Abstract
Objective
Sarcopenic obesity (SO), a combination of low muscle mass and high fat mass, is considered as risk factor for mortality in general population. It is unclear if SO affects mortality in maintenance hemodialysis (MHD) patients. In this study, we aimed to determine whether body composition as assessed by currently available SO definitions are related to all-cause mortality in MHD subjects. We also examined the impact of applying different definitions on the prevalence of SO in our MHD database.
Design
Retrospective analysis
Subjects and Settings
Adult patients on MHD for at least 3 months with no acute illness studied in the clinical research center between 2003 and 2011.
Intervention
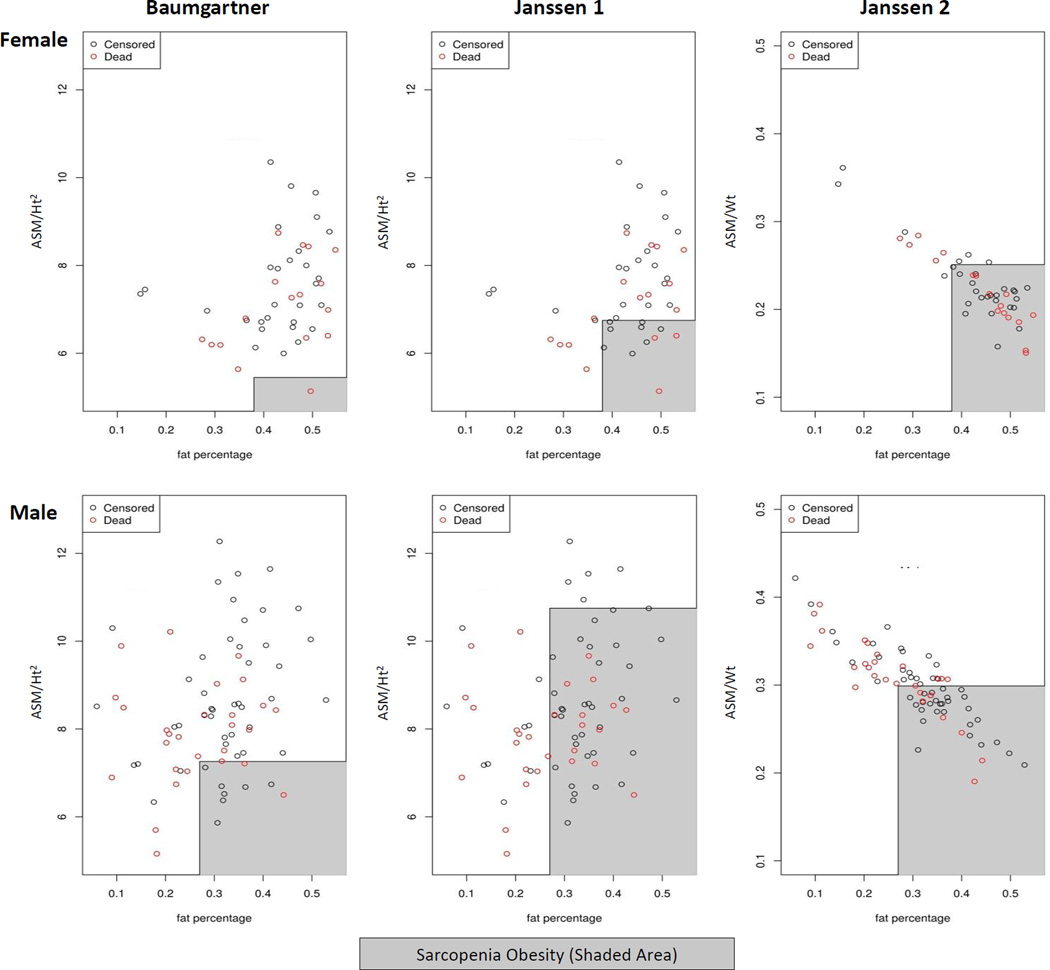
Assessment of body composition was performed using dual energy x-ray absorptiometry (DEXA). SO (Appendicular Skeletal Mass (ASM): arm lean mass + leg lean mass and fat mass) was defined according to Baumgartner definition, Janssen Criteria 1 and Janssen Criteria 2.
Main Outcome Measure
All-cause mortality and prevalence of SO. Patient deaths were ascertained from medical records and United States social security death index.
