSA to BL-derived cells and showed that a VAR2CSA drug conjugate efficiently killed the BL-derived cell lines in vitro. These results identify ofCS as a novel therapeutic BL target and highlight how VAR2CSA could be used as a tool for the discovery of novel approaches for directing BL therapy.
Keywords: Burkitt Lymphoma, VAR2CSA, Cancer, malaria, Plasmodium falciparum, Chondroitin Sulfate A, CSA, chondroitin sulfate proteoglycan
Introduction
Burkitt lymphoma (BL) is a highly aggressive B-cell lymphoma, which affects children between the ages of 0-15 years (1, 2). The disease is associated with Epstein-Barr virus (EBV) infection of the malignant cells and is most frequently found in areas with holoendemic malaria. Most people become infected with EBV during their lifetime without any apparent consequences (3). However, latent EBV-infected B-cells can be reactivated in settings of congenital or acquired immunosuppression leading to the establishment and polyclonal outgrowth that result in BL (4-8). The high BL incidence in areas with holoendemic Plasmodium falciparum suggests a possible link between malaria infection and BL in equatorial Africa, where the disease is termed endemic BL (eBL) (9-11). Epidemiological studies have suggested that the risk of eBL is highest during childhood when exposure to malaria peaks as measured by antibody levels and that the risk diminishes as children acquire antimalarial immunity and thereby gain immunological control of the blood stage parasites (12-15).
We have recently shown that the placental syncytiotrophoblast and the majority of all cancers produce a distinct oncofetal type of chondroitin sulfate (ofCS), which is absent from normal cells and tissues (16). CS consists of long glycosaminoglycan (GAG) chains that can be attached to a diverse panel of protein cores, which together are termed chondroitin sulfate proteoglycans (CSPGs) (17). The CS chains bind several ligands and receptors, and CSPGs thereby act as major factors in tumor growth and invasion (18-23). EBV-positive BL-derived cell lines have recently been shown to express a broad repertoire of CSPGs (24). However, the functional significance of CSPG expression in BL is still largely unknown.
In the placenta, P. falciparum malaria-infected erythrocytes exploit the presence of ofCS to anchor themselves to the endothelial lining thereby avoiding splenic clearance during blood circulation (25). The accumulation of malaria parasites in the placenta causes pregnancy-associated malaria with severe consequences for the woman and the developing foetus (26). Placental cytoadhesion of infected erythrocytes to ofCS is mediated by the VAR2CSA protein expressed by malaria parasites on the surface of infected erythrocytes (27). Protection against pregnancy-associated malaria is acquired as a function of parity and correlated to elevated levels of anti-VAR2CSA IgG (28-31). Despite the highly specific adhesion in the placenta, studies have shown that naturally acquired antibodies against VAR2CSA are not restricted to pregnant women as low-titers can also be detected in a small subset of men and children (32-34). Thus, low levels of VAR2CSA-espressing parasites may be present in the bloodstream of malaria-infected children but rapidly cleared by the spleen, as they cannot adhere to the vascular endothelial lining.
In the light of our novel finding that most cancers produce the ofCS to which VAR2CSA specifically adheres, we initially hypothesized that the presence of Burkitt lymphoma may provide a niche for the accumulation of VAR2CSA-expressing parasites in malaria infected non-pregnant individuals. In this study, we show that BL cell lines as well as patient biopsies present the specific ofCS recognized by the recombinant VAR2CSA protein (rVAR2). We further evaluated if VAR2CSA-expressing infected erythrocytes could bind to the tumor site, and finally, whether a rVAR2-drug conjugate could kill ofCS-expressing BL-derived cells in vitro. These data highlight how the molecular biology of malaria can potentially be used in the development of novel tools for the treatment of BL.
Materials and methods
Immunohistochemistry of BL tissue for placenta-like chondroitin sulfate
Formalin-fixed and paraffin–embedded (FFPE) BL tumor tissues (n=16) obtained in the EMBLEM Study (http://emblem.cancer.gov/) were retrieved in tissue microarray format (TMA) to study rVAR2 binding and sequestration of infected erythrocytes in BL tissue.
Four microns sectioned paraffin-embedded 1mm tissue cores were stained with 500 pM recombinant DBL1-ID2a (rVAR2) without antigen retrieval (35). After a secondary incubation with 700× diluted monoclonal anti-V5 mouse antibody followed by an incubation with UMAP anti-mouse HRP, rVAR2 binding was detected by staining with the ChromoMap DAB Kit on a Ventana Discovery platform. Samples were counterstained with hematoxylin.
Additional paraffin–embedded tissue samples were stained with hematoxylin and eosin (H&E) and Giemsa and evaluated for the presence of P. falciparum infected erythrocytes by light microscopy.
Immunofluorescence staining of ofCS in BL
For immunofluorescence staining, confirmed BL tissues from the SEER Residual Tissue Repository from the United States was used. FFPE tissue was deparafinized and re-hydrated with a series of xylene and ethanol solutions. After blocking, the tissue was incubated with 50 nM rVAR2, followed by incubation with anti-V5-FITC antibody (Invitrogen). The specificity of rVAR2 binding was tested by outcompeting protein binding with 400 μg/ml CSA (Sigma). An additional specificity control was performed by treating the tissue with 0.5 U/ml Chondroitinase ABC (ChABC, Sigma) for 1 hour at 37 °C, before blocking. Samples were counterstained with DAPI.
Imaging was performed on a Nikon C1 confocal microscope with a 60× oil objective. Each image is a composite of the following channels: blue (nuclei), green (ofCS), red (auto-fluorescence of the tissue).
In vitro cultures
The P. falciparum FCR3 parasites were cultured in RPMI 1640 (Sigma) supplemented with 0.125 μg/ml Albumax II (Invitrogen), 5% hematocrit of human blood (group 0+), and 2% normal human serum, as previously described (36). Infected erythrocytes were maintained in a mixture of 2% O2 and 5% CO2 in nitrogen at 37°C. VAR2CSA expression was ensured by panning on BeWo cells (37) and genotype was validated using a nested polymerase chain reaction (PCR) with GLURP and MSP-2 primers.
The sporadic BL derived cell lines (38) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2mM L-glutamine, 100U/mL penicillin and 100μg/mL streptomycin. Cell lines were passaged to a cell density of 2×105/ml on a regular basis and maintained at 5% CO2 at 37°C.
BL cells and FCR3 parasites were regularly tested for mycoplasma and shown free of contamination (Lonza).
Flow cytometry of BL-derived cell lines
Binding of rVAR2 to sporadic BL-derived cell lines was evaluated using 2×106 cells/ml incubated with his-tagged 400-1.56 nM recombinant DBL1-ID2a (rVAR2) in PBS containing 2% FBS for 30 minutes at 4°C. After a secondary incubation using a 500 times diluted anti-penta his alexa fluor 488 antibody (Qiagen), binding was analyzed using a FC500 flow cytometer (Beckmann Coulter). To test specificity of rVAR2 binding to the BL-derived cell lines, cells were stained with rVAR2 (200nM) in the presence of increasing amounts (2-fold dilution, from 200-0 μg/ml) of CSA (Sigma) and HS (Sigma). After a secondary incubation using a 500 times diluted anti-V5-FITC (Invitrogen), binding was analyzed using a FC500 flow cytometer (Beckmann Coulter). An additional specificity control was done by pre-incubating the cells with 0.5 U/ml ChAC (Sigma) for 1 hour at 37 °C. Lastly, a domain of the VAR2CSA protein known not to be involved in the binding (rDBL4) was also used as a control.
