Abstract
Extracellular vesicles (EVs) are a heterogeneous collection of membrane-bound carriers with complex cargos, including proteins, lipids and nucleic acids. While release of EVs was previously thought to be only a mechanism to discard nonfunctional cellular components, increasing evidence implicates EVs as key players in intercellular and even interorganismal communication. EVs confer stability and can direct their cargoes to specific cell types. EV cargoes also appear to act in a combinatorial manner to communicate directives to other cells. This review will focus on recent findings and knowledge gaps in the area of EV biogenesis, release, and uptake. In addition, we highlight examples whereby EV cargoes control basic cellular functions, including motility and polarization, immune responses, and development, as well as contribute to diseases, such as cancer and neurodegeneration.
Keywords: extracellular vesicles, exosomes, microvesicles
Introduction
The cellular release of molecules in association with membrane vesicles has been recognized for some time, together with the question as to what extent this represents “cell debris” [1]. It has become increasingly clear that cells release vesicles of varying sizes through both the endosomal pathway and by budding from the plasma membrane. These vesicles are referred to by a variety of names, including exosomes, microvesicles (ectosomes), microparticles and oncosomes, collectively termed extracellular vesicles (EVs). A large amount of work has been directed at understanding their protein and lipid components (see EVpedia: https://www.ncbi.nlm.nih.gov/pubmed/25388151, http://student4.postech.ac.kr/evpedia2_xe/xe/index.php?mid=Home; Vesiclepedia: http://microvesicles.org/browse; and Exocarta: http://exocarta.org/), as well as their physiological relevance. Interest in EVs was recently stoked by the finding that they contain RNA [2,3], with the implication that their protein and RNA content might be transferred between cells as a previously unrecognized form of intercellular communication. Indeed, the structure of EVs allows for protected and directed transfer of informative molecules between cells. Initial studies found both mRNAs and non-coding RNAs (ncRNAs), such as miRNAs, stably contained within EVs and showed that these molecules together with other EV cargo could be transferred to recipient cells in culture with functional consequences. Several reviews on current cell biological issues in the field are available (e.g. [4–6]). In the present review we point out some of the caveats in the field, and then focus on studies that address cellular functions of EVs, referencing recent, representative papers from the literature.
By way of caveats, the field is young and has a number of “black holes” in data that confound interpretation. For example, EVs of varying size, mode of biogenesis, and cargo can be released from a single cell, and these can change with the physiologic state of the cell. Different cell types may also produce distinct repertoires of vesicles. A current focus of the field is development of improved methods of isolation of these different EV subtypes, in parallel with identification of protein and lipid biomarkers to distinguish them (e.g. [7,8]). This could help to further assess the spatiotemporal fate of EVs in vivo, one of the important black holes in EV research. Also, EV cargo composition is complex consisting of hundreds to thousands of different proteins, unique lipids, some DNA and many small non-coding RNAs (sncRNAs), such as microRNA (miRNA), small nucleolar RNA, Y RNA, mitochondrial RNA, and vault RNA, as well as long ncRNA and mRNA (mostly fragmented) [9]. The possible informational content of most of these ncRNAs remains to be determined. Proteins are found on both the outside and inside of EVs and are likely indicators of both the biogenesis mechanisms and potential communicative functions. Thus, the “language” of EVs is by its nature combinatorial, multifaceted and contextually complex.
Different cell types are continuously exchanging EVs over short and long distances in vivo. In addition to their functions in communication, these vesicles also serve to eliminate molecules from cells, such as modified RNA [10] and amyloid proteins [11]. These “discarded cargoes”, however, can have consequences to neighboring cells, such as spreading of neuropathological diseases through the brain through toxic amyloids [11]. As a means of communication, the “code” by which EVs are addressed to specific recipient cells is only beginning to be deciphered [12]; however, it likely involves specific ligand-receptor interactions and glycoproteins.
EV subtypes and why vesicles are unique vehicles
Most EV studies have been carried out in cell culture, due to greater ability to obtain purified EV samples from defined cell types, as well as better control over experimental conditions. Moving into the future this will serve as an important platform for understanding their role in cell-to-cell communication in vivo [13]. Analysis is complicated by the wide array of communication mechanisms operating among cells which can be hard to distinguish. Cells traditionally have been considered to secrete some proteins, through the secretory pathway (e.g. endoplasmic reticulum (ER) to Golgi to plasma membrane), as well as to release and take up small molecules through transport channels and additional post-Golgi secretory vesicles, collectively termed the secretome. Other modes of cell-to-cell interaction (Fig. 1) include direct cell-to-cell contact with both ligand-receptor signaling and transport of small molecules, including miRNAs, across gap junctions [14]. Cells separated by short distances can also pass macromolecules, organelles, and nuclei through “tunneling” nanotubes [15], and microtubes [16].
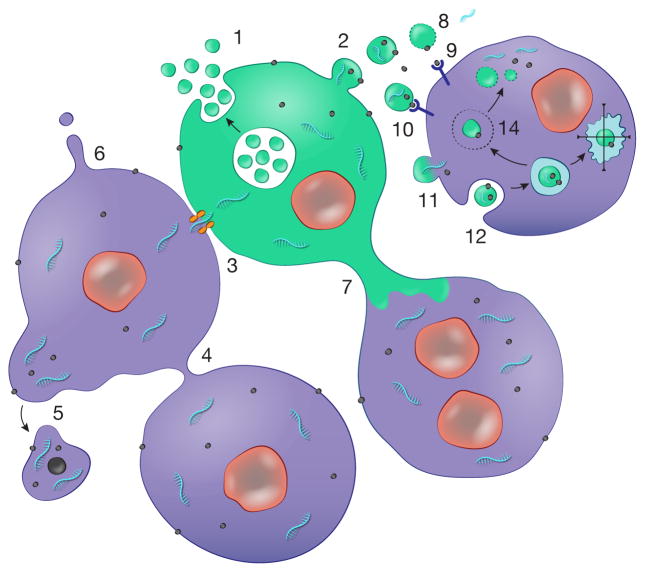
Fig. 1. Cellular sharing of macromolecular information.
Cells have a number of ways of exchanging molecules which are facilitated by being maintained within a membrane boundary. These include deployment of EVs by: 1) release of exosomes through fusion of MVBs with the plasma membrane, and 2) budding of microvesicles off the plasma membrane. 3) In addition, cells in physical contact can form gap junctions allowing exchange of small molecules, including miRNAs. Other modes include: 4) connection of cells through nanotubes; 5) blebbing off of larger vesicles, especially from cancer cells e.g. oncosomes; 6) formation of membrane protrusions which release vesicles from their tips; and 7) larger diameter microtubes connecting cells. In the case of EVs there are a number of ways for information transfer: 8) lysis of vesicles in the extracellular space releasing their contents, including 9) free ligands and 10) ligands on the surface of vesicles, which stimulate receptors on the cell surface. Uptake of EV cargo can occur through: 11) fusion of the vesicle with the plasma membrane or 12) uptake by different types of endocytosis. In the latter case the fate of the vesicle and its content can be: 13) progression through the degradative pathway to lysosomes; and/or 14) escape from the endosome compartment to release contents into the cell cytoplasm where they may be functional. References for these pathways are given in the text.