Results
Of 122 participants, 62% were male; mean age was 46 (interquartile range [IQR] 40, 54) in men and 50 (44, 61) in women. Prevalence of SO ranged from 12% to 62% in men and 2% to 74% in female according to different definitions. SO prevalence was lowest using the Baumgartner criteria (all: 8%, men 12%, women: 2%), and highest according to the Janssen Criteria 2 (all: 57%, men 46%, women 74%). There were 45 deaths during a median follow-up period of 44 (20, 76) months. SO by any definition was not statistically significantly associated with mortality during follow up.
Conclusions
The current SO definitions are not applicable to predict increased risk of death in MHD patients. We found high degree of variation in the rates of SO when using different definitions. Future studies should focus on establishing MHD population-specific thresholds of muscle mass and adiposity for accurate prognostication.
Introduction
Low muscle mass, also termed as sarcopenia, is common in maintenance hemodialysis (MHD) patients and contributes to increased morbidity and mortality (1–3). In addition, sarcopenic MHD patients exhibit reduced physical functioning and are at an increased risk of hospitalization (4–6). Over the past decade, with increasing prevalence of obesity, researchers have started to focus on potential interplay of muscle and fat mass and its association with mortality (12–14). In fact, the term “sarcopenia obesity (SO)” has been coined to describe the concurrence presence of lower muscle mass and higher fat mass in an individual. Several SO definitions developed in healthy young or elderly populations have been studied extensively, especially regarding their association with clinical outcomes (15, 16). However, the applicability of these current definitions to predict mortality in MHD subjects has yet to be fully investigated (17, 18). While obesity is known to be an independent risk factor for cardiovascular disease and mortality in the general population (19, 20), this may not be true in MHD patients where presence of excessive fat mass seems to be protective and obese MHD patients have more favorable clinical prognosis (21, 22).
In this study, we hypothesized that the currently proposed SO definitions based on low muscle and high fat mass can predict increased all-cause mortality in MHD subjects. We also determined the impact of using different SO definitions on prevalence estimates.
Methods
This is a retrospective study conducted at large, tertiary care university hospital. The Institutional Review Board committee at the Vanderbilt University Medical Center (VUMC) approved each study that the data was collected from. Written informed consent was obtained from each participant.
Study Population
In this study, 122 MHD patients were enrolled who had formerly participated in a variety of metabolic studies at the VUMC between 2003 and 2011, and who had data available on body composition. All eligible patients were aged 18 years or older and were on MHD therapy for more than 3 months. Pregnant women and patients with clinical signs of overt infection, vasculitis or liver disease, and those who hospitalized within 1 month prior to enrollment into the studies were excluded from the analysis. We recorded demographic, comorbidities, anthropometric, clinical, dialysis vintage and laboratory data at the time of study enrollment.
Body Composition Data
Assessment of body composition was performed by Dual Energy X-Ray Absorptiometry (DEXA), which offers a rapid, non-invasive three-compartment evaluation that quantifies fat mass, lean body mass, and bone mineral content with minimal radiation exposure. All DEXA measurements were done on a non-dialysis day using a Lunar Prodigy iDEXA machine, v.11.40.004 (software versions 2003 to 2011, General Electric, Madison, WI). Appendicular skeletal mass (ASM) was defined as the sum of total lean mass of the both arms and legs divided by the square of the height or weight. SO was defined based on three research definitions: Baumgartner (ASM/Ht2 + % FM: Men <7.26 kg/m2 & >27%FM and Women <5.45 kg/m2 & >38%FM); Janssen Criteria 1 (ASM/Ht2 + % FM: (CLASS 1 Men 8.51–10.75 kg/m2 & >27%FM and Women 5.76–6.75 kg/m2 & >38%FM) and (CLASS 2 Men <8.5 kg/m2 & >27%FM and Women <5.75 kg/m2 & >38%FM)) and Janssen Criteria 2: (ASM/Wt + % FM: Men <29.9%SMM & >27%FM) (Table 1) (15, 16, 23, 24). We have chosen these definitions as they were most commonly used in epidemiological research and has defined threshold cut-offs for muscle and fat mass. We excluded definitions which were derived from fewer than 50 participants. SO definitions were further applied to our MHD database to determine comparisons of prevalence estimates.
