Abstract
Introduction
Tobacco use remains a leading modifiable cause of cancer incidence and premature mortality in the U.S. and globally. Despite increasing life expectancy worldwide, less is known about the effects of cigarette smoking on older populations. This study sought to determine the effects of smoking on mortality in older age.
Methods
Associations of mortality with self-reported age at smoking cessation, age at smoking initiation, and amount smoked after age 70 years were examined in 160,113 participants of the NIH-AARP Diet and Health Study aged >70 years. Participants completed a questionnaire detailing their smoking use in 2004–2005, and were followed for mortality through December 31, 2011. Analyses were conducted between 2014 and 2016.
Results
Relative to never smokers, current smokers were more likely to die during follow-up (hazard ratio, 3.18; 95% CI=3.04, 3.31). Furthermore, former smokers had lower risks than current smokers (hazard ratios for quitting between ages 30–39, 40–49, 50–59, and 60–69 years were 0.41 [95% CI=0.39, 0.43], 0.51 [95% CI=0.49, 0.54], 0.64 [95% CI=0.61, 0.67], and 0.77 [95% CI=0.73, 0.81], respectively). Among current smokers, mortality was inversely associated with age at initiation, but directly associated with the number of cigarettes smoked per day >70 years.
Conclusions
As among younger people, lifetime cigarette smoking history is a key determinant of mortality after age 70 years.
INTRODUCTION
Despite successes in tobacco control,1 tobacco use remains the leading modifiable cause of cancer incidence and premature mortality in the U.S. and globally.2 The 2014 Surgeon General’s report estimates that cigarette smoking causes at least 21 different diseases, which result in more than 480,000 deaths per year in the U.S.3; however, recent data suggest that this figure may be an underestimate.4 Globally, 12% of deaths among adults aged 30 years and older are attributable to tobacco use,5 and this burden is projected to escalate as a result of increased use of tobacco in the developing world.6 Reducing the mortality burden of cigarette smoking worldwide requires comprehensive measures that prevent smoking initiation in never smokers and increase cessation rates among current smokers.7, 8
The impact of smoking on mortality in the elderly is of interest, given the aging nature of U.S. and global populations. In the U.S., the number of individuals aged 70 years and older is expected to increase from 29.2 million (9.3% of the population) in 2012, to 63.6 million individuals (15.9%) in 2050.9 Furthermore, because current birth cohorts aged 70 years and older had a very high lifetime prevalence of cigarette smoking,10, 11 they are an ideal population in which to examine the risks associated with smoking, and the benefits of quitting at older ages. Yet, most studies of cigarette smoking and mortality have focused on middle-aged populations, with fewer studies examining the impact of tobacco cessation on disease and mortality risk among the elderly.12, 13 The benefit could be relatively small, as individuals who smoke at age 70 years typically started as teenagers, more than 50 years prior.3 Alternatively, the absolute benefit of smoking cessation may be substantial given the high rates of mortality in this age group.
In this study, the effects of smoking on mortality were examined among 160,113 participants, aged 70–82 years at baseline, of the NIH-AARP Diet and Health Study who completed a detailed lifestyle questionnaire in 2004–2005 and were followed for mortality through December 31, 2011.
METHODS
Study Population
The NIH-AARP Diet and Health study was initiated in 1995 when 566,398 individuals, aged 50–71 years, from six U.S. states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and two metropolitan areas (Atlanta, Georgia and Detroit, Michigan) completed a mailed questionnaire covering a broad range of lifestyle factors.14 Participants were followed by linkage to the National Change of Address database maintained by the U.S. Postal Service.
Between 2004 and 2005, a total of 313,835 participants completed a follow-up questionnaire containing detailed questions about lifetime cigarette smoking. Proxy responses (n=18,603), individuals who died before their questionnaires were scanned (n=436), individuals aged <70 years at the follow-up questionnaire (n=132,767), and those with missing (n=6,299) or inconsistent (n=495) smoking history information were excluded, leaving 160,113 participants. The NIH-AARP study was approved by the Special Studies IRB of the National Cancer Institute. Participants gave informed consent by virtue of completing and returning the questionnaire.
Measures
On the 2004–2005 questionnaire, participants reported if they had ever smoked 100 cigarettes, as well as smoking intensity (one or fewer, one to ten, 11–20, 21–30, 31–40, 41–60, and ≥61 cigarettes per day) during nine age periods (<15 years, 15–19 years, 20–24 years, 25–29 years, 30–39 years, 40–49 years, 50–59 years, 60–69 years and ≥70 years. Current smokers were defined as participants who reported smoking in their 70s or later; age at smoking initiation for current and former smokers, and decade of cessation for former smokers was defined by the first and last age-periods during which smoking was reported, respectively. Maximum cigarettes per day was defined as the maximum smoking intensity reported during any age period. Pack years were calculated as the sum of the products of the years in each age period and the number of cigarettes per day reported for that age period.
