Abstract
The product of the human C21orf57 (huYBEY) gene is predicted to be a homologue of the highly conserved YbeY proteins found in nearly all bacteria. We show that, like its bacterial and chloroplast counterparts, the HuYbeY protein is an RNase and that it retains sufficient function in common with bacterial YbeY proteins to partially suppress numerous aspects of the complex phenotype of an Escherichia coli ΔybeY mutant. Expression of HuYbeY in Saccharomyces cerevisiae, which lacks a YbeY homologue, results in a severe growth phenotype. This observation suggests that the function of HuYbeY in human cells is likely regulated through specific interactions with partner proteins similarly to the way YbeY is regulated in bacteria.
Keywords: YBEY, C21orf57, RNase, yeast
Introduction
huYBEY (C21orf57) is an uncharacterized gene located at 21q22.3 in the smallest human autosome, chromosome 21 [1]. Dysregulation of huYBEY expression levels has been observed in several human diseases, including Down syndrome, and also in various cancers [2–4]. Although little is known about its function, HuYbeY is a member of the highly conserved UPF0054 family [5] and shares 23% sequence identity with Escherichia coli YbeY (EcYbeY) [6]. Homologues of E. coli YbeY are found in nearly every sequenced bacterial strain [7]. Even the small genomes of the unusual bacteria in the recently recognized Candidate Phyla Radiation (CPR) [8], which lack multiple biosynthetic pathways and have divergent 16S rRNA genes, include a ybeY gene [8]. ybeY is one of the 206 genes postulated to comprise the minimal bacterial genome set [9].
Bacterial YbeY proteins were originally defined as metalloproteins based on structural analyses, which revealed that three highly conserved histidine residues (H114, H118 and H124 in EcYbeY) and a water molecule coordinate a metal ion [10–12]. The biological function of bacterial YbeY was first characterized in the nitrogen-fixing plant symbiont Sinorhizobium meliloti. A S. meliloti mutant lacking a functional ybeY gene was found to be defective in symbiosis with its legume hosts and to be sensitive to strikingly broad array of environmental stresses [13]. Subsequent studies demonstrated that EcYbeY is a heat-shock protein [14] and implicated YbeY in translation on the basis of a decrease of 70S ribosomes and polysomes and production of defective 30S subunits in an E. coli ΔybeY mutant [7,14,15]. Our group showed that the maturation of the 5′ and 3′ termini of E. coli 16S rRNA and 5′ termini of 23S and 5S rRNA are severely affected in the absence of ybeY. Furthermore, we demonstrated that ybeY mutations exhibit strong genetic interactions with mutations in rnr, pnp and rnc, which encode RNase R, polynucleotide phosphorylase (PNPase) and RNase III, respectively [7]. An E. coli ΔybeY Δrnr double mutant was found to have a pronounced growth defect and to exhibit a massive impairment of 16S rRNA maturation, the first indication of the involvement of YbeY and RNase R in the critical maturation of the 3′ terminus of 16S rRNA [7,16]. Since then, we have demonstrated that, like EcYbeY, Vibrio cholerae YbeY is an RNase with similar properties and acts together with RNase R in 70S ribosome quality control [17,18]. S. meliloti YbeY has recently been shown to be an RNase as well [19].
In addition, YbeY function is needed for other important bacterial physiological processes including both Hfq-dependent and Hfq-independent regulation of small RNAs [20], transcription antitermination [21], and apoptosis-like death (ALD) in E. coli [22]. Although genome-wide deletion or transposon libraries have revealed ybeY to be essential in several human pathogens [17], only few studies have investigated YbeY function in bacteria beside E. coli or S. meliloti [17,23,24]. Interestingly, reduction of YbeY expression levels in V. cholerae results in defects in rRNA processing and ribosome quality control, changes in small RNA regulation, decreased stress tolerance, and ultimately a strongly compromised virulence potential [17]. Most recently, we have used bacterial 2-hybrid analysis to show that EcYbeY interacts with multiple partners including ribosomal protein S11, the GTPases Era and Der, the stress regulator SpoT and its operon partner YbeZ [25]. Interactions with S11 and Era appear to target the relatively non-specific endoribonuclease activity of YbeY during processing of the 3′-terminus of 16S rRNA, suggesting that interactions with various partner proteins are likely to be important for YbeY’s other physiological functions as well [25].
