Abstract
Aggressive induction chemotherapy followed by autologous haematopoietic stem cell transplant (auto-HCT) is effective for younger patients with mantle cell lymphoma (MCL). However, the optimal induction regimen is widely debated. The Southwesterm Oncology Group S1106 trial was designed to assess rituximab plushyperCVAD/MTX/ARAC (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate) (RH) versus rituximab plus bendamustine (RB) in a randomized phase II trial to select a pre-transplant induction regimen for future development. Patients had previously untreated stage III, IV, or bulky stage II MCL and received either 4 cycles of RH or 6 cycles of RB, followed by auto-HCT. Fifty-three of a planned 160 patients were accrued; an unacceptably high mobilization failure rate (29%) on the RH arm prompted premature study closure. The estimated 2-year progression-free survival (PFS) was 81% vs. 82% and overall survival (OS) was 87% vs. 88% for RB and RH, respectively. RH is not an ideal platform for future multi-centre transplant trials in MCL. RB achieved a 2-year PFS of 81% and a 78% MRD negative rate. Premature closure of the study limited the sample size and the precision of PFS estimates and MRD rates. However, RB can achieve a deep remission and could be a platform for future trials in MCL.
Keywords: mantle cell, bendamustine, Auto-HCT, MRD, hyperCVAD
Introduction
Mantle cell lymphoma (MCL) is an aggressive B cell non-Hodgkin lymphoma that is considered incurable with conventional therapy. It represents about 4% of lymphomas in the US (The Non Hodgkin’s Lymphoma Classification Project 1997). For younger patients who are fit, the therapeutic strategy has been to use aggressive induction chemotherapy followed by autologous haematopoietic stem cell transplantation (Auto-HCT). The benefit of consolidative Auto-HCT was demonstrated by the European MCL network in a trial that compared consolidative Auto-HCT to maintenance interferon and showed improved progression-free survival (PFS) in the Auto-HCT arm (median of 39 months versus 17 months [P = 0.0108])(Dreyling, et al 2005). Although there are a variety of upfront regimens, the optimal induction chemotherapy has not been defined. The goal of induction chemotherapy has been to achieve a high rate of complete response (CR) while minimizing toxicities.
The R-hyperCVAD (RH) regimen pioneered at MD Anderson Cancer Center contains rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate for 6–8 cycles. It yields a high CR rate of 87% and overall response rate (ORR) of 97% in single institution settings (Romaguera, et al 2005). It has also been tested in the multicentre cooperative group setting by the Southwesterm Oncology Group (SWOG) S0213 trial in patients with previously untreated MCL, resulting in a CR of 55%, ORR of 86% and 3 year PFS of 66% (Bernstein, et al 2013). Single centre studies have demonstrated the feasibility of Auto-HCT after abbreviated courses of RH (Till, et al 2008).
Rummel et al (2005) studied the combination of rituximab plus bendamustine (RB) in patients with untreated MCL and showed an ORR of 75% and CR of 50%, with minor haematological toxicities. This regimen was then compared to RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) in a large randomized non-inferiority trial in Europe, which showed similar ORR rates (93% vs. 91%) and CR rates (40% vs. 30%) to RCHOP but improved median PFS rates (35 months vs. 22 months), fewer haematological toxicities (30% vs. 68%), and fewer infections (37% vs. 50%)(Rummel, et al 2013). These results were confirmed by the BRIGHT study, which showed that RB is non-inferior to RCHOP in terms of CR and had less neutropenia (Flinn, et al 2014). However, those trials did not include patients consolidated with Auto-HCT.
S1106 was designed to assess RH and RB to select an induction regimen followed by Auto-HCT consolidation as a platform for development in future trials. The hypothesis was that either RH or RB would yield a higher 2-year PFS rate, have fewer toxicities, and allow sufficient stem cell mobilization for Auto-HCT consolidation. Besides using radiographic imaging to assess for response, we can further characterize response status by the use of minimal residual disease (MRD) monitoring. Deep remission as defined by MRD status post-induction appears to be a powerful predictor of outcome in both single arm (Liu et al 2012) and randomized settings (Pott et al 2010). In this study, we use next generation sequencing (NGS) to evaluate MRD status as an important biological correlative.
Methods
Patients
Patients in this study (#NCT01412879) were required to have untreated stage III, IV, or bulky stage II MCL. Cyclin D1 (CCND1) positivity was confirmed by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH), and all pathological material underwent central histological review through SWOG. Other inclusion criteria were age ≥ 18 ≤ 65 years, adequate organ function, no prior treatment for MCL, bidimensionally measurable disease >1.5 cm by computed tomography (CT), fluorodeoxyglucose (FDG)-avid disease by positron emission tomography (PET), and a Zubrod performance status score of 0 – 2.