A number of EV subtypes have been characterized. Traditionally, exosomes are small EVs (sEVs; < 150 nm) released through multivesicular bodies (MVBs) in the endosomal pathway. Vesicles can also bud off the plasma membrane, apparently in a manner similar to that of retroviruses [17], forming EVs in the 200–500 nm range. These shed vesicles are called microvesicles or ectosomes. However, smaller vesicles (~100 nm) have also been described to bud from the plasma membrane and may be isolated together with exosomes [18]. Other modes of release include formation of EVs at the ends of microvillar-like protrusions, which can be accentuated by increased cellular content of hyaluronan [19]. In cancer cells, even larger EVs (1–10 μm in diameter), termed large oncosomes, can bleb off the cell membrane [20,21]. In addition, when cells undergo apoptosis they dissociate into membrane bound apoptotic bodies of different sizes, which are hard to distinguish from other types of EVs, but may contain relatively more genomic DNA. Due to the often unclear composition of purified vesicle preparations, which are usually isolated based on size and density, the terms sEVs and large EVs (lEVs) have been proposed for studies that do not clearly define the biogenesis mode of the EVs in their preparations [4]. A major challenge for the future and a current focus of the field is to both define and isolate distinct subpopulations, either according to their biogenesis mechanism or their molecular content.
It seems likely that the mode of EV biogenesis will determine their protein, RNA and DNA content. A number of groups are developing techniques to define markers for different types of EVs. Iodixanol gradients allow resolution of EVs of different buoyant densities and sizes, and are typically employed after initial collection of 10,000 × g and 100,000 × g ultracentrifugation pellets. Using such a density gradient method for proteomic characterization of EVs from dendritic and other cell types, it was found that syntenin-1, EHD4, annexin XI and ADAM10 were unique to sEVs, while actinin-4 and mitofilin were highly abundant on lEVs [7]. In another study, EVs released from immortalized mesenchymal stem cells (MSCs) were captured based on lipid-binding ligands on their surface, including GM1 ganglioside which binds to cholera toxin B (CTB), phosphatidylserine to annexin V, and globotriaosylceramide to Shiga toxin B (STB) [8]. The CTB and STB binding populations were apparently distinct, with only the latter carrying RNA and fibronectin. Unfortunately, the consequences of ultracentrifugation on the structural integrity of EVs are unknown. Ultracentrifugation does induce aggregation of multiple EVs [22] and could potentially induce changes in EV structure. Over the next few years, we anticipate a barrage of new EV isolation and characterization methods, as well as insights into their biogenesis, fate and function.
EVs are unique in that they can be viewed as cell “biopsies” that are released as free, relatively stable entities that can distribute over short and long distances within the extracellular spaces and biofluids of organisms. Their stability is attributed in part to the lipid content of their membranes, which are enriched in cholesterol, sphingomyelin, annexin, phosphatidylserine and glycosphingolipids, as compared to the cellular plasma membrane [23]. EVs have the ability to protect internal cargo and the potential to deliver it to specific cell types through ligand-receptor interactions. One intriguing parameter is their potential to cross tissue barriers. Several studies even support their ability to cross the blood-brain barrier (e.g. [24]). Although the mechanism is not known, one possibility is that EVs transcytose through endothelial cells, entering a cell through the endocytic system and exiting through MVBs. Their potential for addressability and barrier penetration makes them promising therapeutic delivery vehicles [25]. Their universal presence in all biofluids, release from all different cell types in the body, changes in molecular content based on cell of origin and pathophysiological state of cells makes them remarkable biomarkers [26].
EV biogenesis and release
EV biogenesis
EVs are thought to be formed by multiple mechanisms. In all cases, lipid curvature must be induced to form either an inward-budding vesicle within the endocytic system (exosomes) or an outward budding vesicle at the plasma membrane (microvesicles). For exosomes, several mechanisms have been described. The best-characterized mechanism involves recruitment of the endosomal sorting complex required for transport (ESCRT) machinery to ubiquitinated proteins in the early endosome. The ESCRT machinery consists of four protein complexes (ESCRT-0, -I, -II, and -III) along with accessory proteins (Alix, VPS4, VTA-1) that sequentially act to bind future exosome cargoes and form intraluminal vesicles (ILVs) incorporating those cargoes [27]. Notably, the ESCRT-III complex forms spirals that induce the inward budding and fission of vesicles in order to form MVBs [28–30]. An alternate pathway of exosome formation, more recently described, involves synthesis of ceramide as a mechanism to induce vesicle curvature and budding [31]. Certain cargoes have been shown to preferentially depend on ceramide synthesis [31,32] for their presence in ILVs of MVB. A third mechanism that has been proposed for exosome biogenesis is tetraspanin-mediated organization of specific proteins, such as the amyloidogenic protein, premelanosome protein (PMEL) [33,34].
The description of multiple mechanisms of biogenesis has created some confusion both for insiders and outsiders of the field. Whereas it was originally thought that the ESCRT machinery consisted of a totally essential set of protein complexes that progressively execute stages of ILV formation, recent data suggest that earlier components of the pathway may be more essential than later ones. Thus, while knock-down of the ESCRT-0 and -I proteins Hrs, STAM1, and TSG101 decreases exosome release, knock-down of other ESCRT components can have no effect or increase exosome release from cells [35]. Furthermore, even simultaneous knock-down of multiple ESCRTs can lead to continued formation of ILVs, although they are fewer, of abnormal size, and do not contain EGFR, a classic ubiquitinated protein sorted by ESCRTs to ILVs [36]. Nonetheless, it is not totally clear how separate the proposed pathways of exosome biogenesis truly are [37]. For example, the proposed ceramide mechanism accounts for membrane curvature but not cargo sorting, except for some self-assorting of cargoes into specific lipid domains. It also seems likely that ESCRT-mediated cargo sorting could synergize with ceramide-induced lipid curvature to create vesicles that utilize both mechanisms. The existing studies have used knock-down approaches and it is possible is that the remaining amounts of ESCRT proteins after knock-down are sufficient to induce some ILV formation. Furthermore, ceramide- and tetraspanin-mediated mechanisms may not be totally independent, but could compensate to allow ILV formation in the absence of ESCRT. This is a tricky area, and gene editing techniques to obtain full knock-outs may help resolve the issue of the essentiality of various components, as well as whether they involve separate or collaborating mechanisms.
For EVs that are formed by direct budding from the plasma membrane, e.g. microvesicles, the molecular mechanisms of biogenesis are even less well characterized. One mechanism involves recruitment of the same ESCRT machinery that promotes formation of ILVs in MVB and viral budding. Thus, both exosomes and microvesicles can be formed and pinched off through recruitment of the negative curvature-promoting ESCRT III proteins [28–30,27]. In one case, the adaptor protein, arrestin domain containing protein 1 (ARRDC1) was shown to recruit ESCRT proteins TSG101 (ESCRT-I) and VPS4 (accessory protein) to the plasma membrane [18]. Vesicle budding can occur in response to plasma membrane wounding, as a mechanism to repair damaged membrane [38,39]. Similar to exosome biogenesis, mechanisms that generate or alter asymmetry of the plasma membrane with respect to lipids also appear to be important [40]. These mechanisms include alterations in activity of enzymes that transfer lipids from one leaflet of the plasma membrane, such as flippases, floppases, and scramblases [41]. For example, in C. elegans, inhibition of a lipid flippase that transfers phosphatidylethanolamine from the outer leaflet of the plasma membrane to the inner leaflet promotes vesicle budding [42,43]. Alterations in ceramide content on the outer leaflet via activation of acid sphingomyelinase can also induce membrane curvature and trigger microvesicle release [44–46]. Shedding of vesicles has been shown to occur from multiple aspects of the plasma membrane, including at microvillar protrusions of intestinal epithelial cells [47] and from cells engineered to overexpress hyaluronan synthase [48], as well as from cilia [49].