Homologues of YbeY are encoded in the genomes of many eukaryotes including mammals and plants although not in yeast or fungi. However, to date, detailed characterizations of YbeY function in eukaryotes have been limited to a single study of a plant [26]. The Arabidopsis thaliana homologue of YbeY (AtYbeY) has been shown to be an RNase that localizes to chloroplasts [26], where it is involved in processing of chloroplast 23S, 16S, and 4.5S rRNAs in the plant. In the absence of YbeY, plants show a defect in seed germination and growth, and leaves become pale-green [26]. In contrast HuYbeY and its mouse ortholog have been reported to be part of the proteome of a different organelle, the mitochondrion [27].
The discovery that both bacterial and plant YbeY proteins are RNases that affect a wide range of important physiological processes suggested that the human YbeY homologue, HuYbeY, might also be an RNase that plays important physiological roles. We show here, for the first time, that HuYbeY is an RNase and that the disruption of its putative RNase active site abrogates its activity. Furthermore, we demonstrate that HuYbeY is able to partially suppress the pleiotropic phenotype of an E. coli ΔybeY mutant strain. We also report that expression of HuYbeY in yeast is toxic, suggesting that the action of YbeY in human cells is likely to be highly controlled.
Materials and Methods
Standard molecular manipulations, protein purifications, RNase assays, and stress tests were done as described before [7,17,18] with minor modifications. Experimental procedures are described in detail in Supplementary Materials.
Results and Discussion
HuYbeY is closely related to bacterial YbeY proteins
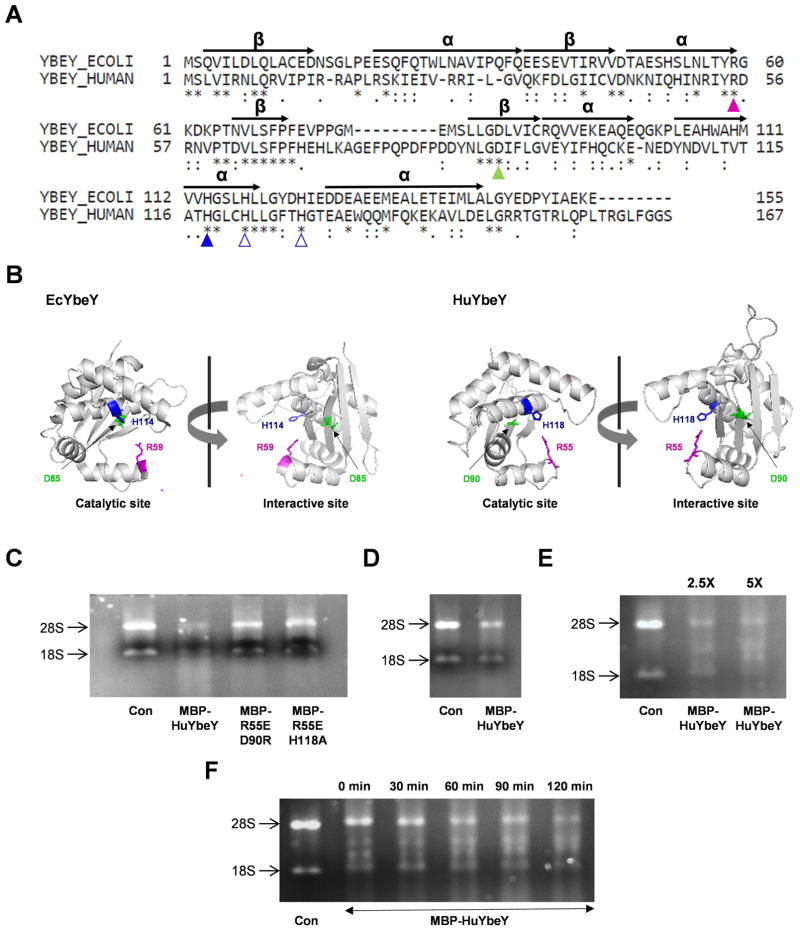
EcYbeY has two well-defined interfaces that are involved in RNA and/or protein interactions (Fig. 1A and 1B). First, the active site of EcYbeY consists of a channel composed of alpha-helixes that includes a highly conserved metal-binding histidine-triad (H3XH5XH motif; H114, H118, H124) and a conserved arginine (R59) at the bottom of the channel [7,18]; mutations of the H3XH5XH motif or R59 result in loss of YbeY’s RNase function. Second, we recently showed that certain residues (e.g. D85) located in a 4-stranded beta-sheet outside of the active site are important for interactions between EcYbeY and ribosomal protein S11; these interactions with the ribosome were also found to be important for EcYbeY’s role in rRNA maturation and stress regulation [25]. In addition, bacterial YbeY proteins share structural similarities with the MID domain of Argonaute (AGO) proteins, consistent with the observed involvement of YbeY in both Hfq-dependent and Hfq-independent small-RNA regulation in bacteria [17,20,28].