Reverse phase-HPLC (RP-HPLC)
5 million cells were frozen in liquid nitrogen, and prepared for HPLC analysis. Samples were digested with Pronase and Benzonase in two separate consecutive overnight incubations at 37 °C. After digestion, the samples were loaded onto a dietilaminoetil (DEAE) column and eluted with 20mM NaOAc, 1M NaCl, pH 5.0. The eluted samples were concentrated by speed-vacuum and precipitated with ethanol saturated with NaOAc (1:3, v:v) overnight at 4°C. Dried samples were treated with 40 mU of ChABC overnight at 37°C and freeze-dried for 2 hours. For AMAC-labeling, 5 ul of 0.1M AMAC were added to the samples for 15 minutes at room temperature. After this incubation, 5 ul of 1M NaCNBH3 were added for 3 hours at 45°C. The samples were then freeze-dried overnight, precipitated with acetone and injected onto the RP-HPLC.
Binding of infected erythrocytes to BL cells
FCR3 parasites were enriched for late trophozoite and schizont stages in a strong magnetic field (Miltenyi Biotec). 5×106 infected erythrocytes and 1×106 BL cells were incubated in complement medium for 2 hours at room temperature. Smears of the suspension were prepared on glass slides and fixed with 100% methanol. Samples were stained with 10% Giemsa stain and analyzed using a Nikon TE 2000-E confocal microscope with 60× oil immersion objective lens (DIC).
Study population
Residual serum was obtained from the historical BL studies conducted by investigators at the National Cancer Institute in Ghana (39, 40). Briefly, children (2-15 years) with histologically or cytologically confirmed BL were enrolled from Korle-Bu Teaching Hospital, Accra, Ghana from 1965 through 1994. The cases were mostly from southern areas of Ghana, where malaria transmission intensity was moderate to high (41). The controls were apparently healthy children of a similar age and sex to the cases enrolled from the same neighborhood, except for a few children referred to the Korle-Bu Teaching Hospital with suspected eBL but found to have benign or a non-lymphoid malignancy (42). These controls were representative of the cases in terms of referral patterns. Verbal consent (or assent for children aged 8 years or older) was obtained prior to enrolment. Age, sex, and date of enrolment were recorded on structured forms. Malaria status was evaluated by light microscopy at the time of the study and children who were positive were treated. A pre-treatment venous blood sample was drawn and separated into aliquots, which were stored at -70°C at the U.S. National Cancer Institute repository until testing. Previously unthawed samples or aliquots of samples that were thawed once only were used in this study. Ethical approval for the current study was obtained from the Office of Human Subject Research at the US National Institutes of Health. No identifying subject data were used in this study.
Sera from P. falciparum exposed individuals from Tanzania (20 children and 21 multigravidae women) and Danish controls naïve to malaria (n=9) were included as technical controls. Antibodies to VAR2CSA antigens were measured using two methods: an enzyme-linked immunosorbent assay (ELISA), and flow cytometry.
ELISA
Nunc MaxiSorpH plates were coated overnight at 4°C with 1 μg/ml recombinant full-length VAR2CSA, produced in baculovirus-transfected insect cells as previously described (43). Following 1-hour incubation in blocking buffer, serum samples were added to the wells in duplicates and incubated for 1 hour at room temperature. After washing the plate, 3000 times diluted horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-human IgG (DAKO, Denmark) was added to the wells and incubated for 1 hour. After incubation with o-phenylenediamine substrate, the reaction was stopped by 2.5 M H2SO4 and the binding was measured at 490 nm using an ELISA plate reader (VersaMax Molecular Devices).
Flow cytometry on infected erythrocytes
The reactivity of the test serum with infected erythrocytes was measured using flow cytometry with VAR2CSA-expressing FCR3 parasites enriched for late trophozoite and schizont stages in a strong magnetic field (Miltenyi Biotec). 2×105 infected erythrocytes were stained with ethidium bromide (Invitrogen) and incubated with 20 times diluted serum, which had been depleted for unspecific binding by pre-incubation with uninfected erythrocytes. After a secondary incubation using a 200 times dilution of goat anti-human IgG FITC antibody (FI3000, Vector), binding was analyzed using a FC500 flow cytometer (Beckmann Coulter).
Statistical methods
Laboratory staff were blinded to the status (case or control) of the samples to minimize bias, and 27% of samples (n=59) were embedded as replicate vials within the same and also in different test plates to measure within- and between-plate assay reproducibility (supplementary figure 1). Within- and between-plate coefficients of variation (CVs), calculated by dividing assay mean by the assay standard deviation of replicate samples, were used to evaluate reliability of IgG assays. Assay results with CVs equal or higher than 30% failed and were excluded from the analyses. Serum reactivity values from ELISA and flow cytometry assays were analyzed with and without log-transformation. The Student's t-test was used when analyzed as continuous variables.
rVAR2 based pull downs of proteoglycans from sporadic BL cell lysates
The pull downs were performed as in (44). In short, BL-derived cells were washed in cold PBS and treated with cell lysis buffer (150mM NaCl, 50 mM tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl), 2.5mM MgCl2, 1mM ethylenediaminetetraacetic acid (EDTA), 1% 3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate (CHAPS), and protease and phosphatase inhibitor cocktails (Roche)). For the analysis of secreted proteoglycans, cells were grown for 3 days in complete media, which were collected for the analysis. Cell lysates of 10×106 cells or media were pre-cleared for unspecific binding by incubation with MyOneC1 Streptavidin Dynabeads (Invitrogen) for 1 hour at 4°C. 5μg biotinylated rVAR2 was added to the sample preparation and incubated overnight at 4°C, after which the rVAR2-bound lysate was incubated with the streptavidin-coated dynabeads for 1 hour at 4°C. Following four gentle washes using a magnet, rVAR2-bound proteoglycans were eluded by boiling the samples in non-reducing LDS buffer (Invitrogen). The proteins were reduced in 1mM 1,4-Dithiothreitol (DTT) and alkylated with 5.5mM Iodoacetamide. The samples were run 1 cm into a Bis-Tris gel and stained with Coomassie Blue. The protein fractions were cut out of the gel, washed and in-gel digested with trypsin. The resulting peptides were captured and washed using a C18 resin in stage-tipping. The peptides were sequenced using a Fusion Orbitrap Mass Spectrometer. Sample analysis and hit verification were performed using the MaxQuant software. All samples were verified against control samples of cell lysates without rVAR2. For the Ingenuity Pathways Analysis (IPA), the verified hits were analyzed using the Ingenuity Pathways Analysis software (Qiagen) with focus on cellular function and disease.
Cytotoxicity
BL-derived cells were cultured in a 96-well plate with 25.000 cells per well in 100ul RPMI supplemented with 10% FBS. rVAR2 conjugated to the microtubule inhibitor (VDC) was prepared in RPMI supplemented with 10% FBS in 3 fold dilutions. Unconjugated cytotoxin 4 was included as a control. Furthermore, ofCS specificity of VDC and toxin were tested by CSA co-incubations. 25ul of 5 times the dose response was spiked into each well in triplicate. Plates were incubated at 37°C, 5% CO2 for 72 hours, following which viability was quantified by a 10 minutes CellTiter-glo reagent incubation and SpectraMax measurement.
Results
Burkitt lymphoma displays oncofetal CS
To investigate whether BL tissue display ofCS, we examined 16 endemic BL tumor biopsies using immunohistochemistry. Binding of rVAR2 was detected in 62.5% (10/16) of the BL cores. Positive samples showed moderate to strong staining in the extracellular matrix of the lymphoma lesions while the staining of the BL cell membrane and cytoplasm was generally low (Figure 1). The same staining pattern was seen when rVAR2 binding was tested using immunofluorescence (Figure 2). To confirm the specificity of rVAR2 for ofCS, rVAR2 binding was outcompeted with soluble CSA. Additionally, a BL tumor biopsy was treated with ChABC before rVAR2 staining. ChABC treatment and CSA inhibition efficiently removed rVAR2 staining (Figure 2). Taken together, this illustrates that rVAR2 is binding specifically to ofCS in BL.