EV release
Extracellular vesicles (EVs) are released from cells by a variety of mechanisms, depending on their mode of biogenesis [50] (Fig. 1). Microvesicles are released quite simply when they pinch off from the cell surface. Exosomes, which derive from the endocytic system, are released when MVBs fuse with the plasma membrane. An alternate fate for MVBs is fusion with lysosomes, which leads to degradation and recycling of their protein, nucleotide and lipid contents. As exosomes carry growth factors and other signaling molecules, fusion of MVB with lysosomes should turn off autocrine signaling. By contrast, fusion with the plasma membrane to release exosomes directly into the cytoplasm could both promote autocrine and paracrine signaling. A number of steps could affect the intracellular decision to degrade or release exosomes, including intracellular transport of MVB along microtubules to the plasma membrane, creation of docking sites at the plasma membrane, or recruitment of Soluble NSF Attachment Protein Receptor (SNARE) proteins that mediate fusion with either lysosomes or the plasma membrane. In addition, autophagosomes have been shown to fuse with MVBs and target them to lysosomes. Molecular regulators implicated in exosome release include multiple molecules implicated in MVB docking including the GTPases Rab27a, Rab27b, Rab35, and RalA [51–53], and the cortical actin regulator, cortactin [54], as well as the fusion regulator synaptotagmin-7 [55].
EV interactions with cells: Binding, uptake, fusion and fate?
The topology of EVs is similar to cells, with extracellular receptors and ligands positioned on the outside, and cytoplasmic proteins and RNAs on the inside. Thus, in order for EVs to functionally communicate with cells, different types of interactions may be involved. This could include release of EV contents in the extracellular space, EV binding to the cell surface, EV-plasma membrane fusion, and uptake by endocytosis (Fig. 1). For stimulation of cell signaling by EV-associated extracellular ligands, EVs may directly interact with cognate receptors located on the plasma membrane of cells (or vice versa). This recognition may also serve as a means of “addressing” EVs to certain cell types [56,12]. Such ligand-receptor interactions likely accounts for many targeted biological effects of EVs, including those caused by EV-carried growth factors, angiogenic factors and extracellular matrix (ECM) proteins. For delivery of RNAs or cytoplasmic proteins, EVs must not only bind to, but also release their contents into recipient cells, either by direct fusion with the plasma membrane or with the endosomal membrane after endocytosis.
Several factors may determine the likelihood of EV-cell fusion occurring. First, close apposition of the two membranes due to ligand-receptor binding or glycoprotein interactions is necessary. Second, the lipid composition of EV and cellular membranes is likely to affect their propensity to fuse. Thus, high lipid raft content in EV may facilitate their fusion with cells [57]. In addition, acidic pH in the extracellular environment has also been shown to enhance EV-membrane fusion [58], suggesting that EV fusion with the plasma membrane may occur preferentially in acidic environments, such as occurs in tumors or in the stomach. It also suggests that the acidic environment found in endosomes may enhance fusion after EV uptake. Uptake of EVs into the endocytic system appears to be the most common mode of uptake and may be promoted by specific stimulation of cellular receptors by EV-bound ligands [57]. Interestingly, a recent study showed that exosomes may initially bind to filopodia and rapidly move inward to be internalized at endocytic hotspots at the filopodia base [59]. Within the cell, the exosome-containing endosomes were found to scan the ER before fusing with lysosomes. It is unclear how cargo delivery takes place within the cell, but it is possible that either a transient “kiss and run” fusion event or full fusion between the EV and endosomal membranes might deliver cargo into the cytoplasm prior to lysosomal fusion with subsequent degradation of cargo. Since the ER has recently been identified as a site of nucleation for the RNA Interference Silencing Complex (RISC) [60,61] through binding of ribosome-bound mRNA [62], an interesting possibility is that the interaction between exosome-containing endosomes and the ER might lead to functional activity of miRNA delivered via exosomes.
Roles of EVs in cellular functions
Cellular migration and invasion
Cellular migration requires well-timed coordination of cellular orientation, protrusion formation and adhesion to ECM proteins, and EVs have been implicated in important roles in all these processes. In vivo, EVs have been shown to promote cell migration. Thus, genetic inhibition of exosome secretion decreased the speed and directionality of cancer cells migrating in a chick embryo model [63]. In addition, EV transfer from malignant cells to less malignant cells was associated with increased migration of recipient cells in xenograft tumors in mice, both locally and at distant sites [64]. In culture, EVs have been shown to enhance the chemotactic response of neutrophils to the bacterial product f-Met-Leu-Phe by generating the lipid signaling molecule, leukotriene B4 (LTB4) via the activity of EV-associated 5-lipoxygenase [65]. EVs can also promote leading edge protrusion via activation of planar cell polarity (PCP) pathways. Thus, Wnt11-loaded CD81-positive vesicles can induce protrusive behavior of breast cancer cells, migration in culture, and metastases in vivo [66]. Finally, EVs can promote migration, invasion, and metastasis through interaction with ECM [63,12].
EV interactions with the ECM are multifaceted. ECM molecules bound to integrins on EVs can be released in an autocrine manner to form an adhesive substrate that facilitates speed of cell migration [63]. Integrins on EVs may also bind to the ECM in tissues as a potential means of creating premetastatic niche sites [12]. The binding of EVs to ECM components is based, at least in part, on ligand-receptor interactions, e.g. α6β4 and α5β1 integrins on EVs bind to matrix molecules laminin-332 and fibronectin, respectively [63,12]. Finally, EV-associated proteases, such as matrix metalloproteinases (MMPs), can degrade the ECM, thereby remodeling tissues and increasing invasion and migration of tumor cells [67,55,68,69].
Immunity
EVs play a complex role in immune responses (Fig. 2) and can influence both adaptive and innate immunity through exchange of EVs among multiple types of immune cells. Intracellular vesicle transport is crucial for MHC class-II mediated antigen presentation [70]. After delivery to the plasma membrane from the Golgi, clathrin-mediated endocytosis of cellular membrane MHC class II complexes can result in the incorporation of MHC class-II αβ dimers into ILVs within MVBs and their limiting membrane. Antigen peptide binding to MHCII occurs within this compartment. Peptide-bound MHCII in the limiting membrane of the MVB can be directly recycled back to the cellular membrane. In addition, some MVBs directly fuse with the plasma membrane releasing the ILVs with incorporated peptide-MHCII into the extracellular space as EVs [71]. These EVs are potent in inducing an immune response, as antigen-specific T-cell activation can be induced by dendritic cell (DC) secreted-EVs, either indirectly through DCs that express the co-stimulatory molecules CD80 (B7-1) and CD86 (B7-2) [72] or directly by ICAM-1-presenting mature DC-derived EVs [73]. EVs purified from DCs have been shown to differentiate T helper cells towards a T-helper 1 (Th1) phenotype and to enhance in vivo immunogenicity [74].
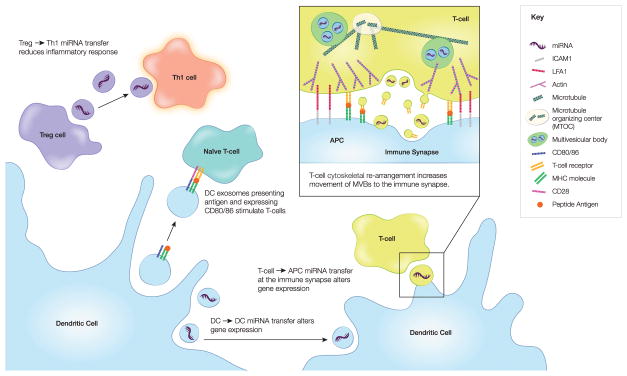
Fig. 2. EV-mediated communication in the immune system.
EV exchange is an important part of the communication between immune cells. In particular, T cells have been shown to both receive and deliver messages via EVs. Thus, peptide-containing MHC on EVs can stimulate naive T cells. In addition, miRNAs transferred in T-cell derived EVs can influence recipient cell gene expression. A critical structure where much of this takes place is the immune synapse, a cell-cell adhesion structure characterized by cytoskeletal reorganization. Both shed EVs containing the T cell receptor (TCR) and exosomes carrying miRNAs are transferred at the immune synapse.