Fig 1. Characterization of human YbeY, C21orf57 (HuYbeY).
(A) Protein sequence alignment of EcYbeY and HuYbeY. α-helices (α) and β-sheets (β) are indicated based on the crystal structure analysis of EcYbeY [10]. (B) PyMOL presentation of EcYbeY (PDB: 1XM5;[10]) showing the catalytic domain (left) and ribosome-binding domain (right). HuYbeY was modelled onto the coordinates of EcYbeY using SWISS-MODEL [6]. EcYbeY residues R59 (magenta), D85 (green) and H114 (blue) correspond to HuYbeY residues R55, D90 and H118, respectively, in (A) and (B). (C)-(F) MBP-HuYbeY degrades total human RNA. (C) RNase activity of MBP-HuYbeY protein in comparison to the mutated forms of MBP-HuYbeY protein; 1 μM concentration of protein was used. RNA was digested with 1 μM (D), 2.5 and 5 μM (E) concentration of MBP-HuYbeY protein, while the time-course assay was performed with 5 μM concentration of MBP-HuYbeY protein (F). Wild-type and mutant MBP-HuYbeY proteins in (C) have been purified from E. coli BL21(DE3) plysS Δrna strain; MBP-HuYbeY in (D, E and F) has been purified from E. coli BL21(DE3) plysS Δrna Δpnp strain. RNA digests were analyzed by agarose gel electrophoresis.
HuYbeY is a 167 amino acids long protein. Based on cDNA analysis, it has three additional isoforms that are smaller in size, each missing a portion of the canonical protein sequence [29] (Fig. S1). The full-length version of HuYbeY protein was aligned with EcYbeY (Fig. 1A) and was structurally modeled using EcYbeY as template (Fig. 1B) [6]. The structural homology model of HuYbeY shows that the overall architecture of bacterial YbeY proteins is well conserved in the human homologue. In particular, key residues in domains responsible for enzyme activity and protein-protein interactions are also conserved, e.g. HuYbeY R55, D90, and H118, which correspond to EcYbeY R59, D85, and H114 respectively. Interestingly, relative to EcYbeY, HuYbeY has an insertion of 9 amino acids (G75-P83) between two conserved beta-sheets. These extra amino acids might possibly serve as an additional protein interaction domain.
HuYbeY is an RNase
Sequence similarity to EcYbeY and other UPF0054 family members suggested that HuYbeY was likely to share a common enzymatic function with its bacterial and chloroplast YbeY counterparts. To assess directly whether HuYbeY is an RNase, we overexpressed and purified recombinant MBP-tagged HuYbeY (MBP-HuYbeY) protein. The MBP was not cleaved from the recombinant protein in order to keep it soluble. Two mutant MBP-HuYbeY proteins were similarly purified: MBP-HuYbeY R55E H118A has two amino acid changes altering the presumptive catalytic site, while MBP-HuYbeY R55E D90R has one mutation altering the presumptive catalytic site and a second one altering the putative protein interaction domain (Fig. S2A). The RNase activity of these purified recombinant MBP-HuYbeY proteins was tested on total RNA extracted from human cells. Wild-type MBP-HuYbeY protein was able to degrade the total RNA within two hours, whereas the mutant MBP-HuYbeY proteins were inactive (Fig. 1C). These results indicated that HuYbeY has an RNase activity that is dependent on the same active site residues as EcYbeY. Nevertheless, since mass spectrometric analysis revealed a small amount of the PNPase contamination in the preparations of both the recombinant wild-type and mutant MBP-HuYbeY proteins (Fig. S2B), we repeated the expression and purification of MBP-HuYbeY from an E. coli strain lacking PNPase to eliminate any possibility that the observed RNA degradation was due to this PNPase contaminant. For this purpose, our standard E. coli expression strain E. coli BL21(DE3) plysS Δrna [18] was further modified by deletion of pnp, generating E. coli BL21(DE3) plysS Δrna Δpnp. Wild-type MBP-HuYbeY purified from this strain retained its RNase activity confirming that, similar to other characterized members of the UPF0054 family, HuYbeY is an RNase (Fig. 1D–F).