Study Design and Treatment
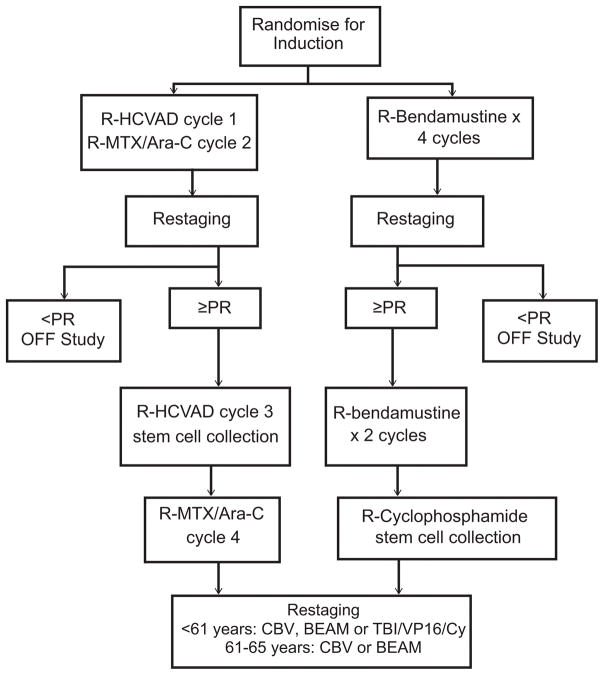
This randomized phase II study was conducted by SWOG through the National Clinical Trials Network (NCTN) adult groups (SWOG/ECOG-ACRIN/Alliance) mechanism. Patients were recruited from January 4, 2012 through June 21, 2013. The study was approved by the Institutional Review Board at each study site, and written informed consent was obtained from all patients prior to any study-specific procedures, per the Declaration of Helsinki. The study was approved and sponsored by the Cancer Therapy Evaluation Program (CTEP). Patients were randomized to 4 cycles of RH or 6 cycles of RB (Fig 1). Randomization was stratified on the MCL International Prognostic Index (MIPI) score (Hoster, et al 2008). The dose of RH and RB were previously described (Bernstein et al 2013; Rummel et al 2005)(Table I). Only patients achieving CR or PR at restaging were eligible to proceed to Auto-HCT consolidation. Patients in the RH arm underwent chemotherapy-based stem cell mobilization after cycle 3 with granulocyte colony-stimulating factor(dose/schedule per institutional standard). Patients in the RB arm underwent stem cell mobilization after cycle 6 (within 8 weeks of last dose of RB) using rituximab (375 mg/m2) plus cyclophosphamide (1.5 mg/m2). Plerixafor or a second mobilization attempt was allowed but not required per protocol. After at least 1.5 × 106 CD34+ cells were collected, patients underwent a second registration and proceeded to the transplant component of planned treatment. For patients between 61 and 65 years old, each site had to select either CBV (carmustine [BCNU]/cyclophosphamide/etoposide) or BEAM (BCNU/etoposide/cytarabine/melphalan) as the sole preparative regimen (Chopra, et al 1993, Reece, et al 1994) for that site. For patients aged less than 61 years, the sites had to select either CBV, BEAM, or total body irradiation/cyclophosphamide/etoposide (Nademanee, et al 1995) as the sole preparative regimen.
Figure 1. Study schema.
Ara-C = cytarabine; BEAM = carmustine [BCNU]/etoposide/cytarabine/melphalan; CBV = carmustine [BCNU], cyclophosphamide, etoposide; Cy, cyclophosphamide; HCVAD = ; HyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate) MTX, = methotrexate; PR = partial response; R = rituximab; TBI = total body irradiation; VP16 = etoposide.
Table I.
Study drugs by dose, days administered, and route.
| Cycle 1 and 3 | Dose | Days | Route |
|---|---|---|---|
| Rituximab | 375 mg/m2 | 1 | IV |
| Cyclophosphamide | 300 mg/m2 | 2–4 | IV |
| Doxorubicin | 16.6 mg/m2 | 5–7 | IV |
| Vincristine | 1.4 mg/m2 (cap 2) | 5 and 12 | IV |
| Dexamethasone | 40 mg | 2–5, 12–15 | IV or PO |
| Cycle 2 and 4 | Dose | Days | Route |
| Rituximab | 375 mg/m2 | 1 | IV |
| Methotrexate | 200 mg/m2 IV over 2 h 800 mg/m2 IV over 22 h |
2 | IV |
| Cytarabine | 3 g/m2 | 3–4 | IV |
| Rituximab | 375 mg/m2 | 1 | IV |
Minimal residual disease (MRD) was assessed at baseline and post-induction from peripheral blood. Genomic tumour DNA was extracted from FPE formalin-fixed paraffin-embedded tissue or bone marrow aspirate mononuclear cells. Polymerase chain reaction (PCR) amplification of IGH VDJ, IGH DJ, and IGK regions was performed followed by next-generation sequencing to determine the tumour clonotype(s) (Adaptive Biotechnologies Corp, South San Francisco, CA)(Faham, et al 2012). DNA from peripheral blood mononuclear cells (PBMC) and plasma was amplified and sequenced to determine lymphoma molecules per million diploid genomes. If either the PBMC or the plasma sample yielded lymphoma clones, then the patient was deemed to be MRD positive.
Study Assessments
Baseline evaluations included documentation of disease-related signs and symptoms, a physical examination, bone marrow biopsy and radiographic studies, including CT of the neck, chest, abdomen and pelvis, and a PET scan. The best clinical response was determined by investigators according to the Revised Response Criteria for Malignant Lymphoma (Cheson, et al 2007). In the RH arm, response was assessed by CT scan after cycles 2 and 4, and a PET scan was done after cycle 4 but prior to Auto-HCT. In the RB arm, CT scans were specified after cycles 4 and 6, and a PET scan was done after cycle 6 but prior to Auto-HCT. After Auto-HCT and after removal from study treatment, long-term follow up assessments (including survival and disease status information) were performed every 3 months for 1 year, every 6 months for 2 year, then annually thereafter until either patient death or 8 years after registration. Patients who discontinued study treatment with stable disease or better had CT scans every 6 months for 3 years and annually thereafter until disease progression.
Safety monitoring included the recording of adverse events and physical examination findings, vital signs, and routine haematology and serum chemistries. Adverse events were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf).