A separate question that assessed how recently participants had quit smoking (within the last year, 1–4 years, 5–10 years, >10 years) was used to examine time since smoking cessation. In these analyses, participants who reported quitting in the past year were classified as current smokers, owing to the difficulty of cessation and the potential for recent quitting to be heavily confounded by disease diagnosis and symptoms.15, 16 Height, sex, race, and education were assessed in the 1995–1996 questionnaire. The 2004–2005 questionnaire also assessed weight and selected medical conditions.
Follow-up began from the date the 2004–2005 questionnaire was scanned and ended at death, or December 31, 2011, whichever came first. Mortality data were obtained by linkage to the National Death Index Plus maintained by the National Center for Health Statistics. ICD-9 and ICD-10 codes were used to define outcomes as follows: lung cancer (162.2–162.9, C34); other smoking-related cancers, to include bladder (188, C67), colorectal (153, 159.0, 154.0–154.1, C18–20, C26.0), esophageal (150, C15), head and neck (140–149, 161, C00–C14, C32), kidney and renal pelvis (189.0–189.1, C64–C65), liver (155.0–155.2, C22), pancreatic (157, C25), and stomach (151, C16) cancers, and acute myeloid leukemia (205.0, 207.0, 207.2, C92.0,C92.4–C92.5, C94.0, C94.2); heart disease (390–398, 401–404, 410–429, 440–448 and I00–I13, I20–I51, I70–I78); stroke (430–438 and I60–I69); diabetes (250, E10–E14); and respiratory disease (e.g., pneumonia, influenza, chronic obstructive pulmonary disease [COPD], and allied conditions; 480–487, 490–496, and J09–J18, J40–J47).
Statistical Analysis
Statistical analyses were conducted between July 2014 and July 2016. Mortality rates were age and sex standardized to the distribution of the entire analytic data set using 5-year age categories. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs, with person years from baseline as the underlying time metric. All models were adjusted for age, sex, education (less or greater than high school education), and alcohol use (none, zero to one, one to three, more than three drinks/day). Additional models were further adjusted for age at smoking initiation or maximum smoking intensity. As the NIH-AARP cohort is predominantly white, adjusting for race did not affect results; therefore, this variable was not included in the final models.
Whether associations of smoking variables with all-cause mortality were modified by sex, age (≤75 years, >75 years), follow-up time (≤5 years, >5 years), or previous condition, defined as diagnosis of either cancer, COPD, stroke, or heart attack, was assessed by repeating all analyses in strata of these variables. Data analysis used SAS, version 9.3; figures were created using Sigmaplot, version 12.5.
RESULTS
Median age at baseline was 75 years (Table 1) and was similar among never, former, and current smokers. Six percent of the cohort were current smokers, and almost 56% were former smokers (Table 1). Of the former smokers, 90% reported quitting prior to age 60 years. Patterns of smoking differed by sex: Men were less likely to be never smokers (31.2% vs 48.0% of women), and male smokers reported smoking more than female smokers (mean pack years of smoking, 18.2 (SD=23.3) for men and 11.6 (SD=18.9) for women). Male smokers were also more likely to start smoking before 15 years (19.0% vs 9.5% of female smokers).
Table 1.
Characteristics of NIH-AARP Participants, Aged Over 70y, Who Completed the 2004–2005 Questionnaire Stratified by Smoking Status
| Former smokers | ||||
|---|---|---|---|---|
| Characteristic | Never smokers | Quit <60y | Quit >60y | Current smokers |
| n (%) | 60,973 (38.1) | 81,137 (50.7) | 8,412 (5.3) | 9,591 (6.0) |
| Demographic variables | ||||
| Mean age (y)a | 74.7 (2.6) | 74.6 (2.6) | 74.9 (2.6) | 74.6 (2.6) |
| Sex (% female) | 51.6 | 31.5 | 43.1 | 50.9 |
| Education level (% college or higher) |
75.0 | 77.6 | 73.5 | 70.7 |
| Race (% non-Hispanic white) | 92.1 | 93.9 | 93.7 | 92.1 |
| Marital status (% married) | 66.6 | 74.1 | 62.6 | 53.5 |
| Overall health (% fair/poor) | 13.2 | 16.6 | 25.3 | 24.6 |
| Age at initiation (% started 15– 19y) |
0.0 | 19.0 | 21.0 | 22.0 |
| Lifetime pack-years | -- | 21.0 (20.0) | 44.6 (27.6) | 42.3 (25.6) |
| Pack-years prior to 70y | -- | 21.0 (20.0) | 44.6 (27.6) | 38.8 (23.8) |
| Alcohol use (grams/day) | 0.5 (1.7) | 1.1 (2.7) | 1.3 (3.1) | 1.4 (3.5) |
| BMI (kg/m2) | 26.5 (4.9) | 26.9 (4.6) | 26.9 (4.9) | 25.2 (4.7) |
| Total activity time (minutes/day) | 25.2 (32.9) | 27.2 (34.3) | 21.3 (29.6) | 17.1 (27.9) |
| Mortality, n (%) | ||||
| All cause | 7,378 (12.1) | 14,957 (18.4) | 2,343 (27.9) | 3,175 (33.1) |
| Lung cancer | 180 (0.3) | 1,464 (1.8) | 375 (4.5) | 630 (6.6) |
| Other smoking cancerb | 357 (0.6) | 821 (1.0) | 88 (1.1) | 148 (1.5) |
| Respiratory infection | 257 (0.4) | 1,299 (1.6) | 394 (4.7) | 540 (5.6) |
| Heart disease | 2,072 (3.4) | 4,015 (5.0) | 565 (6.7) | 722 (7.5) |
| Stroke | 455 (0.8) | 698 (0.9) | 84 (1.0) | 132 (1.4) |
| Previous condition, n (%) | ||||
| Hypertension | 33,502 (58.2) | 47,300 (61.5) | 4,930 (62.0) | 5,070 (55.9) |
| COPD | 2,567 (4.8) | 7,390 (5.2) | 1,866 (24.7) | 2,233 (25.7) |
| Cancer | 17,537 (32.3) | 26,263 (36.1) | 2,698 (35.4) | 2,942 (33.4) |
| Stroke | 2,227 (4.1) | 3,635 (4.9) | 534 (7.1) | 558 (6.4) |
| Heart attack | 10,408 (18.8) | 20,087 (27.0) | 2,203 (28.8) | 1,970 (22.6) |
Means are given (SD)
Other smoking cancers to include all other cancers determined by the Surgeon General’s report to be causally associated with cancer, including bladder, esophageal, stomach, head and neck, colorectal, kidney and renal pelvis, and pancreatic cancers, and acute myeloid leukemia.