Expression of HuYbeY in E. coli ΔybeY partially suppresses the complex phenotype of the mutant strain
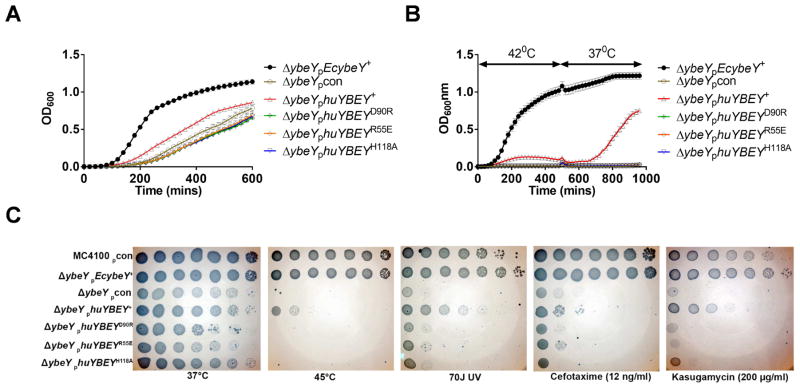
E. coli ΔybeY strains grow slowly compared to the respective wild-type strains and are strikingly sensitive to a wide variety of environmental stresses [7,18]. Ectopic expression of EcYbeY or homologues from various bacterial pathogens or the plant symbiont S. meliloti fully rescues the functional loss of YbeY [7,17,18]. To test whether HuYbeY has sufficient function in common with bacterial YbeY proteins to exert a physiological effect in bacteria, we cloned the cDNA encoding HuYbeY into plasmid pBR322 under the control of a constitutive tetracycline promoter [7]. Three mutated versions of the C21orf57 gene, which encodes huYBEY gene, were also cloned. Each of the mutated genes encodes a HuYbeY protein with a single amino acid change: R55E, D90R or H118A (Fig. 1B). Remarkably, despite its very considerable sequence divergence from EcYbeY, HuYbeY retains sufficient common function to moderately suppress the slow growth phenotype of an E. coli ΔybeY mutant. None of the three HuYbeY mutants had any effect when expressed, indicating that this suppression requires the catalytic function of YbeY as well as very likely the surface near the conserved beta-sheet aspartate D90 of HuYbeY, which corresponds to D85 of EcYbeY.
YbeY is a heat-shock protein that is indispensable at higher temperatures (42°C and higher) [7,14]. We observed that EcYbeY expression, not HuYbeY expression, allows cellular growth of a ΔybeY mutant at 42°C (Fig. 2B). Nevertheless, despite this, HuYbeY is clearly able to provide a protective effect at 42°C since ΔybeY cells that express HuYbeY during the heat treatment are able to resume growth in liquid media following a temperature shift back to 37°C whereas ΔybeY cells cannot (Fig. 2B).
Fig 2. Expression of HuYbeY in E. coli MC4100 ΔybeY changes growth behavior and stress sensitivity.
(A) Growth of ΔybeY cells carrying wild-type or mutant HuYbeY expression plasmids as indicated. Cells were grown in liquid medium at 37°C. (B) Cells were initially grown at 42°C and then shifted to 37°C. Averages of 9 transformants are shown in (A) and (B). None of the three mutants showed any activity. (C) Stress profile of ΔybeY cells carrying the respective HuYbeY expression plasmids grown on solid media at high temperature, after exposure to UV and in the presence of antibiotics cefotaxime and kasugamycin, respectively.