Figure 1. rVAR2 staining of Burkitt lymphoma tissue.
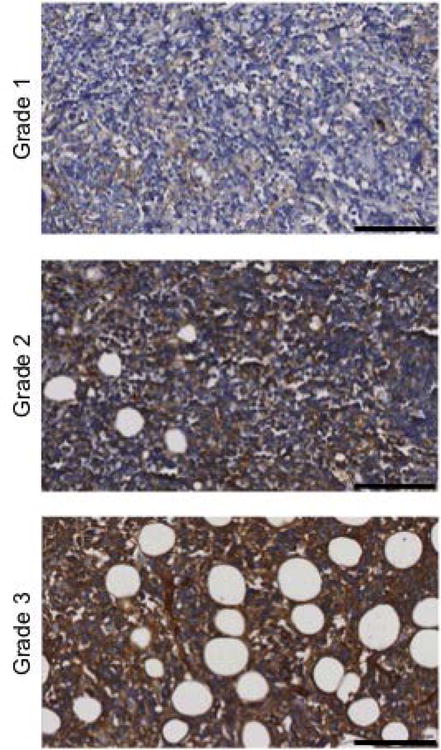
Presence of ofCS in BL tissues was analyzed by rVAR2 staining using the Ventana Discovery Platform. rVAR2 staining intensity was scored as negative (grade 0), weak (grade 1), moderate (grade 2), or strong (grade 3). Pictures are representatives of differentially scored tissues of grade 1-3. Staining intensities of ≥2 were considerate positive. The scale bars represent 100μm.
Figure 2. rVAR2 binding to Burkitt lymphoma tissue is specific to ofCS.
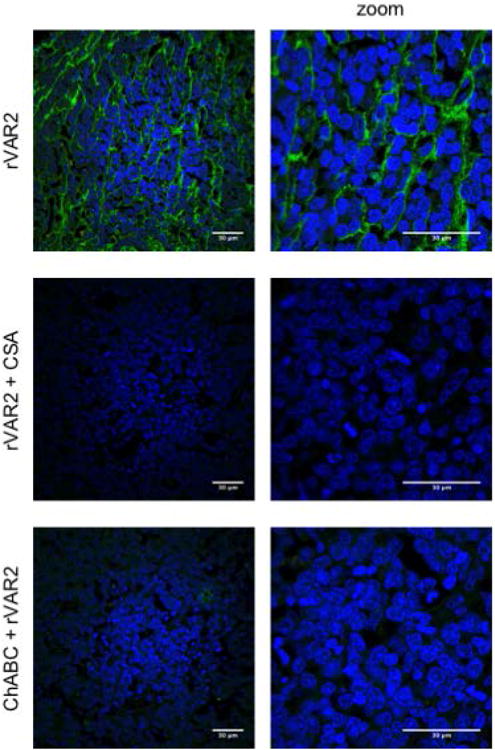
Specificity of rVAR2 binding to ofCS in BL tissue was tested by immunofluorescence. Tissue was incubated with rVAR2 either after treatment with Chondroitinase ABC (ChABC), or co-incubated with soluble CSA. Tissue was counterstained with DAPI (blue). Panel on the right represents zoomed pictures (2.5×). The scale bars represent 30μm.
The rVAR2 BL stainings are consistent with our previous observation that ofCS is often found as a part of the cancer-modified CSPGs in the tumor stroma (16). The results indicate that BL tissue, if accessible, could in theory provide a binding niche for adhesion of VAR2CSA-expressing infected erythrocytes. Therefore, we proceeded to examine the BL tissue cores for the presence of P. falciparum infected erythrocytes in the vascularized parts of the cancer tissue. None of the BL tissue cores, including five from subjects who were confirmed as malaria positive at the time of tissue sampling, showed sequestration of infected erythrocytes using H&E and Giemsa stains (data not shown). Thus, this study did not provide evidence for sequestration of infected erythrocytes within the BL tissue despite the presence of ofCS.
Anti-VAR2CSA IgG levels are slightly decreased in serum samples from Burkitt lymphoma patients
In general, children from malaria endemic areas have low serum levels of anti-VAR2CSA IgG, since VAR2CSA-expressing parasites have no medium for sequestration and therefore are effectively cleared early in the infection (34). In the case that VAR2CSA-expressing parasites can utilize BL tissue for sequestration in a similar way as the placenta during pregnancy-associated malaria infections, it would be expected that BL patients acquired an elevated IgG response to VAR2CSA.
To investigate this theory, we tested serum anti-VAR2CSA antibody levels in samples from Ghanaian BL patients and endemic controls (Supplementary table 1). Two assays were employed. In the first we used ELISA to test the IgG response towards recombinant full-length VAR2CSA protein. The anti-VAR2CSA IgG serum levels were not higher in the BL patients; in fact, IgG reactivity towards immobilized full-length VAR2CSA was slightly but significantly lower in the BL patients than the controls (p=0.012) (Figure 3A). Furthermore, in the analysis of anti-VAR2CSA IgG levels by age, the BL cases showed consistently lower antibody means at all ages examined (Supplementary figure 2). To assure that this was not an observation solely related to the recombinant protein, we tested the IgG levels against native VAR2CSA expressed on P. falciparum infected erythrocytes by flow cytometry. In flow cytometry, serum samples likewise showed a slightly decreased VAR2CSA-reactivity in the BL samples compared to serum from controls (p=0.02) (Figure 3B). The two analytical methods showed a significant positive correlation (r= 0.29, p < 0.0001) using the Bonferonni-adjusted pairwise correlation. However, the effect size was small and there was considerable overlap between the groups. Control serum samples of Tanzanian children furthermore showed lower levels of anti-VAR2CSA IgG levels than what was found for the BL patients (Supplementary figure 3). Thus, the slight decrease in anti-VAR2CSA IgG levels for BL serum samples might not be sufficient to have an actual biological impact on malaria manifestations.
Figure 3. Serum samples of Burkitt lymphoma patients show a decrease in VAR2CSA-specific IgG.
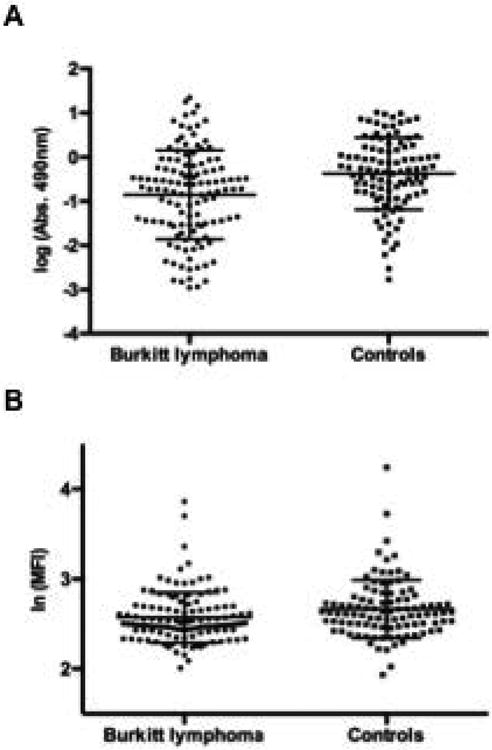
(A) IgG reactivity to recombinant VAR2CSA in human serum samples from BL patients (n=121) and endemic controls (n=101). Reactivity was detected using anti-human IgG HRP and measured by ELISA. Bars represent means with standard deviations, (B) IgG binding to VAR2CSA-expressing infected erythrocytes in human serum samples from BL patients and endemic controls. Geometric mean fluorescence intensity (MFI) was assessed by flow cytometry using anti-human IgG FITC. Bars represent means with standard deviations.