Statistical Analysis
The primary objective of this Phase II design was to select a regimen for further development, and the primary endpoint is estimated 2-year PFS. Recent SWOG experience in this patient population in S0213 (Bernstein, et al 2013) and S0601 (Till, et al 2016) suggested a historical 2-year PFS of 68%. In order to proceed with development, the observed 2-year PFS on the corresponding arm must be at least 75%. Under the original design and sample size, this threshold corresponds not only to a 1-sided 0.05 level test of the null hypothesis that the true 2-year PFS is 68%, but also approximates the outcomes observed with the transplant regimen of the Nordic Lymphoma Group (Geisler, et al 2008).
Interim analyses of stem cell collection data on both treatment arms was conducted by the statistical centre. Either arm would be considered unacceptably toxic if the true stem cell collection failure rate exceeded 10%. If, among the first 20 eligible patients on either arm, more than 4 (20%) were unable to receive transplant because of inadequate stem cell collection, then protocol accrual would be held and consideration would be given to modifications or closure of that arm. These analyses correspond to 1-sided 0.05 level tests of the null hypothesis that the true failure rate is 10% or less.
Secondary endpoints included ORR, CR, OS, toxicities and MRD analysis. The probability of response or a particular toxicity can be estimated to be within at worst 11% (95% confidence interval [CI]) with 80 patients per arm. Any adverse event with at least a 5% probability would be seen at least once (98% chance). This protocol also assessed the association of MRD status at the end of induction therapy, prior to stem cell transplant, with PFS. PFS and OS estimates (with 95% CI) were calculated using Kaplan-Meier methods (Kaplan and Meier 1958). To minimize selection bias, a landmark analysis at 3 months for RH and at 6 months for RB was used for comparing PFS between transplanted and non-transplanted patients. In this analysis, only patients on RH with at least 3 months of follow-up without progression or patients on RB with 6 months of follow-up without progression were included, and the subsequent progression-free time after 3 months of follow-up for RH and after 6 months of follow-up for RB were compared. Four patients on RB who did not receive Auto-HCT were excluded from this landmark analysis, as three patients were censored and one patient progressed within the 6-months landmark time. The estimated 2-year PFS for patients on RB who achieved MRD-negative status at the end of induction was also calculated by Kaplan-Meier methods using landmark analysis at time of specimen collected for MRD testing. Comparisons of response rate and toxicity rate between treatment arms were performed using Fisher’s exact test with two-sided alpha of 0.05. Data as of 10 February 2016 were included in the analyses.
Results
Patients
Patient characteristics are detailed in Table II. This study was closed after 53 patients were accrued: 18 in RH and 35 in RB arms (RH arm closing first). One patient on RH who did not receive any randomized therapy due to the closure of RH arm for safety was excluded from all endpoint analyses. The groups were well-balanced (Table II) with the exception of more females in the RH group. The median age was 57 (range, 33–64) years in the RB arm and 58 (range, 44–66) years in the RH arm. Ninety-one per cent of the patients in the RB arm and 94% of the patients in the RH arm had stage IV disease. Eighty-six per cent of the patients in the RB arm and 82% of the patients in the RH arm had bone marrow involvement. Ninety-one per cent of the patients in the RB arm and 88% of the patients in the RH arm had extra-nodal disease. Lastly, 37% of the patients in the RB arm and 35% of the patients in the RH arm were considered intermediate/high risk by MIPI score. Approximately 20% of the patients in each arm were considered high risk as determined by a Ki67 score > 30%.
Table II.
Patient characteristics
| Characteristics | RH (N=17)* | RB (N=35) | P-value |
|---|---|---|---|
| Age, years | 59 (44–66) | 57 (33–64) | 0.23 |
|
| |||
| Male/female | 9 (53%)/8 (47%) | 32 (91%)/3 (9%) | 0.003 |
|
| |||
| Performance status | |||
| 0 | 11 (65%) | 26 (74%) | 0.52 |
| 1 | 6 (35%) | 9 (26%) | |
|
| |||
| Disease stage | |||
| III | 1 (5.9%) | 3 (8.5%) | 1.00 |
| IV | 16 (94.1%) | 32 (91.4%) | |
|
| |||
| Bulky disease | 1 (6%) | 3 (9%) | 1.00 |
|
| |||
| B symptoms | 6 (35%) | 10 (29%) | 0.75 |
|
| |||
| Bone marrow involvement | 14 (82%) | 30 (86%) | 1.00 |
|
| |||
| Extranodal involvement | 15 (88%) | 32 (91%) | 1.00 |
|
| |||
| Elevated lactate dehydrogenase | 5 (29%) | 9 (26%) | 1.00 |
|
| |||
| Ki67 (N=46) | |||
| <10% | 20% | 10% | |
| 10–30% | 60% | 68% | 0.58 |
| >30% | 20% | 22% | |
|
| |||
| MIPI score | 1.00 | ||
| Intermediate/high | 6 (35%) | 13 (37%) | |
| Low risk | 11 (65%) | 22 (63%) | |
MIPI, mantle cell lymphoma International Prognostic Index; RB, rituximab plus bendamustine; RH, R-HyperCVAD (rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate)
18 patients randomized to RH. However, 1 patient randomized to RH on the day RH arm was closed. That patient did not receive RH and instead received RB as per investigator choice. Thus only 17/18 patients were included in analysis.