NIH-AARP, National Institutes of Health-AARP Study; y, years; COPD, chronic obstructive pulmonary diseases
Current smokers and former smokers who quit between age 60 and 70 years were more likely to report fair/poor health and COPD than never smokers and former smokers who quit before age 60 years (Table 1). However, the distribution of other previous conditions varied across categories of lifetime smoking use. For example, 22.6% of current smokers reported a previous heart attack, compared with 28.8% of former smokers who quit between age 60 and 70 years, 27.0% of former smokers who quit prior to age 60 years, and 18.8% of never smokers, with similar patterns observed for a prior diagnosis of cancer, stroke, or hypertension.
Mean follow-up time was 6.4 years (range, 0.03–7.23 years). There was high mortality during follow-up, with 25,146 participants dying (15.7% of the cohort). Differences in mortality were observed by baseline smoking status (Table 2): 12.1% of never smokers died, whereas 16.2%, 19.7%, 23.9%, and 27.9% of former smokers who quit between ages 30–39, 40–49, 50–59, and 60–69 years died, respectively. The highest mortality was observed in current smokers, of whom 33.1% died. In multivariable-adjusted models, current smokers were more than three times more likely to die than never smokers (HR=3.18, 95% CI=3.04, 3.31).
Table 2.
Age at Smoking Cessation and Mortality in Participants of the NIH-AARP Study Aged Over 70
| Smoking status | ||||||
|---|---|---|---|---|---|---|
| Cause of death | Current (n=9,591) |
Quit 60–69y (n=8,412) |
Quit 50–59y (n=19,046) |
Quit 40–49y (n=21,424) |
Quit 30– 39y (n=21,553) |
Never (n=60,973) |
| Death from all causes | ||||||
| Number of deaths (%) | 3,175 (33.1) | 2,343 (27.9) | 4,553 (23.9) | 4,214 (19.7) | 3,483 (16.2) |
7,378 (12.1) |
| Rate per 100,000 | 5,900.22 (5,690.38, 6,110.06) |
4,588.35 (4,402.06, 4,774.65) |
3,821.28 (3,710.15, 3,932.41) |
3,017.59 (2,925.23, 3,109.96) |
2,349.6 (2,268, 2,431.2) |
1,884.4 (1,840.46, 1,928.34) |
| Multivariable-adjusted hazard ratioa |
1.00 (ref) | 0.77 (0.73, 0.81) |
0.64 (0.61, 0.67) |
0.51 (0.49, 0.54) |
0.41 (0.39, 0.43) |
0.32 (0.30, 0.33) |
| Death from lung cancer |
||||||
| Number of deaths (%) | 630 (6.6) | 375 (4.5) | 630 (3.3) | 441 (2.1) | 260 (1.2) | 180 (0.3) |
| Rate per 100,000 | 1,155.63 (1,063.28, 1,247.98) |
741.47 (666.21, 816.72) |
528.9 (487.55, 570.25) |
318.33 (288.21, 348.44) |
177.09 (154.56, 199.62) |
44.8 (38.1, 51.49) |
| Multivariable-adjusted hazard ratio |
1.00 (ref) | 0.64 (0.56, 0.73) |
0.46 (0.41, 0.51) |
0.28 (0.25, 0.32) |
0.16 (0.14, 0.19) |
0.04 (0.03, 0.05) |
| Death from other smoking related cancersb |
||||||
| Number of deaths (%) | 205 (2.1) | 149 (1.8) | 346 (1.8) | 361 (1.7) | 332 (1.5) | 619 (1.0) |
| Rate per 100,000 | 282.13 (235.74, 328.52) |
175.63 (138.82, 212.44) |
198.92 (173.62, 224.21) |
159.19 (138.19, 180.19) |
123.69 (105.95, 141.44) |
94.04 (84.1, 103.98) |
| Multivariable-adjusted hazard ratio |
1.00 (ref) | 0.72 (0.60, 0.87) |
0.73 (0.62, 0.84) |
0.66 (0.57, 0.77) |
0.57 (0.49, 0.66) |
0.46 (0.40, 0.53) |