Much of the EV exchange between T cells and antigen presenting cells (APCs) takes place at the immune synapse, a site of cell-cell adhesion that promotes T-cell activation (Fig. 2). In addition to the EV flow from APCs to T-cells for the purpose of antigen presentation, T-cells also release EVs at the immune synapse in order to influence target cells. T-cells have been shown to release both exosomes and microvesicles. In the case of exosomes, T cell MVBs were shown to translocate to the immunological synapse before fusion with the plasma membrane [75]. These exosomes contain miRNAs (e.g miR-335) that are internalized into the APCs and subsequently reduce target mRNA expression levels, as detected in a miR-335 target SOX4-luciferase reporter assay. In addition, through a combination of live cell imaging, correlative light-electron microscopy and biochemical experiments, T cell receptor (TCR)-containing EVs were found to bud from T cells at the immune synapse and engage peptide-bound MHC in recipient antigen-presenting B-cells [76]. These experiments suggest that EVs are part of a dynamic interchange between immune cells that includes communication taking place at a distance, such as stimulation of transmembrane receptors.
In addition to ligand-receptor interactions, the RNA cargo of DC EVs can influence immune cell function. miRNA transfer from DC to DC has been shown to involve EVs and to lead to alterations in recipient cell gene expression [77]. Recently, it was also shown that T regulatory (Treg) cells reduce Th1 (CD4+IFNgamma+) inflammatory responses by EV transfer of miRNA (especially let-7d) to Th1 cells [78]. Double knock-out of Rab27a and Rab27b, shown to reduce exosome release [51], reduced Treg EV release and subsequently reduced the Th1 modulation of Treg cells. miRNAs carried by EVs can also activate Toll-like receptor (TLR) 8 signaling which induces cytokine secretion, by presumably mimicking viral RNA [79]. Conversely, EV transfer of miRNAs from MSCs to macrophages suppresses the macrophage immune response by altering NFkB signaling and downstream reduction of TLR (MyD88-dependent) pattern recognition [80].
Overall, the combination of RNA and ligand-receptor interaction between diverse immune and non-immune cell types is likely to contribute to complex immune responses. One could imagine that dynamic feedback via EVs may lead to both transient and more long-term immune modulatory responses that fine-tune the ability to fight diverse pathogens, while preserving host tissue.
Physiological roles of EVs
Increasing evidence supports a fundamental role for EVs in cell-cell communication during normal development and adult physiology.
Development
During development, EVs may have diverse roles in setting up the body plan and determining tissue organization [81]. Morphogen gradients control cell fate during body patterning. While soluble molecules are implicated in this process, the long distances over which the gradients are generated and maintained has suggested that passive gradient generation alone may be insufficient. The contribution of membranous components called argosomes was first described as a possible mechanism to actively generate stable gradients in Drosophila [82]. The discovery that the key morphogen molecules Wingless (Wnt) and Hedgehog are trafficked through MVBs and carried by exosomes further supports the idea that EVs may be an important component of morphogen gradients [83–85]. Nonetheless, further experimental support will be required to solidify the role of EVs in morphogenesis. Notably, mice deficient for several key proteins involved in EV biogenesis (neutral sphingomyelinase2) or secretion (Rab27a/b) regulators are viable and fertile, albeit with defects in growth and bone ossification (nSMase2) or in immune, platelet, and pigmentation functions (Rab27a/b) [86–91]. Conversely, knock-out of the ESCRT-0 and ESCRT-1 genes, Hrs and Tsg101 that promote exosome biogenesis leads to embryonic lethality [92,93] with developmental (Hrs) and implantation (TSG101) defects. It is unclear whether the differences between these phenotypes reflects EV-independent roles of ESCRT proteins, redundancy or tissue-specific compensation by some EV regulators, selectivity of some EV regulators for a subset of EVs, or lack of requirement for EVs in development. Nonetheless, this is an intriguing area for future investigation.
The tissue patterning that occurs in response to morphogen gradients involves cell polarization –that is, the structural specification of the anterior, posterior, ventral and/or dorsal sides of a cell. Recently EVs have been implicated in several aspects of cell polarity, including planar cell and front-back polarity during cell migration [66,63,65]. A cellular structure associated with cell polarization in tissues is the primary cilium, a nonmotile cilium structure that is present in most nondividing cells as one cilium per cell. Primary cilia serve as cellular antennae, receiving and responding to chemical, mechanical, and thermal signals from the environment [94]. In most organisms, they are also critical for Hedgehog signaling [95]. The importance of primary cilia during development has been recognized by the discovery of diseases, such as polycystic kidney disease and congenital cardiac asymmetry disease that occur due to primary cilium dysfunction [94].
Recently, primary cilia have been identified as sites of vesicle release [96–100]. Notably, key polycystic kidney disease molecules, the polycystins are cargos of primary cilia EVs and regulate Ca++ and mechanotransduction signaling in cells [99]. In related flagellar structures of the unicellular organism Chlamydomonas sporangia, these EVs are clearly shed “ectosomes” (microvesicles), as they have been observed budding from the cilia by differential interference contrast (DIC) microscopy [49]. In C. elegans, the lack of MVB near the primary cilium combined with lack of effect of ESCRT-0 and -1 inhibition on cilia-associated vesicle shedding suggests that C. elegans neuronal cilia EVs are also likely to be ectosomes [97]. In addition, EM images of primary cilia from mammalian neuroepithelial progenitor cells are suggestive of budding as the mechanism of EV release from primary cilia [100,101].
Fertilization and mating behavior
EVs are known to promote sperm-egg fusion, dependent on the tetraspanin CD9 [102]. After fusion, EV shedding from the fertilized egg inhibits polyspermy by rapid removal of the sperm receptor from the plasma membrane [103]. In the early embryo, EVs released by stem cells in the inner cell mass contain laminin and fibronectin which bind to integrins on the trophoblasts, leading to activation of JNK and focal adhesion kinase (FAK) pathways promoting migration and implantation [104].
EVs can also affect mating behavior. In Drosophila, remating of females can be inhibited by exosomes released from male reproductive glands in response to bone morphogenetic protein signaling [105]. Although the responsible cargoes are unknown, the male EVs interact with female reproductive tract epithelium and alter female mating behavior. Genetic studies in C. elegans on EVs released by ciliated neurons also suggest that EVs control mating behavior. Thus, male animal-derived EVs affect male-male tail chasing behavior [97] and mate-searching behavior, in which males leave food in order to search for mates if no hermaphrodites are present [98]. While the mechanism that underlie C. elegans mating behavior changes are unknown, the presence of the polycystin PKD-2 in EVs appears to be essential.
Nervous system
Communication is the essential function of the nervous system, requiring multiple types of interactions between diverse cell types. Thus, it is not surprising that EVs play a role in various aspects of neuronal communication (Fig. 3). For the most part, EVs have been implicated in extrasynaptic control of cellular interactions. For example, exosomes released from nerve terminals at the developing Drosophila neuromuscular junction (NMJ) drive synaptic morphological expansion, although they do not directly affect synaptic signaling (Fig. 3) [106–108]. A Wingless (Wg)-Evi complex on the released exosomes interacts with muscle cells and promotes synaptic growth [107]. Due to its hydrophobicity, it would be difficult for soluble Wg to access its receptors on the muscle junctional region; thus, exosome-linked Wg is thought to travel more easily to its target site.
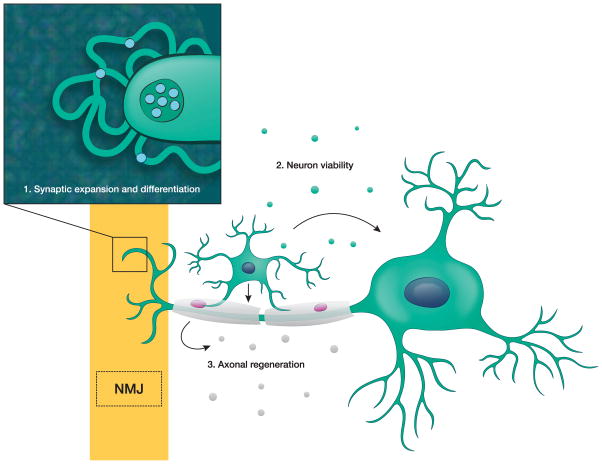
Fig. 3. EV-mediated communication in the nervous system.