VAR2CSA binds Burkitt lymphoma cell lines
To test whether BL-derived cells display ofCS we tested eight cell lines, including three that were EBV-positive, for binding of rVAR2 in flow cytometry. All cell lines were bound by rVAR2 to some degree, however, the intensity of staining varied considerably (Figure 4a). rVAR2 did not bind to non-malignant B-cells (data not shown). We found no correlation between rVAR2 staining intensity and EBV status of the cell lines. For example, the EBV-negative EW36 and EBV-positive KK124 cell lines both showed intense staining with rVAR2, while the EBV-positive LP120 and the EBV-negative cell lines CA46 and BML895 cell lines showed intermediate and low staining, respectively. We confirmed the specificity of rVAR2 binding to ofCS in a competitive assay by adding soluble CSA to the rVAR2-incubated cells (Figure 4b).
Figure 4. VAR2CSA binds to ofCS on Burkitt lymphoma cell lines.
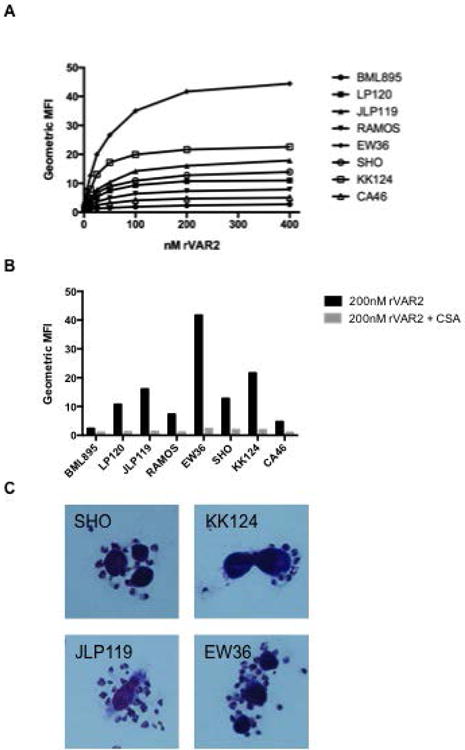
(A) rVAR2 binding to eight different BL cell lines. Signal-to-noise ration of the geometric mean fluorescence intensity (MFI) was tested using an anti-penta his alexa 488 antibody and flow cytometry. The graph is a representative of three independent experiments. (B) rVAR2 binding to BL cell lines with or without the co-incubation of 400 μg/ml soluble CSA in flow cytometry. (C) Binding of VAR2CSA-expressing P. falciparum infected erythrocytes to the BL cell lines as shown by microscopy after Giemsa staining.
To further demonstrate the ofCS specificity of rVAR2 binding to BL-derived cell lines, 4 of the cell lines were stained with 200 nM rVAR2 in the presence of increasing amounts of CSA and HS. The data showed that co-incubation of rVAR2 with soluble CSA inhibits the binding of rVAR2 to the cells, even at low concentrations. Contrary to this, HS did not compete for rVAR2 binding to ofCS even at 200 μg/ml (Figure 5a-e). Pre-treating the cells with ChAC efficiently removed binding of rVAR2 to the cells (Figure 5e). A domain of the VAR2CSA protein that is known to not to be involved in the binding of VAR2CSA to ofCS (rDBL4) was used as a protein control, and showed no binding to the cells (Figure 5e). Taken together, these data demonstrate that rVAR2 specifically binds to ofCS in the BL-derived cell lines.
Figure 5. VAR2CSA specifically binds to ofCS on Burkitt lymphoma cell lines.
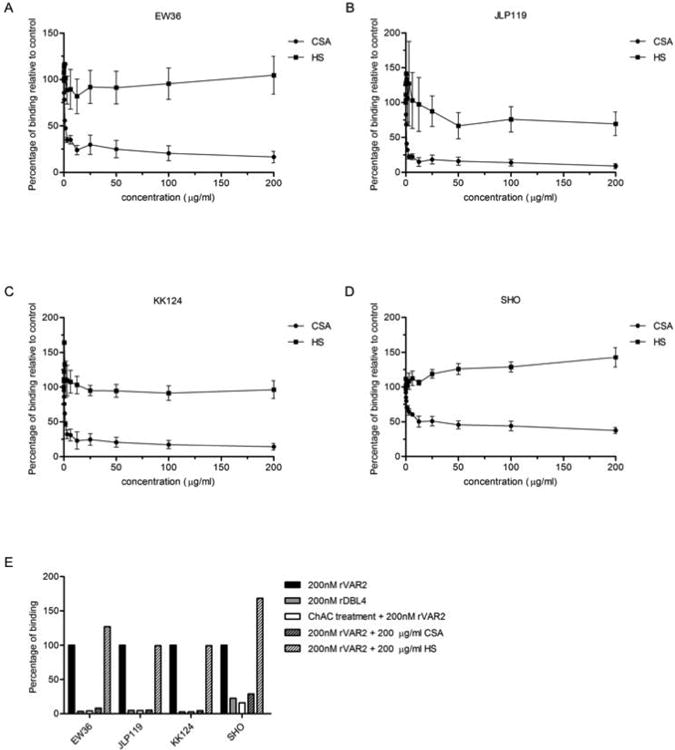
(A) Specificity of rVAR2 binding to the EW36 cell line was tested in flow cytometry by co-incubating 200 nM rVAR2 with a 2-fold dilution of CSA and HS. Percentage of binding towards the control is shown. Data shown as mean (two or three independent experiments) ± SEM. (B) Same as (A) for the JLP119 cell line. (C) Same as (A) for the KK124 cell line. (D) Same as (A) for the SHO cell line. (E) Specificity of rVAR2 binding to four different BL cell lines. The graph shows percentage of binding of rVAR2 to the different cell lines. A control with a domain of the protein not involved in binding to ofCS is shown (rDBL4). Additionally, specificity controls after ChAC treatment and with co-incubation of rVAR2 with 200 ug/ml CSA and HS are also shown.
The individual BL cell lines showed varying staining with rVAR2. To see if this difference was due to differences in the cells overall CS content or an observable difference in CS structure, the 4 BL cell lines were subjected to CS structural analysis using AMAC labelling and HPLC. The cell lines all showed different CS concentrations and structure (Table 1), however there was no apparent link between this and the rVAR2 binding.
Table 1. HPLC analysis of the CS structure in selected BL cell lines.