Table 1.
Definition of Sarcopenic Obesity Criteria’s
| BAUMGARTNER CRITERIA | ASM/Ht2 and % FM | Men <7.26 kg/m2 & >27%FM Women <5.45 kg/m2 & >38%FM |
| JANSSEN CRITERIA (1) | ASM/Ht2 and % FM | CLASS 1 Men 8.51–10.75 kg/m2 & >27%FM Women 5.76–6.75 kg/m2 & >38%FM CLASS 2 Men <8.5 kg/m2 & >27%FM Women <5.75 kg/m2 & >38%FM |
| JANSSEN CRITERIA (2) | ASM/Wt and % FM | Men <29.9% & >27%FM Women <25.1% & >38%FM |
Appendicular Skeletal Mass (ASM) = Arm Lean Mass + Leg Lean Mass; FM= fat mass
Outcome variable
The primary outcome was all-cause mortality. Patient deaths were determined from VUMC medical records. Deaths at outside hospitals were screened from United States social security death index. Subjects were censored if they received kidney transplantation, or no survival data were available.
Statistical Analysis
Continuous variables were expressed as the mean (SD) or median and IQR and analyzed by unpaired t test or the Wilcoxon Rank-Sum test, as appropriate. Categorical variables are expressed as absolute (n) and relative (%) frequency and analyzed by Chi-square test or Fisher’s exact test. Correlation analysis was performed by Spearman correlation coefficient. Kaplan-Meier survival curves with log-rank test are presented to compare mortality by SO definition status (yes or no). Cox-proportional hazard model was used to predict mortality adjusting for age, gender and SO definitions. Statistical analysis was performed using SPSS software Version 21.0 (SPSS Inc., Chicago, IL, USA) and R 3.3.0 (R Core Team, Vienna, Austria).
Results
Study cohort characteristics
Clinical, demographic and body composition characteristics at baseline are summarized in Table 2 and Table 3. Of 122 participants, 62% were male and 38% female. The median (IQR) age was 46 (40, 54) years in men and 50 (44, 61) years in women. The study subjects were predominantly African-American (76%), and 34% (n=42) were diabetic. The median duration of dialysis was 31 (10, 68) months.
Table 2.
Patient characteristics by Sarcopenia Obesity Definitions
| Total | Baumgartner Criteria | Jansen Criteria 1 | Jansen Criteria 2 | |||||
|---|---|---|---|---|---|---|---|---|
| NO | YES | NO | Yes | NO | YES | |||
| Class 1 | Class 2 | |||||||
| Total Subjects, N | 122 | 112 | 10 | 64 | 29 | 29 | 53 | 69 |
| Age (years) | 48±13 | 48±13 | 49±18 | 49±13 | 45±13 | 49±13 | 46±13 | 50±13 |
| Gender, n (%) | ||||||||
| Male | 76 (62) | 67 (60) | 9 (90) | 29 (45) | 19 (66) | 28 (97) | 41 (77) | 35 (51) |
| Female | 46 (38) | 45 (40) | 1 (10) | 35 (55) | 10 (34) | 1 (3) | 12 (23) | 34 (49) |
| Dialysis Vintage (months) | 31 (10–68) | 31 (9–67) | 42 (17–66) | 21(8–56) | 32 (39–52) | 51 (42–58) | 47 (39–55) | 50 (42–60) |