Efficacy
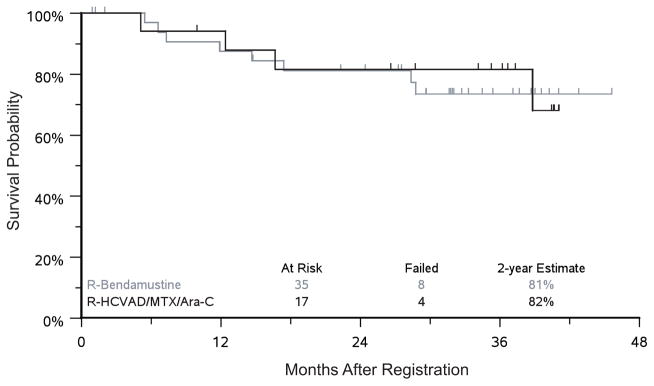
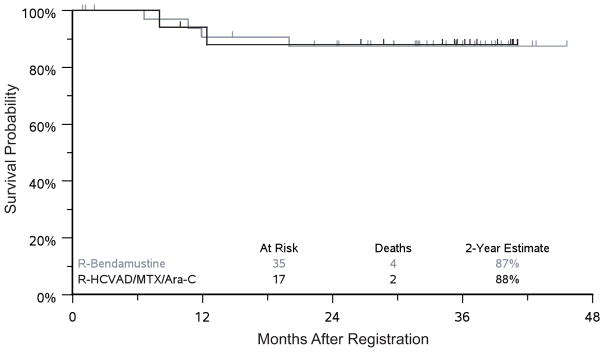
The ORR was 94.1% in the RH arm versus 82.9% in the RB arm (p=0.4). The CR rates were 40% and 35% for RB and RH, respectively, and the PR rates were 43% and 59% for RB and RH, respectively. One patient in the RH arm and 6 patients in the RB arm were not evaluable and considered to be non-responders (did not receive radiographic imaging). The median follow-up was 37 months in the RH arm and 33 months in the RB arm. The estimated 2-year PFS was 81% vs. 82% and estimated 2-year OS was 87% vs. 88% for RB and RH respectively (Figs 2–3). In the RH arm, 5 patients received Auto-HCT on protocol, and 4 patients received Auto-HCT off protocol (discontinued therapy early for toxicities). The 2-year PFS estimates from the landmark at 3 months of follow-up are 75% vs. 88% (p=0.43) for patients that underwent Auto-HCT versus those who did not. In the RB arm, 21 patients received Auto-HCT on protocol, 2 received Auto-HCT off protocol, and 12 patients did not receive Auto-HCT. The 2-year PFS estimates from the landmark at 6 months of follow-up for patients who did not or did receive Auto-HCT was 60% vs. 81% (p=0.20).
Figure 2. Two-year progression-free survival.
HCVAD/MTX/Ara-C = HyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate); R = rituximab.
Figure 3. Two-year overall survival.
HCVAD/MTX/Ara-C = HyperCVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate); R = rituximab.
Safety
The RH arm had significantly more marrow toxicity compared to the RB arm, with increased Grade 3 or 4 thrombocytopenia (71% vs. 17%) (p<0.001), anaemia (59% vs. 8.6%) (p<0.001), neutropenia (65% vs. 34%) (p=0.07), and febrile neutropenia (29% vs. 14%) (p=0.26) (Table III). The RB arm also had fewer observed Grade 3 or 4 non-haematological adverse events, but numbers are too small for a definitive comparison. Grade 3 or 4 non-haematological adverse events that occurred in greater than 5% of the patients on the RH arm were hypokalaemia (29%), hypophosphataemia (24%), hyperglycaemia (12%), aspartate transaminase (AST) elevation (5.9%), alanine transaminase (ALT) elevation (5.9%), catheter-related infection (5.9%), dehydration (5.9%), diarrhoea (5.9), epistaxis (5.9%), nausea (5.9), rash (5.9%) and syncope (5.9%). The sole grade 3 or 4 non-haematological adverse event that occurred in greater than 5% of patients on the RB arm was hypokalaemia (5.7%).
Table III.
Adverse events
| Grade 3/4 toxicities (induction phase) | RH (N=17) | RB (N=35) |
|---|---|---|
| Anaemia | 59% | 8.6% |
| Neutropenia | 65% | 34% |
| Febrile neutropenia | 29% | 14% |
| Thrombocytopenia | 71% | 17% |
| Hypokalaemia | 29% | 5.7% |
| Hypophosphataemia | 24% | 2.9% |
| Hyperglycaemia | 12% | 0% |
| ALT increased | 5.9% | 0% |
| AST increased | 5.9% | 0% |
| Catheter-related infection | 5.9% | 2.9% |
| Dehydration | 5.9% | 0% |
| Diarrhoea | 5.9% | 0% |
| Epistaxis | 5.9% | 0% |
| Nausea | 5.9% | 0% |
| Rash | 5.9% | 2.9% |
| Syncope | 5.9% | 0% |
ALT, alanine transaminase; AST, aspartate transaminase; RB, rituximab plus bendamustine; RH, R-HyperCVAD (rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate)
Twenty-six patients did not finish the course of induction chemotherapy followed by Auto-HCT. On the RH arm, 7/17 patients could not finish induction chemotherapy, mostly because of delayed platelet recovery (Table IV). An additional 5 patients could not mobilize an adequate number of stem cells after cycle 2 of induction chemotherapy. This failure to collect an adequate number of stem cells triggered the early stopping rules, and the RH arm was closed. On the RB arm, 8/35 could not finish induction chemotherapy and 6 did not undergo Auto-HCT per protocol (Table IV). However, only 2 patients could not mobilize an adequate number of stem cells. The detailed reasons for stopping therapy early are listed in Table IV. The entire study was halted and closed because the RH arm encountered the early stopping rule. A result was a smaller sample size of the whole study, which limited the precisions around our PFS estimates.
Table IV.