Neurons communicate with a variety of cells via EVs: 1) At the neuromuscular junction (NMJ), neuron-derived EVs promote synaptic expansion and differentiation; 2) In the central nervous system, oligodendrocyte-derived EVs promote neuronal viability and firing rate; and 3) In the peripheral nervous system, Schwann cell EVs promote axon regeneration.
EVs are also important for glia-neuron communication. In the rat peripheral nervous system, EVs released from Schwann cells promote regeneration of nerve axons [109], both in culture and in vivo. In vivo, injection of Schwann cell EVs enhanced axon regeneration after sciatic nerve crush injury. One potential mechanism for this effect could be EV-mediated inhibition of RhoA, a known suppressor of axon regeneration. In addition, transfer of ribosomes in EVs from the Schwann cells into the damaged axon augments new protein synthesis [110]. In the central nervous system, exosomes secreted by oligodendrocytes have been associated with enhanced neuronal viability and an increase in neuron firing rate [111,112]. Less is known about the role of EVs in neuron-neuron communication, although recently neuronal EVs carrying Ephrin B2 have been shown to induce growth cone collapse, a critical component of axon pathfinding [113]. Together, these studies suggest that an important role of EVs in the nervous system is to promote structural remodeling in a diverse set of circumstances.
EVs in pathophysiological processes
EVs have been implicated in the pathogenesis of multiple diseases. Two particularly active areas of research include the roles of EVs in cancer and in neurodegeneration. Here we highlight the emerging role of EVs in both diseases. However, many of the discussed articles are the initial reports of a specific phenomenon. Confirmation by independent groups will be required to assess all the pathophysiological effects of disease-specific EVs. This analysis is complicated as standardized methods to isolate and/or identify EV subtypes in culture are not fully developed. It remains to be determined if the described effects are generalizable to all EVs in culture and in vivo, or if the reported observations are due to a specific EV subpopulation that may only be present under certain circumstances.
Cancer
The tumor microenvironment includes different normal cell types that function as an ecosystem to support tumor growth, invasion, and metastasis. These tumor-associated cells include endothelial cells, fibroblasts, pericytes, histiocytes and infiltrating immune cells [114], as well as astrocytes, microglia and infiltrating macrophages in the brain. While cell-to-cell communication is often attributed solely to soluble chemokines, cytokines, small molecules and growth factors [114], in recent years a multitude of studies have implicated EVs as having a significant impact in the tumor microenvironment (Fig. 4) [115].
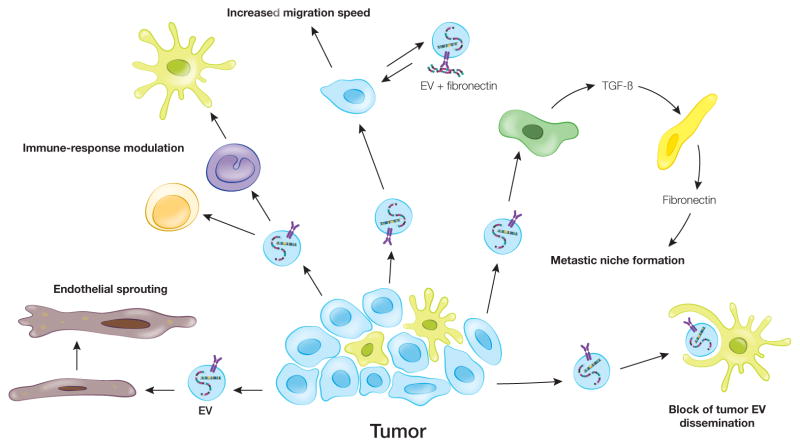
Fig. 4. Role of EVs in cancer.
Cancer-derived EVs influence both stromal and tumor cells. These EVs can induce endothelial sprouting and neovascularization. Incubation of cancer-EVs with T-cells leads both to apoptosis in CD8+ T-cells and the expansion of CD4+ towards T-regulatory cells. Monocytes differentiate towards a more tumor supportive phenotype after incubation with cancer-EVs. As cancer cells migrate, EVs from tumor cells can accelerate this process. EVs from malignant cancer cells induce less-malignant cells to migrate faster; however, the mechanism for this is unknown. Tumor cells load fibronectin onto EVs in an autocrine manner facilitating adhesion formation and rate of migration. Tumor-derived EVs can also set up metastatic niches at a variety of locations. In the liver, cancer-EVs induce TGF-beta production by Kupffer cells, which promotes fibronectin production by hepatic stellate cells. This fibrotic environment enhances retention of neutrophils and macrophages in the liver creating a favorable metastatic niche. In some cases, local cells may counter the effect of cancer-EVs, such as subcapsular macrophages that limit dissemination of cancer-EVs from lymph nodes.
As tumors progress, they outgrow the in situ vascular network, thereby generating hypoxic regions. Hypoxia induces the release of neo-vascularization-stimulating factors. In culture, after uptake of glioblastoma-derived EVs, levels of vascular endothelial growth factor (VEGF) increase in endothelial cells and activate the VEGF-receptor 2 in an autocrine manner [116]. Under hypoxic conditions, glioblastoma-derived EVs are also enriched for hypoxia-regulated mRNAs and proteins. Compared to EVs produced under normoxic conditions, the EVs released from hypoxic tumors act to increase the activation of the ERK1/2 MAPK, PI3K/AKT, and FAK pathways in recipient endothelial cells, resulting in more endothelial cell sprouting - an early step in neo-vascularization [117].
Myofibroblasts are fibroblasts that are activated to express α-smooth muscle actin (α-SMA). Myofibroblasts are relatively absent in normal tissue, but highly present in the tumor microenvironment. Similar to their function in non-cancer fibrotic diseases, their pro-tumor function is probably in large part due to their secretion of different ECM components [114]. In prostate cancer, cultured fibroblasts can be differentiated into myofibroblasts by uptake of prostate cancer EVs containing transforming growth factor (TGF)-β1 [118]. In vivo, the synergy between fibroblasts and tumor cells in promoting tumor growth is reduced by knock-down of Rab27a in prostate cancer cells, a potential regulator of exosome release. However, it cannot be ruled out that the observed effects may be due to the effects of Rab27a knockdown on additional cellular processes or secretion modes.
The tumor microenvironment contains many immune cells, which are often inhibited in their ability to kill tumor cells. Recently, immune checkpoint therapy which targets inhibitory PD-1 and CTLA-4 signaling has been successful in treating subsets of patients with multiple tumor types by reactivating suppressed T and NK cells [119]. Tumor-associated macrophages (TAMs) are often implicated in this suppression and can also induce apoptosis in T cells through the expression of TRAIL and Fas ligand (FasL) [120]. Tumor-derived EVs are likely to influence this behavior by promoting an immunosuppressive macrophage phenotype. Thus, EVs from primary glioblastoma cells were shown to skew monocyte-to-macrophage differentiation towards a tumor supportive phenotype in culture [121]. The presence of tumor cells also increased levels of miR-21 in microglia and macrophages in culture and in vivo, in association with changes in their phenotype [122]. In an in vivo melanoma model, macrophages more distant from the tumor have also been found to interact with tumor-derived EVs in association with suppression of tumor growth. For example, subcapsular macrophages in lymph nodes can limit tumor growth by absorbing tumor-derived EVs and preventing them from interacting with pro-tumor B cells [123].