| Death from respiratory infection | ||||||
| Number of deaths (%) | 540 (5.6) | 394 (4.7) | 602 (3.2) | 369 (1.7) | 206 (1.0) | 257 (0.4) |
| Rate per 100,000 | 984.85 (899.87, 1,069.83) |
769.42 (693.25, 845.6) |
510.72 (469.87, 551.58) |
269.6 (241.68, 297.52) |
138.54 (118.74, 158.33) |
64.2 (56.16, 72.23) |
| Multivariable-adjusted hazard ratio |
1.00 (ref) | 0.78 (0.68, 0.89) |
0.52 (0.46, 0.58) |
0.28 (0.25, 0.32) |
0.16 (0.13, 0.18) |
0.07 (0.06, 0.08) |
| Death from heart disease |
||||||
| Number of deaths (%) | 722 (7.5) | 565 (6.7) | 1,210 (6.4) | 1,159 (5.4) | 939 (4.4) | 2,072 (3.4) |
| Rate per 100,000 | 1,358.68 (1,257.43, 1,459.94) |
1,104.7 (1,013.36, 1,196.04) |
1,010.01 (953.03, 1,066.98) |
817.19 (769.55, 864.83) |
623.14 (581.55, 664.74) |
540.09 (516.36, 563.83) |
| Multivariable-adjusted hazard ratio |
1.00 (ref) | 0.79 (0.71, 0.89) |
0.73 (0.66, 0.80) |
0.60 (0.55, 0.66) |
0.46 (0.42, 0.51) |
0.39 (0.35, 0.42) |
| Death from stroke | ||||||
| Number of deaths (%) | 132 (1.4) | 84 (1.0) | 178 (0.9) | 189 (0.9) | 180 (0.8) | 455 (0.8) |
| Rate per 100,000 | 246.14 (203.22, 289.07) |
163.74 (128.64, 198.83) |
149.86 (127.82, 171.91) |
138.72 (118.63, 158.81) |
124.3 (105.26, 143.34) |
111.47 (100.97, 121.96) |
| Multivariable-adjusted hazard ratio |
1.00 (ref) | 0.66 (0.50, 0.86) |
0.61 (0.49, 0.77) |
0.57 (0.45, 0.71) |
0.52 (0.42, 0.65) |
0.45 (0.37, 0.55) |
Multivariable-adjusted hazard ratios adjusted for age at baseline, sex, education level, and alcohol use
Other smoking cancers to include all other cancers determined by the Surgeon General's report to be causally associated with cancer, including bladder, esophageal, stomach, head and neck, colorectal, kidney and renal pelvis, and pancreatic cancers, and acute myeloid leukemia.
NIH-AARP, National Institutes of Health-AARP Study
Relative to current smokers, the risk of all-cause mortality was lowest among never smokers (HR=0.32, 95% CI=0.30, 0.33) and former smokers who quit at age 30–39 years (HR=0.41, 95% CI=0.39, 0.43). The benefits of quitting at an older age were less, although still substantial, with observed HRs of 0.51 (95% CI=0.49, 0.54), 0.64 (95% CI=0.61, 0.67), and 0.77 (95% CI=0.73, 0.81) for quitting between ages 40–49, 50–59, and 60–69, respectively. Similar findings were observed for each examined smoking-related cause of death. Adjustment for age at initiation and maximum smoking intensity had little effect on these risk estimates (data not shown).
Mortality rates were lower for women than men at each level of smoking use; however, the HRs for smoking were similar in both sexes (Figure 1, Appendix Table 1). HRs were similar among participants who died in the first 5 versus 6–7 years of follow-up, and participants who were aged ≤75 or >75 years at study baseline. For participants who quit smoking in their 60s, the protective effect of smoking cessation on mortality was slightly stronger when restricted to participants who had not been previously diagnosed with cancer, COPD, stroke, or heart disease. This was most evident for death from heart attack, stroke, and respiratory infection.