| Follow-up Duration (months) | 44 (20–76) | 44 (20–73) | 41 (13–103) | 44(24–77) | 38 (17–66) | 48 (18–81) | 31 (14–68) | 30 (9–64) |
| Ethnicity, n (%) | ||||||||
| Non-Hispanics | 116 (95) | 107 (96) | 9 (90) | 0(0) | 25 (86) | 27 (93) | 51 (96) | 65 (94) |
| Hispanic | 6 (5) | 5 (4) | 1 (1) | 64(100) | 4 (14) | 2 (7) | 2 (4) | 4 (6) |
| Race, n (%) | ||||||||
| African American | 93 (76) | 90 (80) | 3 (30) | 55(86) | 23 (79) | 15 (52) | 44 (83) | 49 (71) |
| Caucasian | 25 (20) | 21 (19) | 4 (40) | 9(14) | 5 (17) | 11 (38) | 8 (15) | 17 (25) |
| Diabetes, n (%) | 42 (34) | 40 (36) | 2 (20) | 25(39) | 10 (34) | 7 (24) | 11 (21) | 31 (45) |
| CAD, n (%) | 35 (31) | 32 (31) | 3 (30) | 22(38) | 5 (19) | 8 (29) | 14 (28) | 21 (33) |
| Etiology of ESRD, n (%) | ||||||||
| DM | 22 (18) | 21 (19) | 1 (10) | 31 (20) | 6 (21) | 3 (10) | 5 (9) | 17 (25) |
| HTN | 57 (47) | 55 (49) | 2 (20) | 29 (45) | 15 (52) | 13 (45) | 32 (60) | 25 (36) |
| GN | 17 (14) | 14 (12) | 3 (30) | 10 (16) | 3 (10) | 4 (14) | 9 (17) | 8 (12) |
| PCKD | 4 (3) | 3 (3) | 1 (10) | 2 (3) | 0 (0) | 2 (7) | 1 (2) | 3 (4) |
| Others | 9 (7) | 7 (6) | 2 (20) | 3 (5) | 2 (7) | 4 (14) | 3 (6) | 6 (9) |
| Total death, n (%) | 45 (37) | 42 (38) | 3 (30) | 29 (45) | 6 (21) | 10 (34) | 25 (47) | 20 (29) |
Continuous variables are expressed as mean ± SD or median (inter-quartile range) and categorical variables as n (%)
Abbreviations: DM= diabetes mellitus; HTN= hypertension; GN= glomerulonephritis; PCKD= polycystic kidney disease; CAD = coronary artery disease
Table 3.
Body Composition, nutritional and inflammatory markers by Sarcopenia Obesity Definitions
| Total | Baumgartner Criteria | Jansen Criteria 1 | Jansen Criteria 2 | |||||
|---|---|---|---|---|---|---|---|---|
| NO | Yes | NO | Yes | NO | Yes | |||
| Class 1 | Class 2 | |||||||
| BMI (kg/m2) | 30.4±7.6 | 30.8±7.7 | 25±3 | 30.7±9 | 32.8±5.4 | 27.2±4.3 | 25.5±5.1 | 34.1±7.0 |
| BSA (m2) | 2.0±0.3 | 2.01±0.3 | 1.87±0.16 | 1.95±0.3 | 2.0±0.3 | 1.98±0.2 | 1.89±0.22 | 2.1±0.3 |
| Arm Lean (g) | 6183±1847 | 6270±1858 | 5210±1097 | 5853±1772 | 6817±2336 | 6276±1241 | 6761±1736 | 5738±1817 |
| Leg Lean (g) | 16806±4177 | 17060±4233 | 13957±1956 | 16554±4283 | 17985±5020 | 16181±2636 | 17226±3873 | 16483±4397 |
| Total Fat mass, % | 35±12 | 35±12 | 36±7 | 33±14 | 40±7 | 35±5 | 25±9 | 42±7 |
| ASM/Ht2 (kg/m2) | 8.0±1.4 | 8.13±1.42 | 6.5±0.7 | 8.1±1.5 | 8.42±1.6 | 7.5±0.9 | 8.0±1.5 | 8.0±1.4 |
| ASM/Weight | 0.3±.06 | 0.3±0.06 | 0.3±.04 | 0.3±.06 | 0.3±.05 | 0.3±.03 | 0.3±.04 | 0.2±.04 |
| 6 min Walk, m | 1385±403 (50/122) |
1372±379 (44/112) |
1478±588 (6/10) |
1291±381 (44/64) |
1474±345 (13/29) |
1484±487 (13/29) |
1467±398 (25/53) |