Like TAMs, tumor cell-derived EVs can also directly induce apoptosis in activated T-cells. For example, co-incubation of head and neck squamous cell carcinoma or melanoma cell line-derived EVs, but not normal DC-derived EVs, induced apoptosis in CD8+ T-cells [124]. The tumor-derived EVs were variably loaded with FasL, suggesting EV-FasL induced apoptosis is part of this cell death mechanism [124]. Another mechanism by which EVs can suppress anti-tumor immunity is by altering the differentiation of CD4+ T helper cells. Thus, co-incubation of CD4+ T-cells with tumor EVs was shown to induce their differentiation towards CD4+CD25+FOXP3+ Treg cells that can suppress the cytotoxic T-cell response [124]. Besides ligand-receptor interactions, the lipid composition of the EV-bilayer may also determine the effect of EVs on T-cells, as in ovarian cancer cell-derived EVs, phosphatidylserine linked to the outer leaflet of the EV bilayer is responsible for arresting the T-cell signaling cascade [125].
Tumor cell migration and invasion can be enhanced by EVs and likely facilitate local and distant spread of tumor cells. Some organ sites appear to be more hospitable to the metastatic seeding of particular tumor cells, termed “metastatic organotropism”. Prior to formation of metastatic tumors by actual metastatic cells, these tissue sites may be primed by factors released from the primary tumors and are referred to as “pre-metastatic niches” [126]. Recent studies suggest that tumor-derived EVs may be important priming factors that help set up metastatic niches, typically by interacting with normal host cells at the metastatic site. For example, EVs from pancreatic ductal adenocarcinoma cells can initiate liver pre-metastatic niche formation by transferring migration inhibitory factor (MIF) to Kupffer cells in the liver [56]. These Kupffer cells then secrete TGF-β that subsequently stimulates hepatic stellate cells to produce fibronectin. Fibronectin supports retention of macrophages and neutrophils in the liver, setting up an environment favorable for metastasis. In a syngeneic mouse model of melanoma, melanoma-derived exosomes induced lung vascular leakiness and recruited bone marrow-derived macrophages to metastatic sites [127]. In addition to the role of tumor derived EVs implicated in tumor growth and progression EVs derived from normal cells can support tumor growth. For example, in metastases to the brain, EVs from astrocytes appear to have an important role in supporting local tumor growth [128]. Astrocyte EV-mediated transfer of miR-19a to metastatic breast cancer cells reduced the levels of one of its target mRNAs encoding the tumor suppressor PTEN. In addition, uptake of astrocyte EVs by the metastatic cells induced chemokine CCL2 secretion promoting influx of microglial and myeloid-derived cells into the tumor microenvironment in vivo. Consistent with the tumor supportive role of these infiltrating cells, co-cultures of metastatic breast cancer cells and a microglial cell-line enhanced proliferation and inhibited apoptosis of the tumor cells [128].
The possible mechanisms through which certain EVs home to specific target organs and induce site-specific metastasis remains a major question. Recently, quantitative mass-spectrometry of EVs of breast or pancreatic cancer cell-lines that primarily metastasize to the lung, liver or both identified EV-integrin patterns associated with organotropic metastatic potential [12]. EVs expressing α6β4 and α6β1 integrins were associated with lung metastasis, whereas those with αvβ5 integrins were linked to liver metastatic potential. Conceptually, the idea that adhesion proteins can mediate metastasis to diverse sites has a certain appeal, as integrin-ECM or integrin-cell (e.g. Kupffer cells in the liver) interactions could target EVs to specific tissues or cell-types within tissues. However, it is difficult to accurately track the distribution of tumor-derived EVs in the body, and it seems likely that additional factors, such as selective vascular leakiness and the ECM of specific organs are critical in organotropism of metastasis [127]. Together these studies suggest a role for tumor derived EVs in promoting both primary tumor growth and metastatic spread.
Neurodegenerative diseases
A number of neurodegenerative diseases are associated with infectious isoforms or misfolded proteins, including prion (PrP conformational isoform: PrPSC), Alzheimer’s (β-amyloid plaques) and Parkinson’s disease (α-synuclein fibrils). Since in many of these diseases the aggregating proteins are carried by EVs, EVs are considered to have a role in cell-to-cell transmission of the toxic proteins and (pre-)fibrils [129,130]). In prion-related diseases - the transmissible spongiform encephalopathies, beta-sheet conformations of prion protein (PrPSC) can propagate and be transmitted between both cells and organisms [131]. PrP (both PrPSC and the non-infectious normal isoform PrPC) are GPI-anchored on the surface of EVs [132]. EV release was recently shown to increase cell-to-cell PrPSC infection, indicating that EV-associated PrPSC has infectious potential [131]. In Alzheimer’s disease, in vivo reduction of plaque formation was reported after administration of a drug inhibiting neutral sphingomyelinase 2 (nSMase2), which acts in part to reduce EV biogenesis [133,27]. This suggests disease-enhancing potential of EVs in Alzheimer’s disease. However, a protective function of EVs in Alzheimer’s disease has also been reported, as PrPC, present on the surface of EVs serves as a receptor for toxic Abeta42 peptide [134] and can thus sequester β-amyloid assemblies in vivo [135].
The role of EVs in Parkinson’s disease is also dichotomous. In juvenile-onset parkinsonism PARK9/ATP13A2, an ATPase ion pump localized to MVBs, is mutated [136]. Loss of PARK9/ATP13A2 function results in diminished release of exosome-associated α-synuclein, which may normally allow for disposal of toxic oligomers. In this context, exosomes may reduce intra-cellular α-synuclein levels in Parkinson’s disease [137,138]. However, other reports (e.g. [139]) support the transfer of toxic α-synuclein oligomers from cells of origin to other cells, hence spreading the disease via EVs. It is currently unknown if EV-associated α-synuclein relates to the recently discovered α-synuclein plasma membrane binding partner lymphocyte-activation gene 3 (LAG3) [140] which could mediate either endocytosis of α-synuclein preformed fibrils associated with EVs or lead to subsequent targeting of α-synuclein preformed fibrils to EVs after endocytosis. In Alzheimer’s and Parkinson’s disease, the final presentation of disease may be due in part to an imbalance in the disease stimulating and reductive potential of EVs. Thus, yin-yang studies suggest both that EVs can be involved in transmission of disease-causing proteins between cells and even organisms, and that they can serve as disposal routes for toxic proteins or otherwise mitigate the toxic effects of these proteins. More research is needed to elucidate the balance between reducing toxicity and propagating pathology by EVs in different degenerative diseases.
Concluding Remarks
The recent explosion of EV studies has demonstrated their potentially critical role in cell-to-cell communication. Due to the diversity of EV biogenesis mechanisms and the complexity of EV cargo content, many outstanding challenges and questions remain (see Outstanding Questions). One challenge for the future will be to ascribe specific functions to subclasses of EVs and identify the underlying molecular mechanisms. Technological advances in EV purification and analysis as well as advances in the ability to control EV biogenesis and cargo content – all active areas of research -- will likely move this fast-growing field forward. It will be important to rigorously evaluate experiments in this field to avoid over-interpreting tantalizing findings.
Outstanding Questions box.
What are the unique features of exosomes and can they be differentiated from other subgroups of extracellular vesicles (EVs)? Exosomes, microvesicles and large oncosomes each probably represent distinct subpopulations of EVs. Development of techniques to accurately separate these subpopulations will greatly facilitate elucidation of their unique properties.
Which molecular cargoes mediate specific EV functions and do they do so singly or in combination with other EV cargoes?
What are the spatiotemporal properties of EVs in vivo? Currently most EV studies are performed in culture or in vivo by addition or injection of EVs harvested from cells in culture. These studies may not reflect the actual spatiotemporal properties or concentration of EVs active in normal physiology in vivo.
What are the mechanisms that mediate addressability of specific EVs to diverse cell types and tissues? For EV-mediated drug delivery as well as for understanding cell-cell communication, the identification of mechanisms by which EVs are preferentially taken up in specific target organs will be crucial.