Reasons for discontinuations
| Reasons for discontinuing treatment/stopping before Auto-HCT | RH (N=17) 12/17 |
RB (N=35) 14/35 |
|---|---|---|
| Failure to collect stem cells | 5 | 2 |
| Thrombocytopenia | 5 | |
| Patient choice | 4 | |
| Progressive disease | 2 | |
| Pancytopenia | 1 | |
| Neutropenia | 1 | |
| Allergy | 1 | |
| Seizure | 1 | |
| Insurance denial | 1 | |
| Others | 1 | 2 |
Auto-HCT, autologous haematopoietic stem cell transplantation; RB, rituximab plus bendamustine; RH, R-HyperCVAD (rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone, alternating with high dose cytarabine and methotrexate)
MRD analysis
This study also analysed MRD status post-induction therapy via NGS. Twenty-seven patients consented to the optional MRD analysis, with 12 paired specimens (pre- and post-induction therapy, 2 in RH and 10 in RB). In the RH arm, 2 patients were MRD positive at baseline; both became MRD negative at the end of induction. In the RB arm, 1 patient was MRD negative at baseline, and 9 patients were MRD positive. Seven out of nine patients converted to MRD negativity at the end of induction (MRD negative rate of 78%). Interestingly, the remaining 2 patients that were MRD positive at end of induction had discordant MRD results between the PBMC and plasma samples. For both patients, MRD analysis of the PBMC sample yielded low positive MRD at the end of induction (One patient had a 6570-fold reduction and another patient had 22000-fold reduction in the lymphoma clonotype per million diploid genome). However, the MRD analysis of the plasma sample yielded an MRD negative result. These two patients remain alive and in remission. One patient on RB was MRD negative at baseline and remained MRD negative at the end of induction. This patient eventually developed progressive disease.
There were 3 additional patients that were missing the baseline MRD analysis specimens (not included in the 12 paired samples), but were MRD negative at the end of induction. One of the 10 patients who was MRD negative at end of induction died approximately 3 months after receiving protocol Auto-HCT, and the rest remain in remission, with a 2-year landmark PFS of 90% (95% CI: 47.3%, 98.5%).
Discussion
In this randomized multicentre phase II prospective trial, we showed that both R-hyperCVAD (RH) and R-bendamustine (RB) are active induction regimens with high overall response rates. While the observed complete remission rates were lower than historical controls, this may reflect that functional imaging was not mandatory at the first restaging, and several patients stopped the study early for toxicity and thus did not finish the induction therapy or undergo the post induction FDG scan (Bernstein, et al 2013, Romaguera, et al 2005). There were significant differences in toxicity, with the RH arm displaying significantly more grade 3 and 4 anaemia, neutropenia and thrombocytopenia. Furthermore, almost one-third of patients on the RH arm failed to mobilize even 1.5 × 106 stem cells, prompting protocol-specified early termination of accrual to the RH arm. Historically, R-hyperCVAD was developed as an 8-cycle regimen without stem cell transplantation consolidation. Single institution studies shortened the RH regimen to 4 cycles, followed by Auto-HCT, which appeared feasible (Till, et al 2008). However, our prospective multicentre trial clearly shows that abbreviated RH followed by Auto-HCT is not a viable induction strategy when stem cell transplantation is planned. In contrast, the toxicity profile of the RB arm was similar to prior trials (Rummel et al 2005; Rummel et al 2013; Flinn et al 2014) and well tolerated. However, 40% of the patients did not proceed to Auto-HCT as expected (6% for mobilization failure, 6% for progressive disease, 6% for other reasons, 9% for toxicity and 14% for patient choice or insurance denial). Although the total number of 14 appears to be high, the numbers were low (1–2) for each individual reason given, with the exception of patient choice. Because of the small sample size from early trial closure, the true significance of this data is unclear. The most common reason for patients not proceeding to Auto-HCT was patient choice. It is unclear as to why patients chose not to proceed with Auto-HCT. One possibility could be the transition from induction treatment physician to a different transplant physician as many centres have a separate transplantation team. It also may simply represent the challenges of conducting a randomized multi-step trial involving Auto-HCT in the cooperative group setting. However, given that the ORR rate appears similar in both RH and RB arms, but RH has more haematological toxicity and stem cell mobilization insufficiency, our study supports RB as a more favourable induction backbone than RH for MCL.
The trial’s primary endpoint was to target a 2-year PFS estimate of 75%. The 2-year PFS estimates of both RH and RB arms were similar at 82% and 81%, respectively. Because the study closed prematurely, the precision around the PFS estimates is limited. However, the 2-year PFS estimate of 81% is higher than the planned 75%. The 2-year OS was also similar in both arms at 87% vs. 88%, suggesting that both induction regimens followed by auto-HCT may be similar in terms of efficacy, although the small sample sizes limit inference. In the RH arm, 5 patients received Auto-HCT on protocol, and 4 patients received Auto-HCT off protocol. The 2-year landmark PFS estimates are 75% vs. 88% for patients that underwent Auto-HCT versus those who did not, suggesting that Auto-HCT may not substantially affect 2-year PFS. However, this analysis is limited by the small sample size. In the RB arm, 21 patients received Auto-HCT on protocol, 2 received Auto-HCT off protocol and 12 patients did not receive Auto-HCT. Although the 2-year landmark PFS estimates were appearing more promising for patients who received Auto-HCT (81% vs. 60%) (p=0.20), this difference does not meet statistical significance.