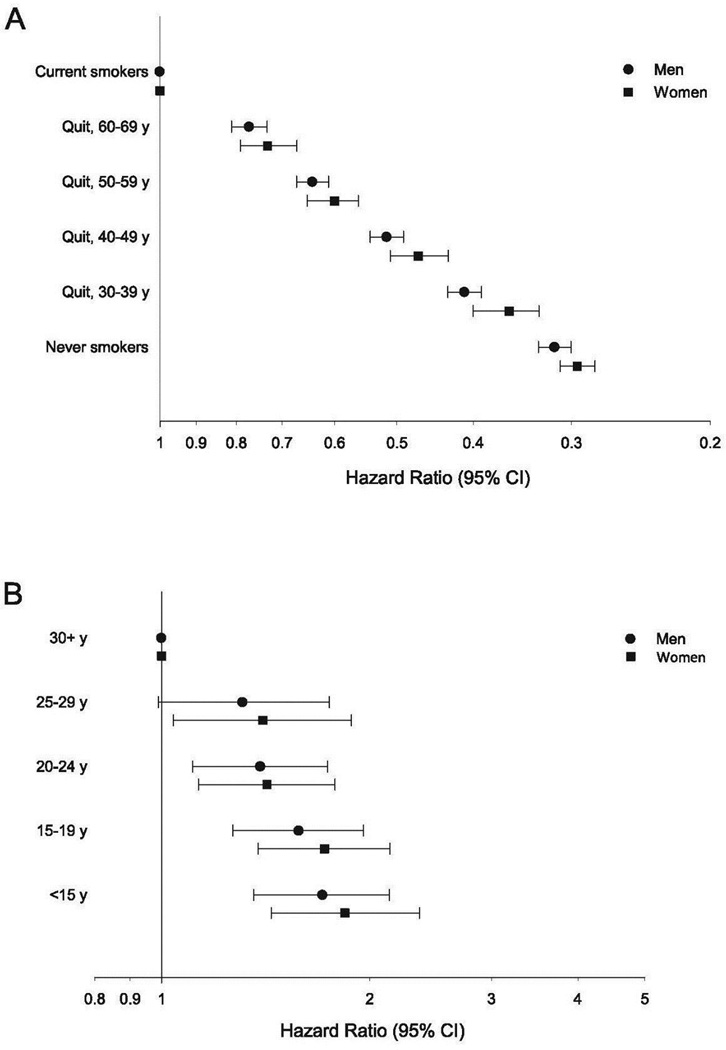
Figure 1.
Risk of all-cause mortality associated with age at smoking cessation for males and females (A), and, in current smokers only, with age at smoking initiation, for males and females (B).
Associations with time since smoking cessation were examined (Appendix Table 2). Participants who reported quitting smoking 1–4 years before baseline were more likely to report being in fair/poor health (28.8%) than current smokers (23.7%), former smokers who quit 5–10 years before baseline (23.8%), or former smokers who quit >10 years before baseline (16.6%), suggesting that disease symptoms was one of the main contributors to quitting in this age group. Relative to current smokers, former smokers who quit >10 years before baseline had substantially reduced risk of all-cause mortality (HR=0.66, 95% CI=0.64, 0.69). Similar findings were observed for each examined smoking-related cause of death. By contrast, former smokers who quit 5–10 years before baseline had similar mortality risks as current smokers (HR=1.07, 95% CI=1.01, 1.14), whereas former smokers who quit 1–4 years before baseline had higher risks than current smokers (HR=1.35, 95% CI=1.26, 1.46). There were similar patterns for death from lung cancer, other smoking-related cancers, and respiratory disease.
Among current smokers, age at smoking initiation and cigarettes smoked per day after age 70 years were examined (Figure 1, Table 3). There was a strong inverse association of mortality with age at initiation. Relative to participants who started smoking after age 30 years, HRs for all-cause mortality for participants who started smoking between ages 25–29, 20–24, 15–19, and <15 years were 1.36 (95% CI=1.09, 1.71), 1.44 (95% CI=1.21, 1.72), 1.59 (95% CI=1.34, 1.89), and 1.74 (95% CI=1.45, 2.08), respectively. HRs were similar across each examined cause of mortality, although the number of deaths in some categories were low, resulting in wide CIs.
Table 3.
Age at Smoking Initiation and Mortality Among Current Smokers Aged Over 70, in the NIH-AARP study
| Age at initiation (y) | |||||
|---|---|---|---|---|---|
| Cause of death | <15 (n=2,105) |
15–19 (n=3,847) |
20–24 (n=2,485) |
25–29 (n=526) |
30+ (n=628) |
| Death from all causes | |||||
| Number of deaths (%) | 791 (37.6) | 1,328 (34.5) | 757 (30.5) | 154 (29.3) | 145 (23.1) |
| Rate per 100,000 | 6,563.23 (6,053.34, 7,073.11) |
5,862.03 (5,546.16, 6,177.89) |
5,306.42 (4,916.89, 5,695.95) |
5,030.64 (4,220.65, 5,840.64) |
3,705.65 (3,061.08, 4,350.22) |
| Multivariable-adjusted hazard ratioa |
1.74 (1.45, 2.08) |
1.59 (1.34, 1.89) |
1.44 (1.21, 1.72) |
1.36 (1.09, 1.71) |
1.00 (ref) |
| Death from lung cancer | |||||
| Number of deaths (%) | 154 (7.3) | 281 (7.3) | 146 (5.9) | 28 (5.3) | 21 (3.3) |
| Rate per 100,000 | 1,237.49 (1,022, 1,452.98) |
1,244.19 (1,098.49, 1,389.89) |
1,033.95 (861.4, 1,206.51) |
931.48 (580.59, 1,282.37) |
480.81 (265.17, 696.45) |
| Multivariable-adjusted hazard ratio |
2.35 (1.48, 3.73) |
2.36 (1.51, 3.68) |
1.92 (1.22, 3.04) |
1.69 (0.96, 2.98) |
1.00 (ref) |
| Death from other smoking related cancersb | |||||
| Number of deaths (%) | 52 (2.5) | 87 (2.3) | 45 (1.8) | 11 (2.1) | 10 (1.6) |
| Rate per 100,000 | 383.78 (267.20, 500.37) |
382.91 (302.31, 463.51) |
326.19 (228.30, 424.09) |
375.27 (149.66, 600.88) |
280.22 (94.26, 466.19) |
| Multivariable-adjusted hazard ratio |
1.17 (0.66, 2.08) |
1.25 (0.73, 2.15) |
1.04 (0.59, 1.83) |
1.00 (0.47, 2.14) |
1.00 (ref) |
| Death from respiratory infection | |||||
| Number of deaths (%) | 164 (7.8) | 230 (6.0) | 107 (4.3) | 22 (4.2) | 17 (2.7) |
| Rate per 100,000 | 1,450.07 (1,203.78, 1,696.37) |
1,023.19 (890.75, 1,155.64) |
706.26 (568.18, 844.35) |
679.49 (390.51, 968.47) |
435.94 (214.9, 656.98) |
| Multivariable-adjusted hazard ratio |