Disaccharide analysis by AMAC labelling and HPLC of CS isolated from the cells. The disaccharides were identified by comparison to commercially available standards. The table shows the CS concentration as well as the percentage 4-O- or 6-O-sulfation for the different cell lines.
| CS [pmol/million cells] | % 4-O-sulfation | % 6-O-sulfation | |
|---|---|---|---|
| Cell line | |||
| EW36 | 160.0 | 35.0 | 6.4 |
|
| |||
| JLP119 | 312.7 | 43.9 | 5.8 |
|
| |||
| KK124 | 267.3 | 59.2 | 4.5 |
|
| |||
| SHO | 152.4 | 24.8 | 10.2 |
Finally, we showed that VAR2CSA-expressing infected erythrocytes bound to the BL-derived cell lines (Figure 4c), suggesting that the native VAR2CSA is capable of mediating infected erythrocytes binding to ofCS on BL cells.
rVAR2 binds to CSPGs displayed and secreted by the BL-derived cancer cell lines
CSPGs are implicated in cancer through their involvement in proliferation, migration, apoptosis, adhesion, and invasion. As CSPGs are also found in non-malignant tissues, their oncogenic potential has been ascribed to a general up regulation as well as modifications of the CS structure. ofCS is a common modification on many proteoglycans in cancer (16). To gain insight into which proteoglycans carry ofCS in BL, we performed rVAR2-based pull-downs of CSPGs from the cell lysates of four BL cell lines as well as from their conditioned media. The samples were incubated with biotinylated rVAR2, potential rVAR2 binding proteins were isolated using streptavidin-coated magnetic beads and identified by Mass Spectrometry (Table 2). All cell lines expressed multiple rVAR2-bound CSPGs in line with a previous study that showed that malignant lymphoid cells in general express more types of cell-associated proteoglycans than normal lymphocytes (45). Among the hits were several well-known cancer-associated CSPGs such as CD44, CD47, CD74, and APP (46-50). Furthermore, several VAR2-bound CSPGs including serglycin and biglycan were identified in the media of the cell cultures. The heterogeneity between the cell lines in what proteoglycans are modified with an of-CS chain could be the reason the differences in the level of rVAR2 binding to the cell lines (Figure 4)
Table 2. Proteomic analysis of the rVAR2-based CSPG pull-downs.
Pull-downs of cell lysates and secretions from four different BL-derived cell lines were analyzed by Mass Spectrometry as in (44). Protein hits shown to carry CS are listed by unique peptide count. Moreover, the peptide covered sequence percentages are listed as well as the signal ratio to the control sample (non-rVAR2 coated beads). NA: ratio not applicable due to lack of signal in the control sample.
| Protein Name | Gene | Peptide Count | Seq. coverage (%) | Ratio to Neg. |
|---|---|---|---|---|
| EW36 | ||||
|
| ||||
| Leukocyte surface antigen CD47 | CD47 | 3 | 8,7 | NA |
| Amyloid-like protein 2 | APLP2 | 3 | 5,5 | NA |
| Syndecan-1 | SDC1 | 2 | 11,6 | NA |
| Versican core protein | VCAN | 1 | 0,3 | NA |
| EW36 medium | ||||
|
| ||||
| Serglycin | SRGN | 2 | 17,1 | NA |
| Biglycan | BGN | 2 | 5,7 | NA |
| Amyloid-like protein 2 | APLP2 | 2 | 3,8 | NA |
| Neuropilin-1 | NRP1 | 1 | 1,8 | NA |
| Amyloid beta A4 protein | APP | 1 | 1,6 | NA |
| CD44 antigen | CD44 | 1 | 1,1 | NA |
| SHO | ||||
|
| ||||
| HLA class II histocompatibility antigen gamma chain | CD74 | 7 | 23,3 | 32,9 |
| Amyloid-like protein 2 | APLP2 | 4 | 6,2 | NA |
| Leukocyte surface antigen CD47 | CD47 | 3 | 8,7 | 42,1 |
| Amyloid beta A4 protein | APP | 3 | 4,3 | NA |
| Neuropilin-1 | NRP1 | 1 | 1,8 | NA |
| SHO medium | ||||
|
| ||||
| Biglycan | BGN | 4 | 13,3 | NA |
| Amyloid-like protein 2 | APLP2 | 4 | 5,5 | 5,8 |
| Neuropilin-1 | NRP1 | 1 | 1,8 | NA |
| Basement membrane-specific heparan sulfate proteoglycan core protein | HSPG2 | 1 | 0,5 | NA |
| JLP119 | ||||
|
| ||||
| HLA class II histocompatibility antigen gamma chain | CD74 | 7 | 23,3 | 14,7 |
| Serglycin | SRGN | 2 | 17,1 | NA |
| JLP199 medium | ||||
|
| ||||
| Biglycan | BGN | 2 | 8,7 | NA |
| Agrin | AGRN | 1 | 0,6 | NA |
| KK124 | ||||
|
| ||||
| HLA class II histocompatibility antigen gamma chain | CD74 | 7 | 23,3 | NA |
| Leukocyte surface antigen CD47 | CD47 | 3 | 8,7 | NA |
| Serglycin | SRGN | 2 | 17,1 | NA |
| CD44 antigen | CD44 | 2 | 3,2 | NA |
| Syndecan-1 | SDC1 | 1 | 5,5 | NA |
| Sushi repeat-containing protein SRPX | SRPX | 1 | 2,2 | NA |
| Versican core protein | VCAN | 1 | 0,3 | NA |
| KK124 medium | ||||
|
| ||||
| Sushi repeat-containing protein SRPX | SRPX | 2 | 4,1 | NA |
| Biglycan | BGN | 1 | 2,7 | NA |
| Versican core protein | VCAN | 1 | 0,3 | NA |
As we have recently described, rVAR2 ofCS specific pull down from cell lysates revealed a number of proteins that included ofCS substituted CSPGs as well as several known CSPG interaction partners, such as integrins (44). To attempt to get an overview over the connection between all the hits as well as how they relate to cellular functions and disease processes in BL, the identified hits were subjected to Ingenuity Pathway Analysis (IPA). This shown an involvement of ofCS in several immune pathways, and in processes related to the development of immunological diseases (Supplementary figure 4).
Toxin-conjugated rVAR2 kills BL-derived cell lines
The high degree of rVAR2 staining in many BL tissues as well as the expression of multiple rVAR2-defined CSPGs on each cell line indicate that rVAR2 can be used to target a broad panel of BL and that binding would not be confined to the expression of a single tumor marker. We have recently shown that rVAR2 coupled to a cytotoxic microtubule inhibitor was effective in specific killing of a number of cancer cells in vitro and in vivo (16). Thus, we tested whether the rVAR2 drug conjugate (VDC) could mediate killing of BL derived cell lines in vitro. VDC effectively killed the BL-derived cell lines with EC50 values of 0.4nM for EW36, 1.9nM for KK124, 2.2nM for SHO and 7.3nM for JLP119 (Figure 6). Killing efficacy aligned with the flow cytometry staining intensity of cell lines, where EW36 and KK124 also displayed the strongest rVAR2 binding. The effect of the rVAR2-induced cytotoxicity must however also be expected to depend on the individual CSPGs targeted on the cell lines. The rVAR2 induced cytotoxicity was shown to be ofCS specific as the cytotoxicity could be inhibited by a co-incubation with soluble CSA. These data highlight the potential of using rVAR2 for therapeutic targeting of BL.
Figure 6. VDC-induced cytotoxicity of BL-derived cells.
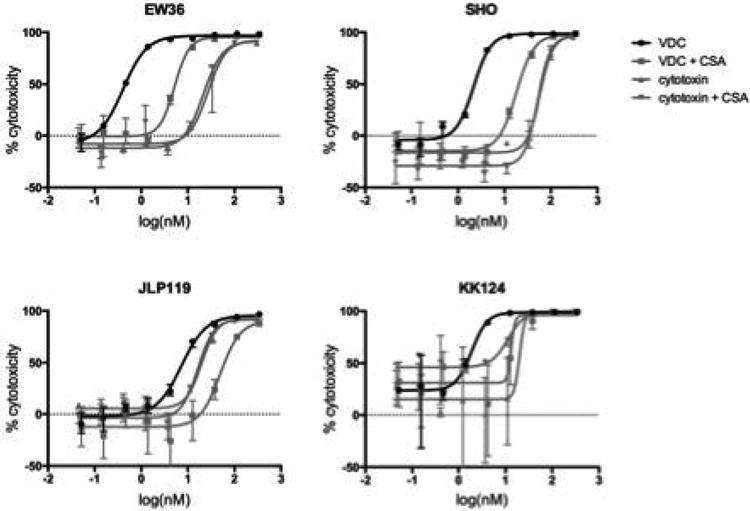
The cytotoxic effect of treatment with VDC was analyzed using CellTiter-glo reagent after 3 days incubation. The EC50 values were estimated at 0.4nM (EW36), 2.2nM (SHO), 7.2nM (JLP119) and 1.9nM (KK124). Unconjugated cytotoxin was included as a control. The ofCS specificity of VDC binding was demonstrated by an addition of 400ug/ml CSA.