Studies in S. meliloti, E. coli, and V. cholerae have shown that the absence or reduced levels of YbeY increase the sensitivity of cells to a broad range of environmental stresses besides heat, including inhibitors of translation (e.g. kasugamycin), cell wall synthesis (e.g. cefotaxime), DNA damage (e.g. UV), and oxidative stress (e.g. H2O2) [7,13,17]. We therefore exposed E. coli ΔybeY strains that express either wild-type HuYbeY or mutant HuYbeYs to heat-treatment or sublethal doses of UV, cefotaxime or kasugamycin and assessed stress sensitivity by standard spotting stress assays on plates; strains grown at 37°C in the absence of any stress agent are shown as controls (Fig. 2C). For all of these diverse stresses, we observed that HuYbeY retained sufficient function in common with EcYbeY to partially suppress the sensitivity of a ΔybeY mutant when it was expressed. In these cases as well, the partial suppression requires the catalytic function of YbeY (EcYbeY R59 and H114/HuYbeY R55 and H118) and possibly the surface near the conserved beta-sheet aspartate (EcYbeY D85/HuYbeY D90).
A hallmark of the phenotype of an E. coli ΔybeY mutant is its severe defect in translation, which is manifested by reduced protein synthesis rates, reduced translational fidelity, a decrease of 70S ribosomes and polysomes, and impaired rRNA maturation [7]. The total rRNA profile of an E. coli ΔybeY mutant shows a significant decrease of mature 16S rRNA and a concomitant increase of 17S rRNA (the unprocessed 16S rRNA precursor), as well as the presence of 16S* rRNA, an aberrant 16S rRNA fragment lacking the mature 3′ terminus [7,18]. HuYbeY expression did not rescue the rRNA maturation defect that is typical for the mutant strain at 37°C (Fig. S3A) but did reduce the amount of 16S* rRNA slightly. Moreover, RNA isolated from cells after a 2-hour incubation at 45°C showed that expressing HuYbeY had the beneficial effect of converting some of the 17S precursor to mature 16S rRNA (Fig. S3B). This observation may account for the improved growth of an E. coli ΔybeY mutant in the presence of HuYbeY after the 42°C heat-shock described above (Fig. 2B). Thus, for all the stressors we tested, HuYbeY retains sufficient activity in common with bacterial YbeY to partially suppress the complex phenotype of an E. coli ΔybeY mutant and that all of these effects require its enzymatic activity.
Expression of HuYbeY in yeast causes severe toxicity
The 19 amino acid long N-terminal sequence of all isoforms of HuYbeY, including the full-length protein (Fig. 1A and Fig. S1), is strongly predicted to be a putative mitochondrial targeting sequence (MTS) by MITOPROT and TargetP v1 [30,31]. Moreover HuYbeY and its mouse ortholog are classified as mitochondrial proteins by both MitoCarta2.0 [27,32] and IMPI [33]. The assignment of HuYbeY and its mouse ortholog to the mitochondrial matrix is supported by multiple pieces of evidence including APEX-labeling and tandem mass spectrometry [27]. However, an assessment of HuYbeY’s subcellular localization is complicated by evidence obtained during antibody-based profiling of human chromosome 21 that HuYbeY localizes to the nucleus, although not the nucleoli [34].