3.50 (2.11, 5.79) |
2.52 (1.54, 4.12) |
1.81 (1.09, 3.02) |
1.70 (0.91, 3.21) |
1.00 (ref) |
| Death from heart disease | |||||
| Number of deaths (%) | 184 (8.7) | 274 (7.1) | 179 (7.2) | 38 (7.2) | 47 (7.5) |
| Rate per 100,000 | 1,534.99 (1,286.15, 1,783.83) |
1,206.9 (1,063.69, 1,350.11) |
1,267.59 (1,076.41, 1,458.78) |
1,257.97 (850.48, 1,665.46) |
1,304.16 (906.02, 1,702.3) |
| Multivariable-adjusted hazard ratio |
1.21 (0.88, 1.68) |
0.99 (0.72, 1.35) |
1.04 (0.75, 1.43) |
1.03 (0.67, 1.58) |
1.00 (ref) |
| Death from stroke | |||||
| Number of deaths (%) | 21 (1.0) | 63 (1.6) | 31 (1.3) | 11 (2.1) | 6 (1.0) |
| Rate per 100,000 | 170.93 (89.42, 252.43) |
274.11 (206.26, 341.96) |
207.3 (132.06, 282.54) |
357.37 (142.11, 572.63) |
151.61 (23.32, 279.89) |
| Multivariable-adjusted hazard ratio |
1.26 (0.50, 3.17) |
1.95 (0.84, 4.51) |
1.54 (0.64, 3.69) |
2.44 (0.90, 6.59) |
1.00 (ref) |
Multivariable-adjusted hazard ratios adjusted for age at baseline, sex, education level, and alcohol use
Other smoking cancers to include all other cancers determined by the Surgeon General’s report to be causally associated with cancer, including bladder, esophageal, stomach, head and neck, colorectal, kidney and renal pelvis, and pancreatic cancers, and acute myeloid leukemia.
NIH-AARP, National Institutes of Health-AARP Study
Finally, current and former smokers with greater cumulative exposure to cigarettes had higher mortality risks than those with a lower exposure (Appendix Table 3). Furthermore, those who smoked more cigarettes per day after age 70 years had higher mortality risk than those who smoked fewer cigarettes per day at this age (Appendix Table 4). Notably, those who smoked 31–40 cigarettes per day had the highest risk of mortality, with lower risk observed in those who smoked ≥40 cigarettes per day.
DISCUSSION
In this study of NIH-AARP participants, behaviors known to be important predictors of mortality among middle-aged populations were also strongly related to mortality among adults aged 70 years and older. Relative to never smokers, current smokers were more than three times more likely to die during follow-up. Furthermore, quitting at any age was associated with lower risk of mortality. Former smokers who quit smoking earlier in life received the largest benefit from cessation. Yet, even participants who quit during their 60s were at substantially decreased risk of death, relative to participants who continued to smoke. This finding is particularly remarkable given that participants who quit smoking in their 60s were as likely as current smokers to report poor/fair health, and were more likely than current smokers to have reported a diagnosis of hypertension, cancer, heart attack, or stroke. These results emphasize the benefit of smoking cessation on mortality even later in life, and provide further evidence that all smokers should be encouraged to quit regardless of their age.
Risk of all-cause mortality was more than three times higher in current smokers than never smokers. This finding parallels results from the Million Women Study,17 the National Health Interview Survey,18 and a pooled analysis of contemporary cohorts, in which the baseline data from the NIH-AARP study were included.19 Importantly, it extends these findings to show that risk of death associated with smoking remains consistent between middle-age and after age 70 years. It should be noted that although these findings are highly relevant for current U.S. birth cohorts, it will be important to continue this research as cohorts with lower lifetime prevalence of smoking advance into middle and older age. The effects of cigarette smoking among older populations with different lifetime smoking histories in other parts of the world should also be examined.