Discussion
Although the link between P. falciparum and BL was made more than 50 years ago, this is the first study to explore the expression of malaria-associated receptors in BL and whether such expression can be exploited to target BL therapy. Taken together, our results suggest that studies of evolutionarily refined pathogen malaria proteins in BL may represent novel opportunities for discovery of ways to target BL therapy.
When we set out to explore the interplay between malaria infections and cancer, we initially hypothesized that a vascularized tumor could possibly act like a placenta by creating a niche for ofCS binding of VAR2CSA-expressing parasites. Experiments confirmed the presence of ofCS in many BL tissues and we showed that rVAR2 binds BL-derived cell lines. Furthermore, we showed that infected erythrocytes expressing VAR2CSA are capable of binding to BL cell lines. However, when we tested the theoretical possibility that VAR2CSA-presenting infected erythrocytes adhere to the vascularized parts of the BL in vivo, we did not find any indications of parasite sequestration in BL tissue from malaria-infected patients. While the small sample size of our study should be considered, one explanation could be that the ofCS expressing compartments of BL are inaccessible to the infected erythrocytes due to the high rate of BL cell proliferation and relatively low vascularity of BL with tendency to early necrosis. An additional explanation could be a difference in the flow rate of the peripheral vascularized tissues compared to the placenta. Furthermore, the placenta is a partly immune restricted region, which might contribute to pregnancy-associated malaria.
Had VAR2CSA-expressing infected erythrocytes been adhering in the BL tissue, we would expect higher levels of anti-VAR2CSA antibodies in the BL patients as is seen in patients suffering from pregnancy-associated malaria. Our results are internally consistent as serum samples of BL patients showed slightly reduced anti-VAR2CSA IgG levels compared to the endemic controls. In general, the anti-VAR2CSA levels were much lower in the BL patients as compared to women who have been exposed to pregnancy-associated malaria infection. Since the BL ECM shows abundant ofCS, it is possible that the tumor tissues leak or actively secret ofCS, which could coat circulating VAR2CSA-expressing infected erythrocytes and inhibit their sequestration, thereby promoting more rapid clearance from circulation by macrophages and the spleen. If so, secreted ofCS could hinder tissue sequestration and reduce the low-level boosting of anti-VAR2CSA antibodies. In line with this, we showed that BL-derived cell lines secrete ofCS carrying CSPGs such as serglycin and biglycan, which could be bound by rVAR2. Serglycin is one of the dominant proteoglycans of hematopoietic cells and was accordingly identified by rVAR2 on JLP119 and KK124 as well as secreted from EW36. Furthermore, rVAR2-based pull-downs identified biglycan in the media from all of the four tested cell lines. Biglycan has been found to be a serum biomarker elevated in esophageal adenocarcinoma, but to our knowledge the protein has not yet been explored in BL (51).
Combined, the lack of evidence for binding of infected erythrocytes in BL tissue and our serological data do not support the notion that cancer tissue provides a niche for binding of VAR2CSA expressing parasites in children with BL. However, we were only able to investigate a limited number of BL needle biopsies from patients with a positive malaria blood slide. Thus, we cannot reject that sequestration might occur in some patients. Furthermore, we cannot rule out that there are sub-microscopic infections of limited clinical and immunological impact in BL tissues.
Inaccessibility to the BL tissue or secretion of ofCS into the blood stream could potentially impair the manifestation of low levels of VAR2CSA-expressing infected erythrocytes. However, it is possible that the rVAR2 protein could reach ofCS in the tumor and thus be used to deliver a toxic payload to BL patients. Though toxic chemotherapy of BL has been successful, BL remains a serious cause of cancer deaths in low-income countries. Therefore, alternative targeting strategies for BL should be explored. The abundant presence of ofCS in BL tissue, combined with the previously shown absence of ofCS in non-malignant tissue (16), suggested that rVAR2 can target BL cells and that a rVAR2 drug conjugate (VDC) could be used in the treatment of BL. Furthermore, our data indicate that VDC could potentially be used to target a broader panel of BL malignancies, as it would not be restricted to the expression of a single tumor target. We found rVAR2 to bind to multiple surface-exposed CSPGs including CD47, CD74, and APP, which all have an oncogenic role. Elevated levels of CD47 have been shown to be important for avoiding macrophage clearance and promoting dissemination of non-Hodgkin lymphoma including BL (48). Likewise, CD74 plays an important role for malignant B-cell proliferation and survival (52). Studies in BL xenografted mice have indicated that anti-CD74 antibodies antagonistic or toxin-coupled can be applied for treatment of BL (49, 53). Finally, APP is highly expressed in EBV-negative BL and seems to be implicated in the rapid proliferation rate of these cells (50). The specific targeting of CSPGs implicated in the oncogenic potential of BL cells was supported by in vitro cytotoxicity. VDC induced BL cytotoxicity with low EC50 values, demonstrating its effective killing of BL cells in vitro.
In summary, we have shown that rVAR2 binds to BL-derived cell lines as well as patient tissue and that the VDC facilitates potent killing of BL cells in vitro. There is a certain irony in the fact that a protein derived from a pathogen such as malaria long suspected to play a role in the pathogenesis of eBL can be modified and used as a potential therapeutic agent against the malignancy.
Supplementary Material
Supplementary figure 1. Reliability of anti-VAR2CSA IgG assays. 59 serum samples were embedded as replicate vials to evaluate within- and between-plate assay reproducibility. Mean coefficients of variation (CVs), calculated by dividing assay mean by the assay standard deviation of replicate samples, were estimated to 14.9% for ELISA (A) and 6.8% for flow cytometry (B). Assay results with CVs equal or higher than 30% failed and were excluded from the analyses.
Supplementary figure 2. Graph showing anti-VAR2CSA antibody levels in BL cases and endemic controls by age as measured by ELISA. Lines represent the age-specific means and 95% CI of the mean.
Supplementary figure 3. IgG reactivity to recombinant VAR2CSA. Mean anti-VAR2CSA IgG levels were tested in serum samples of BL patients (-0.85 ±1.01, n=121), endemic controls (-0.37 ±0.82, n=101), Danish controls (-2.11 ±0.36, n=9), Tanzanian children (-1.45 ±1.02, n=20), and Tanzanian women (1.20 ±0.14, n=21). Reactivity was detected using anti-human IgG HRP and measured by ELISA. Bars represent means with standard deviations.
Supplementary figure 4. Analysis of the pull-down hits using the Ingenuity Pathways Analysis (IPA) software. Protein hits from four different cell lines were analyzed. IPA heat map analysis of the canonical pathways (left) and diseases and biological functions (right) enriched in proteins after rVAR2 were analyzed.
Supplementary table 1. Characteristics of subjects included in the study to evaluate anti-VAR2CSA IgG levels in children with or without BL from Ghana
Novelty and Impact.