1303±399 (25/69) |
| BF BIA | 28(20–47) (66/122) |
29(21–48) (60/112) |
22(16–25) (6/10) |
30(16–51) (50/64) |
39(24–45) (18/29) |
24(21–26) (15/29) |
21(16–30) (31/53) |
41(25–49) (35/69) |
| Insulin (µU/ml) | 13(8–20) (83/122) |
13(7–20) (75/112) |
14 (11–20) (8/10) |
12.3(7–19) (53/64) |
16(12–26) (17/29) |
13(7–20) (19/29) |
8(6–13) (40/53) |
17(12–24) (43/69) |
| Leptin (ng/mL) | 15(6–65) (77/122) |
20(6–67) (70/112) |
9 (6–13) (7/10) |
9(3–69) (50/64) |
50(18–70) (21/29) |
9(7–21) (17/29) |
5(2–9) (34/53) |
53(18–77) (43/69) |
| CRP (mg/dL) | 7 (3–15) (97/122) |
7 (3–15) (89/112) |
11 (4–24) (8/10) |
4(2–14) (59/64) |
10 (6–15) (21/29) |
9 (4–19) (21/29) |
4 (3–15) (46/53) |
9 (5–17) (51/69) |
| Resistin (ng/ml) | 5.4± 3.8 (100/122) |
5.5±3.8 (92/112) |
4.5±3.0 (8/10) |
5.8±4.2 (59/64) |
5.1±2.4 (22/29) |
4.9±3.8 (21/29) |
5.2± 4.0 (45/53) |
5.6±3.6 (55/69) |
| Albumin (g/dL) | 3.9± 0.5 (96/122) |
3.9±0.5 (88/112) |
3.7±0.5 (8/10) |
3.9±0.4 (59/64) |
3.9± 0.7 (21/29) |
3.9± 0.4 (21/29) |
3.9± 0.4 (45/53) |
3.8± 0.5 (51/69) |
| Pre-albumin (mg/dL) | 35.6± 9.5 (83/122) |
35.5±9.6 (78/112) |
36.1±8.8 (5/10) |
34.5±9.4 (57/64) |
37.6± 9.6 (16/29) |
36.2± 9.7 (15/29) |
34.9± 10.2 (41/53) |
36.2± 8.9 (42/69) |
| Creatinine (mg/dl) | 9± 3 (97/122) |
9±3 (89/112) |
8.4±2.6 (8/10) |
8.8±3.1 (59/64) |
9.1± 2.8 (21/29) |
8.9±2.8 (21/29) |
9.7± 3.2 (46/53) |
8.2± 2.6 (51/69) |
| IL-6 (pg/mL) | 9 (4–15) (58/122) |
9 (5–15) (55/112) |
3 (3–28) (3/10) |
7(4–14) (45/64) |
13(6–18) (13/29) |
6 (2–11) (13/29) |
5 (2–13) (25/53) |
9 (6–15) (33/69) |
Continuous variables are expressed as mean ± SD or median (inter-quartile range) and categorical variables as n (%)
Abbreviations: IL-6 = interleukin-6; CRP= C - reactive protein; ASM= appendicular skeletal mass; BSA = body surface area; BMI= body mass index; BIA= bioelectric impedance; m= meter; g= gram
The mean arm lean mass (7.1 vs 4.6 kg), leg lean mass (18.1 vs 14.6 kg) and ASM/Ht2 (8.4 vs 7.4) were higher in men. In contrast, fat mass percentage (43.2 vs 30.0) and BMI (33.6 vs 28.4 kg/m2) were higher in women.
Prevalence of SO based on differing definitions
Applying the three definitions (Table 2), the prevalence of SO ranged from 12% to 62% for men and 2% to 74% for female. SO prevalence was lowest using the Baumgartner criteria (all: 8%, men 12%, women: 2%), intermediate for Janssen criteria 1 (combined class 1 and class 2: all 48%, men 62%, women 24%) and highest according to the Janssen criteria 2 (all: 57%, men 46%, women 74%). Prevalence of the SO was higher in men than women for Janssen criteria 1 (62% vs 24%). Whereas, women were more often diagnosed to have SO by using Janssen criteria 2 (74% vs 46%).