Relative to current smokers, risk of mortality was lower in individuals who quit smoking at earlier ages. This finding is in agreement with studies that have examined age at or time since quitting in elderly former smokers. In the Cancer Prevention II study, former smokers aged 70–79 years and 80 years and older had a lower risk of all-cause mortality than current smokers, and risk of mortality decreased with increased time since smoking cessation.20 Similar patterns have been observed in adults aged 65 years and older21–23 and 60 years and older.12 In the pooled analysis of contemporary cohorts (mean age, 66 years), there was a strong association of age at smoking cessation with all-cause and cause-specific mortality,19 with similar associations for age at cessation and mortality as those observed here.
Evaluations of whether risk of mortality differed by time since quitting showed that, relative to current smokers, individuals who quit smoking more than 10 years prior were at decreased risk of mortality, those who quit 5–10 years ago had similar risk, and those who quit 1–4 years ago were at increased risk. Such findings are indicative of the strong association between smoking cessation and disease symptoms in these age groups, and the difficulties of separating proactive cessation from that due to the onset of symptoms. Given that smoking cessation at later ages was associated with worse health and a higher likelihood of having a chronic disease, the benefits associated with smoking cessation at age 60–70 years are even more remarkable.
Age at smoking initiation was strongly associated with mortality after age 70 years. Current smokers who started smoking earlier were at progressively higher risk of mortality during follow-up, relative to those who started smoking later. It is striking that relatively small differences in age at initiation are associated with such strong differences in mortality risk 40–60 years later. This speaks to the importance of smoking duration as a determinant of mortality risk, even after age 70 years. Given that the majority of U.S. smokers start smoking as teenagers,24 these findings provide support for efforts to prevent adolescent smoking initiation, such as an increase in the minimum age to purchase cigarettes.
No previous studies of age at smoking initiation and mortality have explicitly focused on older adults. However, these findings are consistent with studies conducted in younger populations. In the Nurses’ Health Study, age at smoking initiation was inversely associated with age mortality from all causes, respiratory infection, lung cancer, and other smoking-related cancers.25 Similarly, findings from the Million Women Study observed increased risk of all-cause mortality in women who initiated smoking earlier in life.17 Similar findings have been observed for incidence of lung cancer26 and cardiovascular disease.27
Several previous studies have demonstrated positive associations of amount smoked in later life and risk of all-cause, cardiovascular disease, and cancer mortality in adults aged 65 years and older.21, 22, 28 In this study, the amount smoked after age 70 years was associated with risk of mortality from all causes, lung cancer, and respiratory infection. Interestingly, risk was highest in individuals who smoked 31–40 cigarettes per day, with lower mortality observed in individuals who smoked ≥40 cigarettes per day, although there was substantial overlap in the CIs around these point estimates. These differences may be due to differences in survival, with heavier smokers more likely to die before age 70 years. Furthermore, individuals who smoke more after age 70 years likely smoked more throughout their lives, contributing to their increased risk.
Limitations
This study has several strengths. These include the large size of the NIH-AARP study, which enabled examination of smoking-related predictors of mortality in a large number of elderly individuals, as well as the study’s prospective design and detailed assessment of lifetime cigarette use. This study also has several limitations. Importantly, there was no information on changes in cigarette use. A number of participants likely quit smoking during follow-up, which may have attenuated the associations with current smoking. Participants in the NIH-AARP study are also predominantly white and of higher SES than the general population. However, given the consistency of associations between smoking and mortality across many different populations, the general patterns of association found here should hold to other population groups. Finally, this study had relatively few participants aged older than 80 years (n=707). Future studies in other populations and among participants in their 80s and older are needed.
CONCLUSIONS
These data show that age at smoking initiation and cessation, both key components of smoking duration, are important predictors of mortality in U.S. adults aged 70 years and older. In this study population, younger age at initiation was associated with increased risk of mortality, highlighting the importance of youth and early-adult smoking on lifetime mortality risk, even among people who live to age 70 years. Furthermore, former smokers were at substantially reduced risk of mortality after age 70 years relative to current smokers, even those who quit in their 60s. Therefore, smoking cessation should be emphasized to all smokers, regardless of age.