Are intracellular proteins and nucleic acids specifically incorporated into EVs and if so by which mechanism? Some elective cargo sorting mechanisms have been identified for exosomes. Whether all exosome cargoes are selectively sorted and whether other EV types have sorting mechanisms are important future questions.
Trends box.
Proteins, RNA, DNA, lipids and metabolites can be transferred to recipient cells and exert functional effects on target molecules either immediately (external cargoes) or after EV fusion and/or endosomal uptake (internal cargoes). Functional events for internal cargoes include EV-miRNA inhibition of target mRNA translation and EV-mRNA-to-protein translation.
EVs can serve autocrine and paracrine functions, controlling multiple cell processes in development, proliferation, migration and pathology.
The lipid membrane of EVs may serve to protect and stabilize EV cargoes in the extracellular space.
EV cargoes may be dysregulated and/or have abnormal content in disease states and serve as “snapshots” of diseased cells.
EVs are formed by multiple biogenesis mechanisms, which likely affect their cargo content.
Acknowledgments
Funding acknowledgements: We thank Ms. Suzanne McDavitt for skilled editorial assistance and Ms. Emily Mills for preparation of figures. This work was supported by U19 CA179563 (XOB) through the NIH Common Fund in the Office of Strategic Coordination/Office of the NIH Director, and NIH/NCI P01 CA069246 (XOB). AMW is supported by U19 CA179514, R01 GM117916, R01 CA163592, and R01 CA206458. A Nijbakker-Morra travel stipend and the Dutch Cancer Society (KWF) travel grant to SLNM are also acknowledged. The authors declare no conflicts of interest.
Abbreviations
- α-SMA
α-smooth muscle actin
- APCs
antigen presenting cells
- CTB
cholera toxin B
- DCs
dendritic cells
- DIC
differential interference contrast
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- ESCRT
endosomal sorting complex required for transport
- EV
extracellular vesicle
- FAK
focal adhesion kinase
- FasL
Fas ligand
- ILVs
intraluminal vesicles
- lEVs
large EVs
- LTB4
leukotriene
- MMPs
matrix metallopeptidases
- MIF
migration inhibitory factor
- miRNAs
microRNAs
- MSCs
mesenchymal stem cells
- MVB
multivesicular body
- NMJ
neuromuscular junction
- nSMase2
neutral sphingomyelinase 2
- ncRNA
non-coding RNA
- PCP
planar cell polarity
- RISC
RNA Interference Silencing Complex
- STB
Shiga toxin B
- sEVs
small EVs
- sncRNA
small non-coding RNAs
- SNARE
Soluble NSF Attachment Protein Receptor
- TAMs
tumor associated macrophages
- TCR
T cell receptor
- TLR
Toll-like receptor
- TGF
transforming growth factor
- Th1
T-helper 1
- Treg
T regulatory
- VEGF
vascular endothelial growth factor
- Wnt
Wingless
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cocucci E, et al. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 3.Skog J, et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tkach M, Théry C. Communication by extracellular vesicles: Where we are and where we need to go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 5.Abels ER, Breakefield XO. Introduction to extracellular vesicles - biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo Cicero A, et al. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Kowal J, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–E977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai RC, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. doi: 10.3402/jev.v5.29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Balkom BW, et al. Quantitative and qualitative analysis of small RNAs in human endothelial cells and exosomes provides insights into localized RNA processing, degradation and sorting. J Extracell Vesicles. 2015;4:26760. doi: 10.3402/jev.v4.26760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppers-Lalic D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8(6):1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Coleman BM, Hill AF. Extracellular vesicles--Their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev Biol. 2015;40:89–96. doi: 10.1016/j.semcdb.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitt JM, et al. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126(4):1139–1143. doi: 10.1172/JCI87316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong X, et al. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget. 2015;6(17):15566–15577. doi: 10.18632/oncotarget.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor Y, et al. Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nat Commun. 2015;6:8671. doi: 10.1038/ncomms9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osswald M, et al. A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol. 2016;18(4):479–485. doi: 10.1093/neuonc/now014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gan X, Gould SJ. Identification of an inhibitory budding signal that blocks the release of HIV particles and exosome/microvesicle proteins. Mol Biol Cell. 2011;22(6):817–830. doi: 10.1091/mbc.E10-07-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabhan JF, et al. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109(11):4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rilla K, et al. Hyaluronan-coated extracellular vesicles--a novel link between hyaluronan and cancer. Adv Cancer Res. 2014;123:121–148. doi: 10.1016/B978-0-12-800092-2.00005-8. [DOI] [PubMed] [Google Scholar]
- 20.Minciacchi VR, et al. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Vizio D, et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linares R, et al. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4:29509. doi: 10.3402/jev.v4.29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Record M, et al. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Jarmalavičiūtė A, Pivoriūnas A. Exosomes as a potential novel therapeutic tools against neurodegenerative diseases. Pharmacol Res. 2016 Feb 6; doi: 10.1016/j.phrs.2016.02.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Lener T, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia S, et al. Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Rev Mol Diagn. 2014;14(3):307–321. doi: 10.1586/14737159.2014.893828. [DOI] [PubMed] [Google Scholar]
- 27.Christ L, et al. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci. 2016 Sep 23; doi: 10.1016/j.tibs.2016.08.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Chiaruttini N, et al. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell. 2015;163(4):866–879. doi: 10.1016/j.cell.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCullough J, et al. Structure and membrane remodeling activity of ESCRT-III helical polymers. Science. 2015;350(6267):1548–1551. doi: 10.1126/science.aad8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee IH, et al. Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proc Natl Acad Sci U S A. 2015;112(52):15892–15897. doi: 10.1073/pnas.1518765113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trajkovic K, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 32.Kajimoto T, et al. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 33.Theos AC, et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343–354. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Niel G, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombo M, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 36.Stuffers S, et al. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 37.Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol. 2011;23(4):452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez AJ, et al. ESCRT machinery is required for plasma membrane repair. Science. 2014;343(6147):1247136. doi: 10.1126/science.1247136. [DOI] [PubMed] [Google Scholar]
- 39.Andrews NW, et al. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014;24(1):734–742. doi: 10.1016/j.tcb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark MR. Flippin’ lipids. Nat Immunol. 2011;12(5):373–375. doi: 10.1038/ni.2024. [DOI] [PubMed] [Google Scholar]
- 41.Hugel B, et al. Membrane microparticles: two sides of the coin. Physiology (Bethesda) 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 42.Tuck S. Extracellular vesicles: budding regulated by a phosphatidylethanolamine translocase. Curr Biol. 2011;21(24):R988–990. doi: 10.1016/j.cub.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Wehman AM, et al. The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol. 2011;21(23):1951–1959. doi: 10.1016/j.cub.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bianco F, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28(8):1043–1054. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awojoodu AO, et al. Acid sphingomyelinase is activated in sickle cell erythrocytes and contributes to inflammatory microparticle generation in SCD. Blood. 2014;124(12):1941–1950. doi: 10.1182/blood-2014-01-543652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoehn RS, et al. Acid Sphingomyelinase Inhibition in Stored Erythrocytes Reduces Transfusion-Associated Lung Inflammation. Ann Surg. 2016 Jan 27; doi: 10.1097/SLA.0000000000001648. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McConnell RE, et al. The enterocyte microvillus is a vesicle-generating organelle. Cell Biol. 2009;185(7):1285–1298. doi: 10.1083/jcb.200902147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rilla K, et al. Hyaluronan production enhances shedding of plasma membrane-derived microvesicles. Exp Cell Res. 2013;319(13):2006–2018. doi: 10.1016/j.yexcr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Wood CR, et al. The cilium secretes bioactive ectosomes. Curr Biol. 2013;23(10):906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colombo M, et al. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 51.Ostrowski M, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 52.Hsu C, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyenne V, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211(1):27–37. doi: 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinha S, et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214(2):197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshino D, et al. Exosome secretion isenhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–68. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mulcahy LA, et al. Routes and mechanisms of extracellular vesicle uptake. J Extracell Ves. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parolini I, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heusermann W, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–184. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stalder L, et al. The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. EMBO J. 2013;32(8):1115–1127. doi: 10.1038/emboj.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S, et al. MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell. 2013;153(3):562–574. doi: 10.1016/j.cell.