It was recently shown that diverse types of tumors present an oncofetal type of chondroitin sulfate. In the placenta, the malaria parasite utilizes this molecule for sequestration. We address the key question if cancer tissue in malaria endemic regions can likewise function as a reservoir for placental type malaria parasite and demonstrate that this is not the case. However, Burkitt lymphoma does express the oncofetal chondroitin sulfate, which can be targeted by malaria derived recombinant VAR2CSA, and utilized to deliver a toxic payload.
Acknowledgments
We would like to thank Anne Corfitz for excellent technical assistance. This work was supported by the Harboe Foundation, European Research Councils under the MalOnco program, the Danish Innovation Foundation and the EUREKA program as well as the Danish Cancer Society. The work was also received financial support from the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, and National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services; Grant numbers: N01-CO-12400 and AI-001040.
Abbreviations
- BL
Burkitt Lymphoma
- CSA
Chondroitin Sulfate A
- CSPG
Chondroitin Sulfate Proteoglycan
- eBL
Endemic Burkitt Lymphoma
- EBV
Epstein Barr Virus
- ofCS
Oncofetal Chondroitin Sulfate
- GAG
Glycosaminoglycan
- rVAR2
Recombinant VAR2CSA
Footnotes
Authorship: Contribution: M.Ø.A., T.M.C., A.S., and S.M.M. conceived the idea, designed the research and wrote the manuscript. F.N., J.N., R.J.B., and S.J.R. performed the fieldwork. M.Ø.A., M.A.P., T.M.C., C.P., H.Z.O., C.S., J.R., V.F., and M.A.N. performed the experiments. K.B., G.T., S.T.P, L.A., T.G.T., and M.D. provided useful reagents and helpful discussions.
Conflict-of-interest disclosure: A.S., T.M.C., T.G.T., M.A.N. and M.D. have together with University of Copenhagen submitted a patent application on oncofetal CS as a cancer target. M.Ø.A is co-founder and shareholder of VAR2 pharmaceuticals where the patent is located.
Besides the authors declare no competing financial interests.
References
- 1.Linet MS, Brown LM, Mbulaiteye SM, Check D, Ostroumova E, Landgren A, et al. International long-term trends and recent patterns in the incidence of leukemias and lymphomas among children and adolescents ages 0-19 years. Int J Cancer. 2015 doi: 10.1002/ijc.29924. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe ES, Pittaluga S. Aggressive B-cell lymphomas: a review of new and old entities in the WHO classification. Hematology Am Soc Hematol Educ Program. 2011;2011:506–14. doi: 10.1182/asheducation-2011.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempkes B, Robertson ES. Epstein-Barr virus latency: current and future perspectives. Curr Opin Virol. 2015;14:138–44. doi: 10.1016/j.coviro.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chene A, Donati D, Orem J, Mbidde ER, Kironde F, Wahlgren M, et al. Endemic Burkitt's lymphoma as a polymicrobial disease: new insights on the interaction between Plasmodium falciparum and Epstein-Barr virus. Semin Cancer Biol. 2009;19(6):411–20. doi: 10.1016/j.semcancer.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Donati D, Mok B, Chene A, Xu H, Thangarajh M, Glas R, et al. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol. 2006;177(5):3035–44. doi: 10.4049/jimmunol.177.5.3035. [DOI] [PubMed] [Google Scholar]
- 6.Simone O, Bejarano MT, Pierce SK, Antonaci S, Wahlgren M, Troye-Blomberg M, et al. TLRs innate immunereceptors and Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) CIDR1alpha-driven human polyclonal B-cell activation. Acta Trop. 2011;119(2-3):144–50. doi: 10.1016/j.actatropica.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183(3):2176–82. doi: 10.4049/jimmunol.0901297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam KM, Syed N, Whittle H, Crawford DH. Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet. 1991;337(8746):876–8. doi: 10.1016/0140-6736(91)90203-2. [DOI] [PubMed] [Google Scholar]
- 9.Haddow AJ. An Improved Map for the Study of Burkitt's Lymphoma Syndrome in Africa. East Afr Med J. 1963;40:429–32. [PubMed] [Google Scholar]
- 10.Burkitt DP. Etiology of Burkitt's lymphoma--an alternative hypothesis to a vectored virus. J Natl Cancer Inst. 1969;42(1):19–28. [PubMed] [Google Scholar]
- 11.Wright DH. What is Burkitt's lymphoma and when is it endemic? Blood. 1999;93(2):758. [PubMed] [Google Scholar]
- 12.Aka P, Vila MC, Jariwala A, Nkrumah F, Emmanuel B, Yagi M, et al. Endemic Burkitt lymphoma is associated with strength and diversity of Plasmodium falciparum malaria stage-specific antigen antibody response. Blood. 2013;122(5):629–35. doi: 10.1182/blood-2012-12-475665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carpenter LM, Newton R, Casabonne D, Ziegler J, Mbulaiteye S, Mbidde E, et al. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer. 2008;122(6):1319–23. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- 14.Mutalima N, Molyneux E, Jaffe H, Kamiza S, Borgstein E, Mkandawire N, et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS One. 2008;3(6):e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guech-Ongey M, Yagi M, Palacpac NM, Emmanuel B, Talisuna AO, Bhatia K, et al. Antibodies reactive to Plasmodium falciparum serine repeat antigen in children with Burkitt lymphoma from Ghana. Int J Cancer. 2012;130(8):1908–14. doi: 10.1002/ijc.26203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salanti A, Clausen TM, Agerbaek MO, Al Nakouzi N, Dahlback M, Oo HZ, et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell. 2015;28(4):500–14. doi: 10.1016/j.ccell.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830(10):4719–33. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Wade A, Robinson AE, Engler JR, Petritsch C, James CD, Phillips JJ. Proteoglycans and their roles in brain cancer. The FEBS journal. 2013;280(10):2399–417. doi: 10.1111/febs.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooney CA, Jousheghany F, Yao-Borengasser A, Phanavanh B, Gomes T, Kieber-Emmons AM, et al. Chondroitin sulfates play a major role in breast cancer metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast cancer research : BCR. 2011;13(3):R58. doi: 10.1186/bcr2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Wang Y, Yu L, Sakakura K, Visus C, Schwab JH, et al. CSPG4 in cancer: multiple roles. Current molecular medicine. 2010;10(4):419–29. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- 21.Wegrowski Y, Maquart FX. Chondroitin sulfate proteoglycans in tumor progression. Advances in pharmacology. 2006;53:297–321. doi: 10.1016/S1054-3589(05)53014-X. [DOI] [PubMed] [Google Scholar]
- 22.Theocharis AD, Tsolakis I, Tzanakakis GN, Karamanos NK. Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv Pharmacol. 2006;53:281–95. doi: 10.1016/S1054-3589(05)53013-8. [DOI] [PubMed] [Google Scholar]