Supplementary Material
Acknowledgments
The authors assume full responsibility for the analyses and interpretation of data used in the study; the research presented herein does not necessarily represent the official views of NIH. This work was supported by the National Cancer Institute’s Intramural Program, and its Cancer Prevention Fellowship Program, as well as the Intramural Research Program of the National Institute on Aging. Author responsibilities were as follows: Sarah Nash was responsible for data analysis, interpretation, and drafting the manuscript; Linda Liao was responsible for data acquisition and also helped in drafting the manuscript; Tamara Harris aided in drafting the manuscript; and Neal Freedman was responsible for the design of this study, as well as data interpretation, and drafting the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Holford TR, Meza R, Warner KE, et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 19642013;2012. JAMA. 2014;311(2):164–171. doi: 10.1001/jama.2013.285112. http://dx.doi.org/10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2014. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 3.The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: DHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 4.Carter BD, Abnet CC, Feskanich D, et al. Smoking and mortality--beyond established causes. N Engl J Med. 2015;372(7):631–640. doi: 10.1056/NEJMsa1407211. http://dx.doi.org/10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Geneva, Switzerland: WHO Press; 2012. WHO global report: mortality attributable to tobacco. [Google Scholar]
- 6.Oppeltz RF, Jatoi I. Tobacco and the escalating global cancer burden. J Oncol. 2011;2011:408104. doi: 10.1155/2011/408104. http://dx.doi.org/10.1155/2011/408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ending the tobacco epidemic: a tobaco control strategic action plan for the U.S. DHHS. Washington D.C.: 2010. [Google Scholar]
- 8.Koh HK, Sebelius KG. Ending the tobacco epidemic. JAMA. 2012;308(8):767–768. doi: 10.1001/jama.2012.9741. http://dx.doi.org/10.1001/jama.2012.9741. [DOI] [PubMed] [Google Scholar]
- 9.Ortman JMVV, Hogan H. An Aging Nation: The Older Population in the United States. Washington, DC: U.S. Census Bureau; 2014. pp. 25–1140. [Google Scholar]
- 10.Escobedo LG, Peddicord JP. Smoking prevalence in U.S. birth cohorts: the influence of gender and education. Am J Public Health. 1996;86(2):231–236. doi: 10.2105/ajph.86.2.231. http://dx.doi.org/10.2105/AJPH.86.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900–80. J Natl Cancer Inst. 1983;71(3):473–479. [PubMed] [Google Scholar]
- 12.Gellert C, Schottker B, Brenner H. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med. 2012;172(11):837–844. doi: 10.1001/archinternmed.2012.1397. http://dx.doi.org/10.1001/archinternmed.2012.1397. [DOI] [PubMed] [Google Scholar]
- 13.Gellert C, Schottker B, Holleczek B, Stegmaier C, Muller H, Brenner H. Using rate advancement periods for communicating the benefits of quitting smoking to older smokers. Tob Control. 2013;22(4):227–230. doi: 10.1136/tobaccocontrol-2012-050572. http://dx.doi.org/10.1136/tobaccocontrol-2012-050572. [DOI] [PubMed] [Google Scholar]
- 14.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. http://dx.doi.org/10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 15.Salive ME, Cornoni-Huntley J, LaCroix AZ, Ostfeld AM, Wallace RB, Hennekens CH. Predictors of smoking cessation and relapse in older adults. Am J Public Health. 1992;82(9):1268–1271. doi: 10.2105/ajph.82.9.1268. http://dx.doi.org/10.2105/AJPH.82.9.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitson HE, Heflin MT, Burchett BM. Patterns and predictors of smoking cessation in an elderly cohort. J Am Geriatr Soc. 2006;54(3):466–471. doi: 10.1111/j.1532-5415.2005.00641.x. http://dx.doi.org/10.1111/j.1532-5415.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 17.Pirie K, Peto R, Reeves GK, Green J, Beral V Million Women Study C. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381(9861):133–141. doi: 10.1016/S0140-6736(12)61720-6. http://dx.doi.org/10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128. http://dx.doi.org/10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–364. doi: 10.1056/NEJMsa1211127. http://dx.doi.org/10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990–996. doi: 10.2105/ajph.92.6.990. http://dx.doi.org/10.2105/AJPH.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaCroix AZ, Lang J, Scherr P, et al. Smoking and mortality among older men and women in three communities. N Engl J Med. 1991;324(23):1619–1625. doi: 10.1056/NEJM199106063242303. http://dx.doi.org/10.1056/NEJM199106063242303. [DOI] [PubMed] [Google Scholar]
- 22.Paganini-Hill A, Hsu G. Smoking and mortality among residents of a California retirement community. Am J Public Health. 1994;84(6):992–995. doi: 10.2105/ajph.84.6.992. http://dx.doi.org/10.2105/AJPH.84.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt MT, Cauley JA, Scott JC, Kuller LH, Browner WS. Smoking and mortality among older women: the study of osteoporotic fractures. Arch Intern Med. 1996;156(6):630–636. http://dx.doi.org/10.1001/archinte.1996.00440060050006. [PubMed] [Google Scholar]
- 24.Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General's report: "The health consequences of smoking-50 years of progress": a paradigm shift in cancer care. Cancer. 2014;120(13):1914–1916. doi: 10.1002/cncr.28695. http://dx.doi.org/10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299(17):2037–2047. doi: 10.1001/jama.299.17.2037. http://dx.doi.org/10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegmann KT, Fraser AM, Keaney RP, et al. The effect of age at smoking initiation on lung cancer risk. Epidemiology. 1993;4(5):444–448. doi: 10.1097/00001648-199309000-00010. http://dx.doi.org/10.1097/00001648-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Huxley RR, Yatsuya H, Lutsey PL, Woodward M, Alonso A, Folsom AR. Impact of age at smoking initiation, dosage, and time since quitting on cardiovascular disease in African Americans and whites: the atherosclerosis risk in communities study. Am J Epidemiol. 2012;175(8):816–826. doi: 10.1093/aje/kwr391. http://dx.doi.org/10.1093/aje/kwr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam TH, Li ZB, Ho SY, et al. Smoking, quitting and mortality in an elderly cohort of 56,000 Hong Kong Chinese. Tob Control. 2007;16(3):182–189. doi: 10.1136/tc.2006.019505. http://dx.doi.org/10.1136/tc.2006.019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.