Minimal residual disease (MRD) appears to be a potent predictor of outcome, and was included as an optional correlative study. Historically, real-time quantitative PCR analysis of rearranged immunoglobulin heavy chain (IGH) genes and multicolour flow cytometry have been used for MRD assessment in MCL (Pott 2011). Studies using these methods show that MRD negative status post therapy can predict for long term remission (Bottcher, et al 2008, Pott, et al 2010). However, these approaches have a number of limitations, including low sensitivity, failure of marker identification, and requirement of patient-specific strategies, which is more cumbersome. NGSuses locus-specific primer sets of IGH and IGK regions to amplify and sequence cancer-derived clones (Faham, et al 2012). These cancer-derived sequences are then used as targets that assess for the presence of MRD in follow-up samples. In our study, we evaluated MRD status with NGS technology. Twenty-seven patients consented to MRD testing, with 12 paired samples of both pre- and post-induction material (peripheral blood). Despite this limited sample size, both RH and RB achieved a relatively high rate of MRD negative status. Patients with conversion from MRD positive to MRD negative status post-induction had excellent outcomes with a 2-year landmark PFS of 90%; three patients did not proceed to Auto-HCT. We also observed discordance between MRD results from PBMC and plasma samples. It is unclear whether MRD results from PBMC or plasma samples are more sensitive or specific. Two patients were low-level MRD positive (low number of molecules) by PMBC and negative by plasma at the end of induction. These two patients had a dramatic reduction in their lymphoma clonotype molecules (6570-fold for one and 22000-fold for another) and still remain in remission. The clinical significance of low level MRD positivity is unknown at this time and will need to be evaluated in future studies. Although these provocative findings require confirmation in a larger study, our data suggest that NGS-based MRD-negative status is feasible after RB and could become a new endpoint for future studies and allow for more rapid evaluation of new treatment strategies.
The Nordic Lymphoma Group introduced the concept of adding cytarabine to the induction regimen of MCL. In their single arm prospective study, the addition of cytarabine to induction followed by Auto-HCT resulted in a 6-year OS of 70% and PFS of 66% (Geisler, et al 2008). Of the 160 patients, 145 (91%) were able to collect stem cells and proceed to Auto-HCT. Although this was not a randomized trial, this argues for inclusion of cytarabine in the induction regimen. A recent publication from the European Mantle Cell Lymphoma Network (Hermine, et al 2016) compared 6 cycles of R-CHOP to 6 cycles of alternating R-CHOP and R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin) as induction regimen and suggested that the addition of cytarabine to induction chemotherapy improves outcomes post Auto-HCT. This study showed an improved 5-year PFS in the cytarabine group (65% vs. 44%). It may appear that the result from this study is contradictory to our study, as our conclusion was to avoid RH, a cytarabine-containing regimen. However, there are a number of similarities in both studies, and the difference lies in the interpretation of the data. For example: 1) the confirmed CR rate of R-CHOP/R-DHAP was 38%, which is similar to the confirmed CR of RB (40%); 2) the MRD negative rate from R-CHOP/R-DHAP was 79%, which is similar to the MRD negative rate from RB (78%); 3) the 2-year PFS and OS from R-CHOP/R-DHAP appear similar to the 2-year PFS and OS from RB. These findings would suggest that, although the addition of cytarabine into the induction therapy is advantageous to R-CHOP, it may not be necessary when compared to RB. Finally, the stem cell collection failure rate was much higher in the R-CHOP/R-DHAP arm as compared to the R-CHOP arm (34% vs. 16%). Although the Nordic Lymphoma Group did not have problems with stem cell collection with the inclusion of cytarabine, it appears that the inclusion of cytarabine and cisplatin (R-DHAP) contributes to stem cell mobilization failure.
A variety of novel therapies have been tested in recent years for relapsed/refractory MCL. Bortezomib, a proteasome inhibitor, has demonstrated an ORR of 33% and a CR rate of 8% in this setting (Fisher, et al 2006). Patients treated with the immunomodulatory agent lenalidomide were shown in a phase II trial to have an ORR of 35% and a CR rate of 12% (Zinzani, et al 2013). Ibrutinib, a Bruton tyrosine kinase inhibitor (Wang, et al 2013), has shown an ORR of 68% and a CR of 21%. These agents are now being incorporated into the induction setting as combination therapies. Early results from an induction trial of lenalidomide plus rituximab showed an ORR of 92%, CR of 64% and a 2-year PFS of 85% (Ruan, et al 2015). The ORR and PFS were similar to those in our study and other induction regimens. However, this was a single arm study that excluded patients with high-risk MIPI score (unless there were contraindications to chemotherapy) and required patients to be treated for at least 36 cycles of therapy unless there was excess toxicity or progressive disease. These results need to be confirmed in a larger randomized trial with longer follow-up time. It is possible that the addition of novel therapies into induction regimens could result in greater CR rates, higher MRD negative responses post-induction, and longer PFS. Although the current standard treatment for younger patients is consolidation in first CR post-induction with Auto-HCT, it is possible that inclusion of novel therapies into induction or as consolidation may change this standard in the future. The current European Network younger trial and future US intergroup trials will attempt to answer this provocative question.
Overall, significant progress has been made in understanding the molecular biology of MCL, and that has translated into improved outcome. The median OS of patients with MCL has been extended from 2.7 years to 4.8 years from the time period of 1975–1996 to 1996–2004 (Herrmann, et al 2009). Since then, other trials have further shown an improvement in OS. The Nordic group used an induction regimen of R-maxi-CHOP alternating with cytarabine followed by Auto-HCT, showing a median event-free survival (EFS) of 7.4 years and median OS of greater than 10 years (Geisler, et al 2012). Many novel agents have been discovered and will certainly extend the median OS even further.