SO Status at Baseline and Mortality Outcome
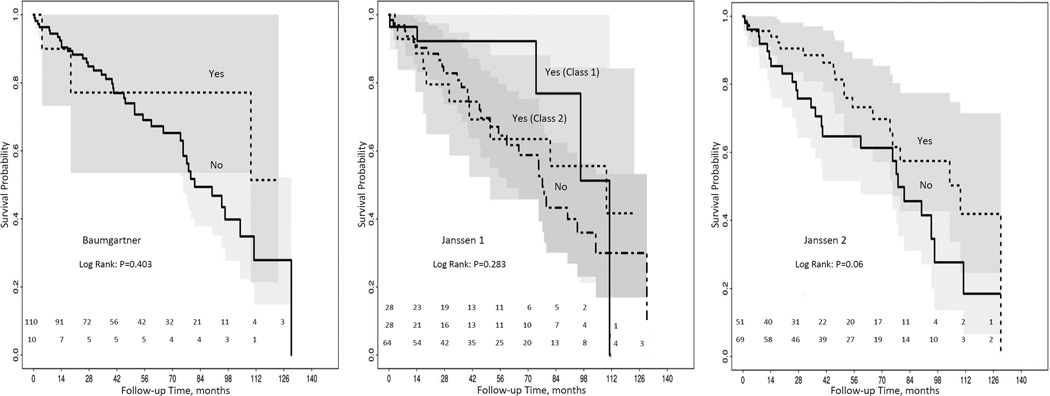
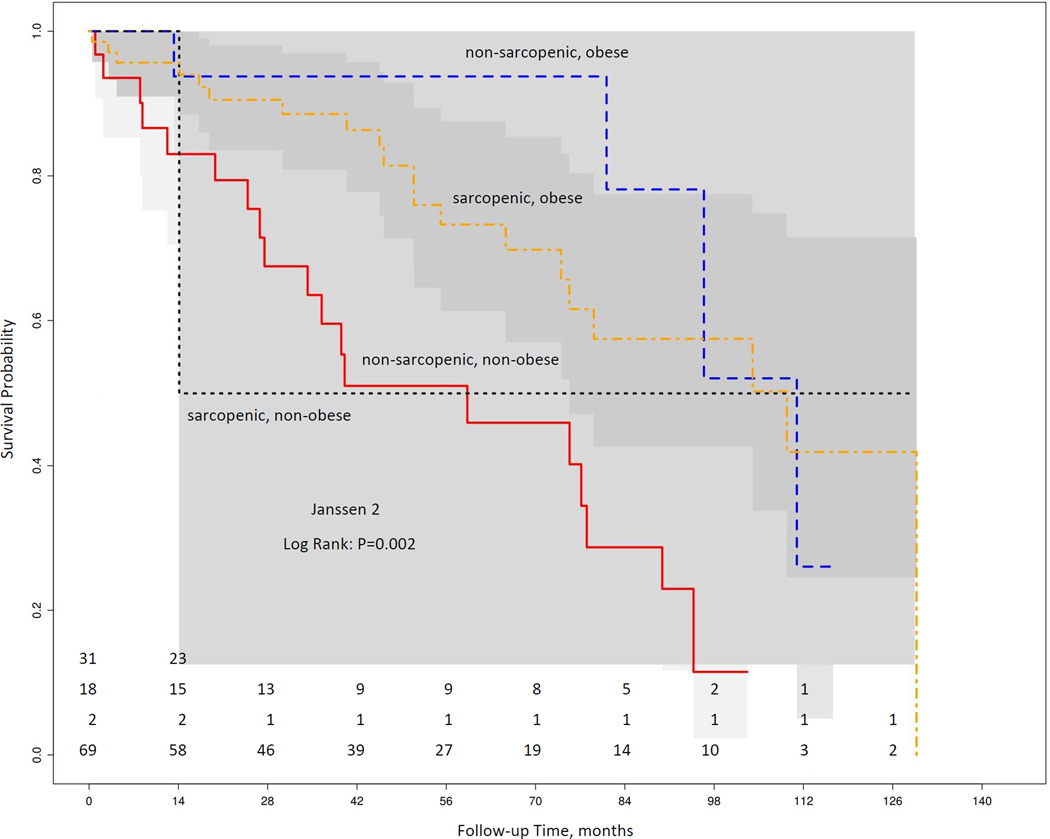
There were 37% (n=45) deaths during a median follow-up period of 44 (20, 76) months. Figure 1 shows the raw data mapping of SO criteria’s and mortality stratified by gender. Kaplan-Meier curves according to the presence of SO was not statistically associated with mortality during follow up (all log rank test P value >0.05) for all 3 definitions (Figure 2). On Cox proportional hazard multivariate analysis, SO definitions were not found to predict increased mortality after adjustment for age and gender (Table 4). Further adjustment for c-reactive protein (CRP) did not change the association appreciably (data not shown). Also, according to other body composition categories: non-sarcopenia obese patients had best survival; whereas, sarcopenia non-obese had the worse survival (log rank p=0.002) (Figure 3).
Figure 1.