- 23.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, et al. Glycosaminoglycans: key players in cancer cell biology and treatment. The FEBS journal. 2012;279(7):1177–97. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsidulko AY, Matskova L, Astakhova LA, Ernberg I, Grigorieva EV. Proteoglycan expression correlates with the phenotype of malignant and non-malignant EBV-positive B-cell lines. Oncotarget. 2015;6(41):43529–39. doi: 10.18632/oncotarget.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272(5267):1502–4. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 26.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 27.Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. The Journal of experimental medicine. 2004;200(9):1197–203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricke CH, Staalsoe T, Koram K, Akanmori BD, Riley EM, Theander TG, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165(6):3309–16. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 29.Staalsoe T, Megnekou R, Fievet N, Ricke CH, Zornig HD, Leke R, et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. J Infect Dis. 2001;184(5):618–26. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 30.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363(9405):283–9. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 31.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71(11):6620–3. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babakhanyan A, Leke RG, Salanti A, Bobbili N, Gwanmesia P, Leke RJ, et al. The antibody response of pregnant Cameroonian women to VAR2CSA ID1-ID2a, a small recombinant protein containing the CSA-binding site. PLoS One. 2014;9(2):e88173. doi: 10.1371/journal.pone.0088173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gnidehou S, Doritchamou J, Arango EM, Cabrera A, Arroyo MI, Kain KC, et al. Functional antibodies against VAR2CSA in nonpregnant populations from colombia exposed to Plasmodium falciparum and Plasmodium vivax. Infect Immun. 2014;82(6):2565–73. doi: 10.1128/IAI.01594-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beeson JG, Ndungu F, Persson KE, Chesson JM, Kelly GL, Uyoga S, et al. Antibodies among men and children to placental-binding Plasmodium falciparum-infected erythrocytes that express var2csa. Am J Trop Med Hyg. 2007;77(1):22–8. [PubMed] [Google Scholar]
- 35.Baik S, Mbaziira M, Williams M, Ogwang MD, Kinyera T, Emmanuel B, et al. A case-control study of Burkitt lymphoma in East Africa: are local health facilities an appropriate source of representative controls? Infect Agent Cancer. 2012;7(1):5. doi: 10.1186/1750-9378-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen MA, Resende M, Alifrangis M, Turner L, Hviid L, Theander TG, et al. Plasmodium falciparum: VAR2CSA expressed during pregnancy-associated malaria is partially resistant to proteolytic cleavage by trypsin. Exp Parasitol. 2007;117(1):1–8. doi: 10.1016/j.exppara.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Haase RN, Megnekou R, Lundquist M, Ofori MF, Hviid L, Staalsoe T. Plasmodium falciparum parasites expressing pregnancy-specific variant surface antigens adhere strongly to the choriocarcinoma cell line BeWo. Infect Immun. 2006;74(5):3035–8. doi: 10.1128/IAI.74.5.3035-3038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain A, Gutierrez MI, Timson G, Siraj AK, Deambrogi C, Al-Rasheed M, et al. Frequent silencing of fragile histidine triad gene (FHIT) in Burkitt's lymphoma is associated with aberrant hypermethylation. Genes Chromosomes Cancer. 2004;41(4):321–9. doi: 10.1002/gcc.20099. [DOI] [PubMed] [Google Scholar]
- 39.Nkrumah FK, Sulzer AJ, Maddison SE. Serum immunoglobulin levels and malaria antibodies in Burkitt's lymphoma. Trans R Soc Trop Med Hyg. 1979;73(1):91–5. doi: 10.1016/0035-9203(79)90137-8. [DOI] [PubMed] [Google Scholar]
- 40.Nkrumah FK, Olweny CL. Clinical features of Burkitt's lymphoma: the African experience. 60. IARC Sci Publ; 1985. pp. 87–95. [PubMed] [Google Scholar]
- 41.Gardiner C, Biggar RJ, Collins WE, Nkrumah FK. Malaria in urban and rural areas of southern Ghana: a survey of parasitaemia, antibodies, and antimalarial practices. Bull World Health Organ. 1984;62(4):607–13. [PMC free article] [PubMed] [Google Scholar]
- 42.Nkrumah FK, Perkins IV. Sickle cell trait, hemoglobin C trait, and Burkitt's lymphoma. Am J Trop Med Hyg. 1976;25(4):633–6. doi: 10.4269/ajtmh.1976.25.633. [DOI] [PubMed] [Google Scholar]
- 43.Khunrae P, Dahlback M, Nielsen MA, Andersen G, Ditlev SB, Resende M, et al. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. J Mol Biol. 2010;397(3):826–34. doi: 10.1016/j.jmb.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clausen TM, Pereira MA, Al Nakouzi N, Oo HZ, Agerbaek MO, Lee S, et al. Oncofetal Chondroitin Sulfate Glycosaminoglycans are Key Players in Integrin Signaling and Tumor Cell Motility. Molecular cancer research : MCR. 2016 doi: 10.1158/1541-7786.MCR-16-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fadnes B, Husebekk A, Svineng G, Rekdal O, Yanagishita M, Kolset SO, et al. The proteoglycan repertoire of lymphoid cells. Glycoconj J. 2012;29(7):513–23. doi: 10.1007/s10719-012-9427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12(10):1167–74. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 47.Gao Y, Foster R, Yang X, Feng Y, Shen JK, Mankin HJ, et al. Up-regulation of CD44 in the development of metastasis, recurrence and drug resistance of ovarian cancer. Oncotarget. 2015;6(11):9313–26. doi: 10.18632/oncotarget.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118(18):4890–901. doi: 10.1182/blood-2011-02-338020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, et al. CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res. 2007;13(18 Pt 2):5556s–63s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 50.Maesako Y, Uchiyama T, Ohno H. Comparison of gene expression profiles of lymphoma cell lines from transformed follicular lymphoma, Burkitt's lymphoma and de novo diffuse large B-cell lymphoma. Cancer Sci. 2003;94(9):774–81. doi: 10.1111/j.1349-7006.2003.tb01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidi AH, Gopalakrishnan V, Kasi PM, Zeng X, Malhotra U, Balasubramanian J, et al. Evaluation of a 4-protein serum biomarker panel-biglycan, annexin-A6, myeloperoxidase, and protein S100-A9 (B-AMP)-for the detection of esophageal adenocarcinoma. Cancer. 2014;120(24):3902–13. doi: 10.1002/cncr.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Starlets D, Gore Y, Binsky I, Haran M, Harpaz N, Shvidel L, et al. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood. 2006;107(12):4807–16. doi: 10.1182/blood-2005-11-4334. [DOI] [PubMed] [Google Scholar]
- 53.Chang CH, Sapra P, Vanama SS, Hansen HJ, Horak ID, Goldenberg DM. Effective therapy of human lymphoma xenografts with a novel recombinant ribonuclease/anti-CD74 humanized IgG4 antibody immunotoxin. Blood. 2005;106(13):4308–14. doi: 10.1182/blood-2005-03-1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1. Reliability of anti-VAR2CSA IgG assays. 59 serum samples were embedded as replicate vials to evaluate within- and between-plate assay reproducibility. Mean coefficients of variation (CVs), calculated by dividing assay mean by the assay standard deviation of replicate samples, were estimated to 14.9% for ELISA (A) and 6.8% for flow cytometry (B). Assay results with CVs equal or higher than 30% failed and were excluded from the analyses.
Supplementary figure 2. Graph showing anti-VAR2CSA antibody levels in BL cases and endemic controls by age as measured by ELISA. Lines represent the age-specific means and 95% CI of the mean.
Supplementary figure 3. IgG reactivity to recombinant VAR2CSA. Mean anti-VAR2CSA IgG levels were tested in serum samples of BL patients (-0.85 ±1.01, n=121), endemic controls (-0.37 ±0.82, n=101), Danish controls (-2.11 ±0.36, n=9), Tanzanian children (-1.45 ±1.02, n=20), and Tanzanian women (1.20 ±0.14, n=21). Reactivity was detected using anti-human IgG HRP and measured by ELISA. Bars represent means with standard deviations.
Supplementary figure 4. Analysis of the pull-down hits using the Ingenuity Pathways Analysis (IPA) software. Protein hits from four different cell lines were analyzed. IPA heat map analysis of the canonical pathways (left) and diseases and biological functions (right) enriched in proteins after rVAR2 were analyzed.
Supplementary table 1. Characteristics of subjects included in the study to evaluate anti-VAR2CSA IgG levels in children with or without BL from Ghana