In conclusion, this SWOG-led NCTN trial showed that RH was difficult to incorporate in a multicentre setting because of toxicity and inadequate stem cell mobilization, and is thus not ideal for future development in this setting. In contrast, RB had high response rates, a 78% MRD negativity rate, and was generally well tolerated with successful stem cell mobilization. In addition, patients achieving MRD negative status after induction had excellent outcomes reflected by a 2-year landmark PFS of 90%. We recognize that 40% of the patients on the RB arm did not proceed to Auto-HCT per protocol and that the sample size was small. Thus, the results of this trial need to be confirmed. The current Eastern Cooperative Oncology Group-led NCTN trial (NCT 01415752) using RB with or without bortezomib, followed by rituximab with or without lenalidomide, could support our observation. Upon confirmation, future trials should focus on MRD analysis as primary endpoints for induction trials, as well as the role of Auto-HCT consolidation therapy in patients who are MRD negative post-induction therapy.
Acknowledgments
This work was supported in part by National Institute of Health, National Cancer Institute grants CA180888, CA180819, CA180821, CA180820, CA189957, CA189821, CA189972, CA189953, CA180846, CA180835, CA189808, CA11083, CA46368, CA46136 CA76462, and in part by Sequenta, Inc. (Adaptive Biotechnologies).
Footnotes
Authorship
Designed research: RC, SJF, BC, SB, LC, JF, MF, RF, BK, ML, TS, JW.
Performed research: RC, SJF, KB, PB, AC, JF, BK, HL, JL, TS, JW.
Collected data: RC, KB, TF, HL, JL.,L.R, JW.
Analysed and interpreted data: RC, SJF, PB, SB, AC, JF, MF, RF, TF, BK, HL, JL, TP. LR, SS, JW.
Performed statistical analysis: HL.
Wrote manuscript: RC, SJF, PB, SB, BC, JF, BK, HL, SS, TS.
Reviewed manuscript: All authors.
Disclosures
No completing financial interests: SJF, KB, PB, SB, BC, LC, RF, SK, HL, ML, TP, LR, SS, JW.
Membership on another entity’s Board of Directors or its advisory committees (whether for profit or not for profit): R.C. serves on Advisory Boards of Merck, Seattle Genetics, and Genentech, and serves on Speaker Bureau for Seattle Genetics, Millennium, and Genentech. J.F. serves on Bayer Data and Safety Monitoring Board; T.S. serves on Advisory Boards of Novartis, Spectrum, Seattle Genetics, and Merck; as consultant for Teva: J.L.; Consulting with Teva and Genentech: B.K.; Consulting with Celgene, Seattle Genetics, Pharmacyclics and Sanofi: T.F.; Research funding through his University from Millennium, Otsuka, GSK, Novartis, and BMS: T.S.; Speakers’ Bureau for Celgene: A.C.; M.F. is Stockholder, Adaptive Biotechnolgies.
References
- Bernstein SH, Epner E, Unger JM, LeBlanc M, Cebula E, Burack R, Rimsza L, Miller TP, Fisher RI. A phase II multicenter trial of hyperCVAD MTX/Ara-C and rituximab in patients with previously untreated mantle cell lymphoma; SWOG 0213. Annals of Oncology. 2013;24:1587–1593. doi: 10.1093/annonc/mdt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher S, Ritgen M, Buske S, Gesk S, Klapper W, Hoster E, Hiddemann W, Unterhalt M, Dreyling M, Siebert R, Kneba M, Pott C. Minimal residual disease detection in mantle cell lymphoma: methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica. 2008;93:551–559. doi: 10.3324/haematol.11267. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V. Revised Response Criteria for Malignant Lymphoma. Journal of Clinical Oncology. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- Chopra R, McMillan AK, Linch DC, Yuklea S, Taghipour G, Pearce R, Patterson KG, Goldstone AH. The Place of High-Dose BEAM Therapy and Autologous Bone Marrow Transplantation in Poor-Risk Hodgkin’s Disease. A Single-Center Eight-Year Study of 155 Patients. Blood. 1993;81:1137–1145. [PubMed] [Google Scholar]
- Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, Metzner B, Truemper L, Reiser M, Steinhauer H, Boiron JM, Boogaerts MA, Aldaoud A, Silingardi V, Kluin-Nelemans HC, Hasford J, Parwaresch R, Unterhalt M, Hiddemann W. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- Faham M, Zheng J, Moorhead M, Carlton VE, Stow P, Coustan-Smith E, Pui CH, Campana D. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120:5173–5180. doi: 10.1182/blood-2012-07-444042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, Epner E, Krishnan A, Leonard JP, Lonial S, Stadtmauer EA, O’Connor OA, Shi HL, Boral AL, Goy A. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. Journal of Clinical Oncology. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M, Kolibaba K, Issa S, Clementi R, Hallman DM, Munteanu M, Chen L, Burke JM. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123:2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Andersen NS, Pedersen LB, Jerkeman M, Eriksson M, Nordstrom M, Kimby E, Boesen AM, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Akerman M, Ehinger M, Sundstrom C, Langholm R, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler CH, Kolstad A, Laurell A, Jerkeman M, Raty R, Andersen NS, Pedersen LB, Eriksson M, Nordstrom M, Kimby E, Bentzen H, Kuittinen O, Lauritzsen GF, Nilsson-Ehle H, Ralfkiaer E, Ehinger M, Sundstrom C, Delabie J, Karjalainen-Lindsberg ML, Brown P, Elonen E. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. British Journal of Haematology. 2012;158:355–362. doi: 10.1111/j.1365-2141.2012.09174.x. [DOI] [PubMed] [Google Scholar]
- Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, Szymczyk M, Bouabdallah R, Kneba M, Hallek M, Salles G, Feugier P, Ribrag V, Birkmann J, Forstpointner R, Haioun C, Hanel M, Casasnovas RO, Finke J, Peter N, Bouabdallah K, Sebban C, Fischer T, Duhrsen U, Metzner B, Maschmeyer G, Kanz L, Schmidt C, Delarue R, Brousse N, Klapper W, Macintyre E, Delfau-Larue MH, Pott C, Hiddemann W, Unterhalt M, Dreyling M. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388:565–575. doi: 10.1016/S0140-6736(16)00739-X. [DOI] [PubMed] [Google Scholar]
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, Reiser M, Forstpointner R, Metzner B, Peter N, Wormann B, Trumper L, Pfreundschuh M, Einsele H, Hiddemann W, Unterhalt M, Dreyling M. Improvement of Overall Survival in Advanced Stage Mantle Cell Lymphoma. Journal of Clinical Oncology. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, Pfreundschuh M, Reiser M, Metzner B, Einsele H, Peter N, Jung W, Wörmann B, Ludwig WD, Dührsen U, Eimermacher H, Wandt H, Hasford J, Hiddemann W, Unterhalt M. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- Kaplan G, Meier P. Non-parametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Liu H, Johnson JL, Koval G, Malnassy G, Sher D, Damon LE, Hsi ED, Bucci DM, Linker CA, Cheson BD, Stock W. Detection of minimal residual disease following induction immunochemotherapy predicts progression free survival in mantle cell lymphoma: final results of CALGB 59909. Haematologica. 2012;97:579–585. doi: 10.3324/haematol.2011.050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nademanee A, O’Donnell MR, Snyder DS, Schmidt GM, Parker PM, Stein AS, Smith EP, Molina A, Stepan DE, Somlo G. High-Dose Chemotherapy With or Without Total Body Irradiation Followed by Autologous Bone Marrow and/or Peripheral Blood Stem Cell Transplantation for Patients with Relapsed and Refractory Hodgkin’s Disease: Results in 85 Patients with Analysis of Prognos. Blood. 1995;85:1381–1390. [PubMed] [Google Scholar]
- Pott C. Minimal residual disease detection in mantle cell lymphoma: technical aspects and clinical relevance. Seminars in Hematology. 2011;48:172–184. doi: 10.1053/j.seminhematol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Pott C, Hoster E, Delfau-Larue MH, Beldjord K, Bottcher S, Asnafi V, Plonquet A, Siebert R, Callet-Bauchu E, Andersen N, van Dongen JJ, Klapper W, Berger F, Ribrag V, van Hoof AL, Trneny M, Walewski J, Dreger P, Unterhalt M, Hiddemann W, Kneba M, Kluin-Nelemans HC, Hermine O, Macintyre E, Dreyling M. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115:3215–3223. doi: 10.1182/blood-2009-06-230250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece DE, Connors JM, Spinelli JJ, Barnett MJ, Fairey RN, Klingemann HG, Nantel SH, O’Reilly S, Shepherd JD, Sutherland HJ. Intensive therapy with cyclophosphamide, carmustine, etoposide +/− cisplatin, and autologous bone marrow transplantation for Hodgkin’s disease in first relapse after combination chemotherapy. Blood. 1994;83:1193–1199. [PubMed] [Google Scholar]
- Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, McLaughlin P, Younes A, Samaniego F, Goy A, Sarris AH, Dang NH, Wang M, Beasley V, Medeiros LJ, Katz RL, Gagneja H, Samuels BI, Smith TL, Cabanillas FF. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. Journal of Clinical Oncology. 2005;23:7013–7023. doi: 10.1200/JCO.2005.01.1825. [DOI] [PubMed] [Google Scholar]
- Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, Christos P, Rodriguez A, Svoboda J, Lewis J, Katz O, Coleman M, Leonard JP. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. New England Journal of Medicine. 2015;373:1835–1844. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel MJ, Al-Batran SE, Kim SZ, Welslau M, Hecker R, Kofahl-Krause D, Josten KM, Durk H, Rost A, Neise M, von Grunhagen U, Chow KU, Hansmann ML, Hoelzer D, Mitrou PS. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. Journal of Clinical Oncology. 2005;23:3383–3389. doi: 10.1200/JCO.2005.08.100. [DOI] [PubMed] [Google Scholar]
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Durk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- The Non Hodgkin’s Lymphoma Classification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- Till BG, Gooley TA, Crawford N, Gopal AK, Maloney DG, Petersdorf SH, Pagel JM, Holmberg L, Bensinger W, Press OW. Effect of remission status and induction chemotherapy regimen on outcome of autologous stem cell transplantation for mantle cell lymphoma. Leukemia and Lymphoma. 2008;49:1062–1073. doi: 10.1080/10428190801923725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BG, Li H, Bernstein SH, Fisher RI, Burack WR, Rimsza LM, Floyd JD, DaSilva MA, Moore DF, Jr, Pozdnyakova O, Smith SM, LeBlanc M, Friedberg JW. Phase II trial of R-CHOP plus bortezomib induction therapy followed by bortezomib maintenance for newly diagnosed mantle cell lymphoma: SWOG S0601. British Journal of Haematology. 2016;172:208–218. doi: 10.1111/bjh.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, Barrientos JC, Chmielowska E, Radford J, Stilgenbauer S, Dreyling M, Jedrzejczak WW, Johnson P, Spurgeon SE, Li L, Zhang L, Newberry K, Ou ZS, Cheng N, Fang BL, McGreivy J, Clow F, Buggy JJ, Chang BY, Beaupre DM, Kunkel LA, Blum KA. Targeting BTK with Ibrutinib in Relapsed or Refractory Mantle-Cell Lymphoma. New England Journal of Medicine. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani PL, Vose JM, Czuczman MS, Reeder CB, Haioun C, Polikoff J, Tilly H, Zhang L, Prandi K, Li J, Witzig TE. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Annals of Oncology. 2013;24:2892–2897. doi: 10.1093/annonc/mdt366. [DOI] [PMC free article] [PubMed] [Google Scholar]