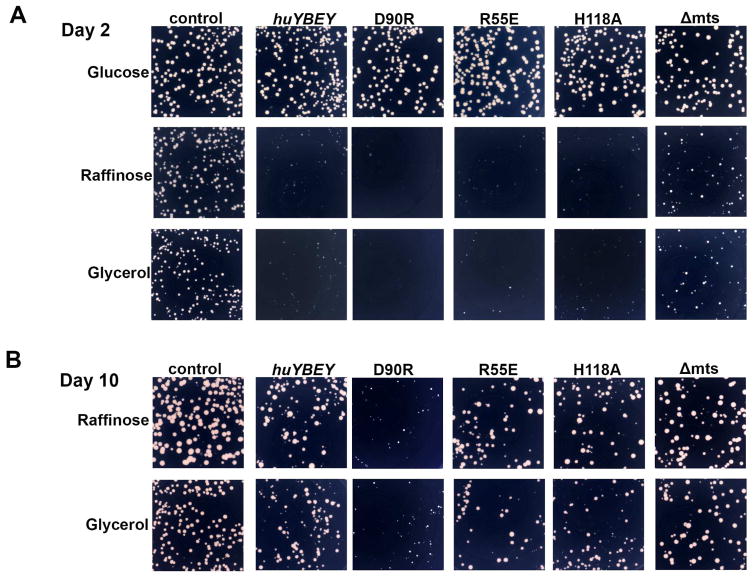
The cDNA encoding full-length HuYbeY was cloned downstream of an inducible GAL1 promoter in an E. coli-yeast shuttle vector and expressed in Saccharomyces cerevisiae. We reasoned that expression of the HuYbeY RNase in a cellular environment lacking the protein partners that normally direct and regulate its activity would likely be highly detrimental. Indeed, expression of HuYbeY in S. cerevisiae proved extremely toxic, indicated by an overall reduction and strong heterogeneity of colony sizes (Fig. 3A). Remarkably, all HuYbeY variants (R55E, D90R or H118A) that carry mutations at residues shown above to be important for HuYbeY function (Fig. 1), presented a similar toxic phenotype after standard growth of transformants at 30°C for 2 days (Fig. 3A). Extended incubation at room temperature revealed an interesting growth phenotype (Fig. 3B): transformants expressing HuYbeY wildtype protein or mutant proteins R55E and H118A with changes in the active site of HuYbeY were able to grow, albeit noticeably slower than transformants containing the empty expression vector; in contrast, expression of HuYbeY D90R, which carries a mutation in the predicted protein-protein interface based on homology with EcYbeY, continued to be extremely toxic for yeast.
Fig 3. Expression of HuYbeY in yeast is toxic.
S. cerevisiae CKY473 transformants expressing wild-type or mutant HuYbeY were grown on synthetic minimal medium containing 0.5% galactose supplemented with either 2% glucose, 2% raffinose or 3% glycerol as indicated. Plates were incubated at 30°C for 2 days (A), followed by a 7-day incubation at room temperature (B). Yeast cells carrying the empty vector are provided as control. Δmts indicates a HuYbeY expression construct lacking the predicted MTS. Assays were carried out in duplicates; a representative experiment is shown.
To provide an independent assessment of the MTS sequence of HuYbeY to direct the protein to mitochondria, we also generated a HuYbeY expression construct lacking the predicted MTS (Δmts) to assay for mitochondrial deficiency manifested through specific formation of small colonies, also known as petite (ρ−) mutants, on a non-fermentable carbon source such as glycerol [35, 36]. Unfortunately, because of the strong toxicity observed in raffinose medium upon induction of wild-type and mutant HuYbeY expression in yeast, we were unable to detect further distress through growth on glycerol (Fig. 3A and 3B). Removal of the predicted HuYbeY MTS causes only a moderate rescue of the severe growth phenotype, but the effects of expressing wild-type or Δmts HuYbeY in yeast were not specific to the nature of the carbon source (fermentable/raffinose vs. non-fermentable/glycerol). As expected, the addition of glucose to the medium repressed HuYbeY expression from the GAL1 promoter and alleviated the toxic phenotype in yeast (Fig. 3A).
Further work will be required to determine whether the report in the Human Protein Atlas [37] that HuYBeY localizes to the nucleoplasm of cells rather than to mitochondria is due to i) as of yet unknown regulatory mechanisms underlying the subcellular localization of HuYbeY, mechanisms that are more complicated than suggested by HuYbeY’s mitochondrial targeting sequence, ii) the particular antibody used, iii) a different isoform of YbeY being detected, or iv) the use of cancer cell lines (osteosarcoma U-2 OS, epithelial carcinoma A-431, malignant glioma U-251 MG) rather than normal tissues.
Concluding remarks
Our results demonstrate that HuYbeY, the protein encoded by the uncharacterized human gene huYBEY (C21orf57), resembles characterized bacterial and chloroplast members of the highly conserved UPF0054 family in possessing an RNase activity and that, despite its very considerable evolutionary divergence, HuYbeY retains sufficient function in common with EcYbeY to partially suppress many aspects of the complex phenotype of an E. coli ΔybeY mutant. We also demonstrate that expression of HuYbeY in S. cerevisiae, a eukaryote that does not encode a YbeY protein, is highly toxic. Our findings suggest that the toxicity of HuYbeY expression in yeast is most likely not linked to its RNase activity, but rather to unfavorable or non-regulated interactions of HuYbeY with proteins or nucleic acids, ultimately affecting cell physiology. These observations are consistent with the notion that HuYbeY function in human cells must be directed and regulated by evolved partners as YbeY function is in bacteria [25] and are a first indication for the possible involvement of HuYbeY in human diseases that are associated with the dysregulation of cellular HuYbeY levels.