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barman B, Bhattacharyya SN. mRNA targeting to endoplasmic reticulum precedes ago protein interaction and microRNA (miRNA)-mediated translation repression in mammalian cells. J Biol Chem. 2015;290(41):24650–24656. doi: 10.1074/jbc.C115.661868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sung BH, et al. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zomer A, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majumdar R1, TTA, Parent CA. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 2016;14(1):e1002336. doi: 10.1371/journal.pbio.1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Luga V, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 67.Mu W, et al. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia. 2013;15(8):875–887. doi: 10.1593/neo.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sedgwick AE, et al. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci Rep. 2015;5:14748. doi: 10.1038/srep14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clancy JW, et al. Regulated delivery of molecular cargo to invasive tumour-derived microvesicles. Nat Commun. 2015;6:6919. doi: 10.1038/ncomms7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol. 2015;15(4):203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buschow SI, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 72.Thery C, et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1145–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 73.Segura E, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 74.Qazi KR, et al. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood. 2009;113(12):2673–2683. doi: 10.1182/blood-2008-04-153536. [DOI] [PubMed] [Google Scholar]
- 75.Mittelbrunn M, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choudhuri K, et al. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507(7490):118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montecalvo A, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okoye IS, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41(1):89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fabbri M, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phinney DG, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greco V, et al. Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106(5):633–45. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- 83.Gross JC, et al. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 84.Beckett K, et al. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic. 2013;14(1):82–96. doi: 10.1111/tra.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vyas N, et al. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci Rep. 2014;4:7357. doi: 10.1038/srep07357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stoffel W, et al. Neutral sphingomyelinase 2 (smpd3) in the control of postnatal growth and development. Proc Natl Acad Sci U S A. 2005;102(12):4554–4559. doi: 10.1073/pnas.0406380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aubin I, et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet. 2005;37(8):803–805. doi: 10.1038/ng1603. [DOI] [PubMed] [Google Scholar]
- 88.Singh RK, et al. A role for Rab27 in neutrophil chemotaxis and lung recruitment. BMC Cell Biol. 2014;15:39. doi: 10.1186/s12860-014-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson SM, et al. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci U S A. 2000;97(14):7933–7938. doi: 10.1073/pnas.140212797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tolmachova T, et al. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15(1):332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tolmachova T, et al. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci U S A. 2007;104(14):5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Komada M, Soriano P. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev. 1999;13(11):1475–1485. doi: 10.1101/gad.13.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wagner KU, et al. Tsg101 is essential for cell growth, proliferation, and cell survival of embryonic and adult tissues. Mol Cell Biol. 2003;23(1):150–162. doi: 10.1128/MCB.23.1.150-162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Satir P, et al. The primary cilium at a glance. J Cell Sci. 2010;123(Pt 4):499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nozawa YI, et al. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev. 2013;23(4):429–437. doi: 10.1016/j.gde.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cao M, et al. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife. 2015:4. doi: 10.7554/eLife.05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol. 2014;24(5):519–525. doi: 10.1016/j.cub.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maguire JE, et al. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Mol Biol Cell. 2015;26(15):2823–2832. doi: 10.1091/mbc.E15-01-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang J, Barr MM. Ciliary extracellular vesicles: Txt msg organelles. Cell Mol Neurobiol. 2016;36(3):449–457. doi: 10.1007/s10571-016-0345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wood CR, Rosenbaum JL. Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol. 2015;25(5):276–285. doi: 10.1016/j.tcb.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dubreuil V, et al. Midbody and primary cilium of neural progenitors release extracellular membrane particles enriched in the stem cell marker prominin-1. J Cell Biol. 2007;176(4):483–495. doi: 10.1083/jcb.200608137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyado K, et al. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci U S A. 2008;105(35):12921–12926. doi: 10.1073/pnas.0710608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bianchi E, et al. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508(7497):483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Desrochers LM, et al. Microvesicles provide a mechanism for intercellular communication by embryonic stem cells during embryo implantation. Nat Commun. 2016;7:11958. doi: 10.1038/ncomms11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corrigan L, et al. BMP-regulated exosomes from Drosophila male reproductive glands reprogram female behavior. J Cell Biol. 2014;206(5):671–688. doi: 10.1083/jcb.201401072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Korkut C, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039–1046. doi: 10.1016/j.neuron.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Korkut C, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koles K, et al. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem. 2012;287(20):16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lopez-Verrilli MA, et al. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 110.Lopez-Leal R, Court FA. Schwann cell exosomes mediate neuron-glia communication and enhance axonal regeneration. Cell Mol Neurobiol. 2016;36(3):429–436. doi: 10.1007/s10571-015-0314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Frühbeis C, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fröhlich D, et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652) doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gong J, et al. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J Cell Biol. 2016;214(1):35–44. doi: 10.1083/jcb.201601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–647. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 115.Zomer A, van Rheenen J. Implications of extracellular vesicle transfer on cellular heterogeneity in cancer: What are the potential clinical ramifications? Cancer Res. 2016;76(8):2071–2075. doi: 10.1158/0008-5472.CAN-15-2804. [DOI] [PubMed] [Google Scholar]
- 116.Al-Nedawi K, et al. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kucharzewska P, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Webber JP, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 119.Zou W, et al. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Vrij J, et al. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. Int J Cancer. 2015;137(7):1630–1642. doi: 10.1002/ijc.29521. [DOI] [PubMed] [Google Scholar]
- 122.van der Vos KE, et al. Directly visualized glioblastoma-derived extracellular vesicles transfer RNA to microglia/macrophages in the brain. Neuro Oncol. 2016;18(1):58–69. doi: 10.1093/neuonc/nov244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pucci F, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352(6282):242–246. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wieckowski EU, et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activatedCD8+ T lymphocytes. J Immunol. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kelleher RJ, et al. Extracellular vesicles present in human ovarian tumor microenvironments induce a phosphatidylserine-dependent arrest in the T-cell signaling cascade. Cancer Immunol Res. 2015;3(11):1269–1278. doi: 10.1158/2326-6066.CIR-15-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang L, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vella LJ, et al. Focus on extracellular vesicles: Exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int J Mol Sci. 2016;17(2) doi: 10.3390/ijms17020173. pii: E173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quek C, Hill AF. The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun. 2016 Sep 19; doi: 10.1016/j.bbrc.2016.09.090. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 131.Mays CE, Soto C. The stress of prion disease. Brain Res. 2016;1648(Pt B):553–560. doi: 10.1016/j.brainres.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 132.Vella LJ, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol. 2007;211:582–590. doi: 10.1002/path.2145. [DOI] [PubMed] [Google Scholar]
- 133.Dinkins MB, et al. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35(8):1792–1800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laurén J, et al. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.An K, et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Aβ assemblies in vivo. Mol Brain. 2013;6:47. doi: 10.1186/1756-6606-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramirez A, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 137.Kong SM, et al. Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Hum Mol Genet. 2014;23(11):2816–2833. doi: 10.1093/hmg/ddu099. [DOI] [PubMed] [Google Scholar]
- 138.Tsunemi T, et al. ATP13A2/PARK9 regulates secretion of exosomes and α-synuclein. J Neurosci. 2014;34(46):15281–15287. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Danzer KM, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mao X, et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353(6307) doi: 10.1126/science.aah3374. pii: aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]