Figure 2.
Table 4.
Cox proportional hazards model evaluating the effect of Sarcopenia obesity definitions and covariates on mortality
| Variable | Hazard ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Age (per year) | 3.54 | 2.18 | 5.72 | <.0001 |
| Gender (female) | 0.28 | 0.08 | 0.95 | 0.03 |
| Baumgartner Criteria | 0.48 | 0.25 | 0.94 | 0.04 |
| Variable | Hazard ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Age (per year) | 3.19 | 1.99 | 5.10 | <.0001 |
| Gender (female) | 0.38 | 0.18 | 0.78 | <0.01 |
| Jansen Criteria Class 1 | 0.41 | 0.15 | 1.10 | 0.07 |
| Jansen Criteria Class 2 | 0.48 | 0.22 | 1.04 | 0.07 |
| Variable | Hazard ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Age (per year) | 3.53 | 2.18 | 5.72 | <.0001 |
| Gender (female) | 0.75 | 0.37 | 1.52 | 0.42 |
| Jansen Criteria 2 | 0.36 | 0.18 | 0.74 | <0.01 |
Data adjusted for age, gender and sarcopenia obesity definitions
Figure 3.
Discussion
In this study, we found that current SO criteria are of limited utility in predicting all-cause mortality in prevalent MHD patients. In fact, the contrary was true and the patients who did not meet the criteria for SO phenotype had worse survival. We also found large differences in SO prevalence rates when using different definitions. These findings indicate the need to develop appropriate cutoff values of muscle and fat mass for predicting mortality in MHD patients.
Our findings are in contrast with a previous published report where SO was associated with presence of systemic inflammation and increased mortality in ESRD population (17). In a sample of 328 HD patients, Honda et al. showed that protein-energy wasting was common in overweight dialysis patient and is associated with high fat and low muscle mass and increased risk of mortality (17). The possible reasoning for this association was attributed to increased pro-inflammatory mediators and oxidative stress observed in obese dialysis patients. However, in our study, we found no association between SO definitions and increased risk of mortality after adjusting for CRP. One of the explanations for our results is that the different ratios for lean to fat mass may have different outcomes and not captured by pre-defined dichotomous values of SO definitions. This is supported by study of body composition and survival by Marcelli et al., where patients with both lean muscle and fat mass within 10th–90th percentile of health population was associated with best survival (18). Whereas, patient with low muscle and low fat indices was associated with poor outcomes. We have also demonstrated similar findings suggesting that sarcopenic non-obese patient had highest mortality (Figure 3).
Our study results have significant clinical and research implications. When we aimed to examine the prevalence of SO in our HD database, we observed lack of consensus and wide variation in prevalence among different diagnostic definitions. The SO definitions used for this analysis were the ones recognized as useful in both research and clinical practice. This result is important as differences in SO ascertainment may affect designing effective nutritional interventions. Several factors may explain wide variability in prevalence estimates including use of different cut-offs for defining sarcopenia between studies, differences in methodology (DEXA or bioimpedance) and diagnostic approach (-2SD, residuals, quantiles) and lack of agreement over using ASM/Wt or ASM/Ht2. We believe that the current working definitions of SO need further refining and must incorporate muscle strength and quality to determine sarcopenia in ESRD patients who represent a different mortality risk profile in terms of their nutrition status. Development of a maintenance dialysis population-specific SO definition, preferably using longitudinal measurements of body composition and function along with the establishment of cut-off related to meaningful long-term outcomes is necessary in ESRD patients. A more appropriately defined SO criteria would also help clinicians identify high-risk patients, inform clinical decisions, and facilitate patient counseling. Clinicians can evaluate baseline risk and thus implement preventive and therapeutic strategies, e.g., exercise, diet and anti-inflammatory approaches.
Our study has several strengths including the long follow-up period (median followup of 44 months) and the use of precise and direct measurement of body composition by DEXA. There are also limitations to our study. First, the relatively small sample size of our MHD database precludes our ability to perform detailed analysis and detect significant associations between body composition indices and mortality. Observational nature of our study would not allow inferring causality. Thirdly, the participants in our study were selected from metabolic studies, thus increasing the potential for selection bias. Fourth, body composition measurements were obtained at baseline. It is possible that patients’ body composition may have changed during follow-up. Finally, a majority proportion of our study subjects were African-American that may limit generalizability to other races/ethnicity.