Although the biological roles of HuYbeY remain uncharacterized, there are indications that HuYbeY may play important roles in human health. For example, huYBEY is one of nine chromosome 21 genes in Down individuals that are expressed more highly than the expected 1.5 fold [2] and it is one of the most highly overexpressed chromosome 21 genes in Down neural progenitor cells [38]. In examples related to cancer, a likely loss-of-function huYBEY mutation has led to huYBEY being classified as a potential causative candidate gene for colorectal adenomatous polyposis and a partial duplication of the 3′ end of the huYBEY gene has been identified in a patient with early-onset breast cancer [3,4]. We hope that the insights and principles reported here will help to guide further investigations of HuYbeY’s physiological and medical significance in humans.
Supplementary Material
Acknowledgments
We would like to thank Prof. Uttam L. RajBhandary for critical comments on the manuscript. This work was supported by a grant from the National Institutes of Health GM31010 to GCW. CK was supported by a grant from the National Institutes of Health GM17151 to Prof. Uttam L. RajBhandary (Massachusetts Institute of Technology). BWD was supported by the Welch Foundation and the U.S. Army Research Office. GCW is an American Cancer Society Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hattori M, Fujiyama A, Taylor TD, et al. The DNA sequence of human chromosome 21. Nature. 2000;405:311–319. doi: 10.1038/35012518. [DOI] [PubMed] [Google Scholar]
- 2.Ait Yahya-Graison E, Aubert J, Dauphinot L, et al. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact on disease phenotypes. Am J Hum Genet. 2007;81:475–91. doi: 10.1086/520000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krepischi ACV, Achatz MIW, Santos EMM, et al. Germline DNA copy number variation in familial and early-onset breast cancer. Breast Cancer Res. 2012;14:R24. doi: 10.1186/bcr3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horpaopan S, Spier I, Zink AM, et al. Genome-wide CNV analysis in 221 unrelated patients and targeted high-throughput sequencing reveal novel causative candidate genes for colorectal adenomatous polyposis. Int J Cancer. 2015;136:E578–E589. doi: 10.1002/ijc.29215. [DOI] [PubMed] [Google Scholar]
- 5.UNIPROT. [accessed 12.14.2016];2016 http://www.uniprot.org/uniprot/W2PC85.
- 6.SWISS-MODEL. [accessed 12.14.2016];2016 https://swissmodel.expasy.org/
- 7.Davies BW, Kohrer C, Jacob AI, et al. Role of Escherichia coli YbeY, a highly conserved protein, in rRNA processing. Mol Microbiol. 2010;78:506–18. doi: 10.1111/j.1365-2958.2010.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown CT, Hug LA, Thomas BC, et al. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature. 2015;523:208–211. doi: 10.1038/nature14486. [DOI] [PubMed] [Google Scholar]
- 9.Gil R, Silva FJ, Pereto J, Pereto J. Determination of the core of a minimal bacterial gene set. Microbiol Mol Biol Rev. 2004;68:518–537. doi: 10.1128/MMBR.68.3.518-537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan C, Fedorov EV, Shi W, et al. The ybeY protein from Escherichia coli is a metalloprotein. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61:959–963. doi: 10.1107/S1744309105031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oganesyan V, Busso D, Brandsen J, et al. Structure of the hypothetical protein AQ_1354 from Aquifex aeolicus. Acta Crystallogr D Biol Crystallogr. 2003;59:1219–1223. doi: 10.1107/s0907444903011028. [DOI] [PubMed] [Google Scholar]
- 12.Penhoat CH, Li Z, Atreya HS, et al. NMR solution structure of Thermotoga maritima protein TM1509 reveals a Zn-metalloprotease-like tertiary structure. J Struct Funct Genomics. 2005;6:51–62. doi: 10.1007/s10969-005-5277-z. [DOI] [PubMed] [Google Scholar]