In conclusion, our data question the utility of current SO definitions to identify high-risk MHD patients. Development of new SO criteria will help target high-risk patients for surveillance and enable clinicians and researchers to evaluate novel diagnostic, preventive, and therapeutic modalities to mitigate the devastating consequences of low muscle mass and fat mass.
Practical Application
The present study indicates that SO defined by current definitions do not seem to predict mortality in MHD patients. Prevalence of SO vary widely with different SO diagnostic criteria’s. Future confirmatory studies are warranted to validate our results.
Acknowledgments
Support and Financial Disclosure:
This work was supported by grants from Department of Veterans Affairs (Merit Award # I01 CX000414) and funds from the Vanderbilt Center for Kidney Disease. S.M.D. is also supported by American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Bàràny P, Heimbürger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clinical Journal of the American Society of Nephrology. 2014 doi: 10.2215/CJN.10261013. CJN. 10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiran VR, Zhu TY, Yip T, Lui SL, Lo WK. Body mass index and mortality risk in Asian peritoneal dialysis patients in Hong Kong—Impact of diabetes and cardiovascular disease status. Peritoneal Dialysis International. 2014;34(4):390–398. doi: 10.3747/pdi.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuzawa R, Matsunaga A, Wang G, Yamamoto S, Kutsuna T, Ishii A, et al. Relationship between lower extremity muscle strength and all-cause mortality in Japanese patients undergoing dialysis. Physical therapy. 2014;94(7):947–956. doi: 10.2522/ptj.20130270. [DOI] [PubMed] [Google Scholar]
- 4.Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014;29(9):1655–1665. doi: 10.1093/ndt/gft070. [DOI] [PubMed] [Google Scholar]
- 5.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. Journal of the American Society of Nephrology. 2013;24(3):337–351. doi: 10.1681/ASN.2012010047. [DOI] [PubMed] [Google Scholar]
- 6.Kim JC, Shapiro BB, Zhang M, Li Y, Porszasz J, Bross R, et al. Daily physical activity and physical function in adult maintenance hemodialysis patients. J Cachexia Sarcopenia Muscle. 2014;5(3):209–220. doi: 10.1007/s13539-014-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P, et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clinical nutrition. 2008;27(4):557–564. doi: 10.1016/j.clnu.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett-Connor E, Lee CG, et al. Evaluation of the Usefulness of Consensus Definitions of Sarcopenia in Older Men: Results from the Observational Osteoporotic Fractures in Men Cohort Study. J Am Geriatr Soc. 2015;63(11):2247–2259. doi: 10.1111/jgs.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamarca F, Carrero J, Rodrigues J, Bigogno F, Fetter R, Avesani CM. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. The journal of nutrition, health & aging. 2014;18(7):710–717. doi: 10.1007/s12603-014-0505-5. [DOI] [PubMed] [Google Scholar]
- 10.Malmstrom TK, Miller DK, Herning MM, Morley JE. Low appendicular skeletal muscle mass (ASM) with limited mobility and poor health outcomes in middle-aged African Americans. J Cachexia Sarcopenia Muscle. 2013;4(3):179–186. doi: 10.1007/s13539-013-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren H, Gong D, Jia F, Xu B, Liu Z. Sarcopenia in patients undergoing maintenance hemodialysis: incidence rate, risk factors and its effect on survival risk. Renal failure. 2016;38(3):364–371. doi: 10.3109/0886022X.2015.1132173. [DOI] [PubMed] [Google Scholar]
- 12.Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Sarcopenic obesity and risk of cardiovascular disease and mortality: a populationbased cohort study of older men. J Am Geriatr Soc. 2014;62(2):253–260. doi: 10.1111/jgs.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68(9):1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 14.Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc Nutr Soc. 2015;74(4):405–412. doi: 10.1017/S002966511500169X. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obesity research. 2004;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 17.Honda H, Qureshi AR, Axelsson J, Heimburger O, Suliman ME, Barany P, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. The American journal of clinical nutrition. 2007;86(3):633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 18.Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol. 2015;10(7):1192–1200. doi: 10.2215/CJN.08550814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56(4):415–425. doi: 10.1016/j.pcad.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. American journal of epidemiology. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 24.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. American journal of epidemiology. 2004;159(4):413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]