- 13.Davies BW, Walker GC. A highly conserved protein of unknown function is required by Sinorhizobium meliloti for symbiosis and environmental stress protection. J Bacteriol. 2008;190:1118–1123. doi: 10.1128/JB.01521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasouly A, Schonbrun M, Shenhar Y, et al. YbeY, a heat shock protein involved in translation in Escherichia coli. J Bacteriol. 2009;191:2649–2655. doi: 10.1128/JB.01663-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasouly A, Davidovich C, Ron EZ. The heat shock protein YbeY is required for optimal activity of the 30S ribosomal subunit. J Bacteriol. 2010;192:4592–4596. doi: 10.1128/JB.00448-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutscher MP. Maturation and degradation of ribosomal RNA in bacteria. Prog Mol Biol Transl Sci. 2009;85:369–391. doi: 10.1016/S0079-6603(08)00809-X. [DOI] [PubMed] [Google Scholar]
- 17.Vercruysse M, Kohrer C, Davies BW, et al. The highly conserved bacterial RNase YbeY is essential in Vibrio cholerae, playing a critical role in virulence, stress regulation, and RNA processing. PLoS Pathog. 2014;10:e1004175. doi: 10.1371/journal.ppat.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob AI, Kohrer C, Davies BW, et al. Conserved bacterial RNase YbeY plays key roles in 70S ribosome quality control and 16S rRNA maturation. Mol Cell. 2013;49:427–38. doi: 10.1016/j.molcel.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saramago M, Peregrina A, Robledo M, et al. Sinorhizobium meliloti YbeY is an endoribonuclease with unprecedented catalytic features, acting as silencing enzyme in riboregulation. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey SP, Winkler JA, Li H, et al. Central role for RNase YbeY in Hfq-dependent and Hfq-independent small-RNA regulation in bacteria. BMC Genomics. 2014;15:121. doi: 10.1186/1471-2164-15-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grinwald M, Ron EZ. The Escherichia coli translation-associated heat shock protein YbeY is involved in rRNA transcription antitermination. PLoS One. 2013;8:e62297. doi: 10.1371/journal.pone.0062297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erental A, Kalderon Z, Saada A, et al. Apoptosis-like death, an extreme SOS response in Escherichia coli. MBio. 2014;34:2331–2336. doi: 10.1128/mBio.01426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leskinen K, Varjosalo M, Skurnik M. Absence of YbeY RNase compromises the growth and enhances the virulence plasmid gene expression of Yersinia enterocolitica O:3. Microbiology. 2015;161:285–299. doi: 10.1099/mic.0.083097-0. [DOI] [PubMed] [Google Scholar]
- 24.Ohyama H, Sakai T, Agari Y, et al. The role of ribonucleases in regulating global mRNA levels in the model organism Thermus thermophilus HB8. BMC Genomics. 2014;15:386. doi: 10.1186/1471-2164-15-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vercruysse M, Kohrer C, Shen Y, et al. Identification of YbeY-protein interactions involved in 16S rRNA maturation and stress regulation in Escherichia coli. MBio. 2016;7:e01785–16. doi: 10.1128/mBio.01785-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Zhou W, Liu G, et al. The conserved endoribonuclease YbeY is required for chloroplast ribosomal RNA processing in Arabidopsis. Plant Physiol. 2015;168:205–21. doi: 10.1104/pp.114.255000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2015;44:1–7. doi: 10.1093/nar/gkv1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey SP, Minesinger BK, Kumar J, et al. A highly conserved protein of unknown function in Sinorhizobium meliloti affects sRNA regulation similar to Hfq. Nucleic Acids Res. 2011;39:4691–4708. doi: 10.1093/nar/gkr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UNIPROT. [accessed 12.14.2016];2016 http://www.uniprot.org/uniprot/P58557.
- 30.Claros MG, Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 31.Emanuelsson O, Brunak S, von Heijne G. Locating proteins in the cell using TargetP, SignalP, and related tools. Nat Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- 32. [accessed 12.14.2016];MITOCARTA: AN INVENTORY OF MAMMALIAN MITOCHONDRIAL GENES. 2016 www.broadinstitute.org/pubs/MitoCarta.
- 33.THE MITOCHONDRIAL PROTEOME (IMPI) [acccessed 12.14.2016];2016 http://www.mrc-mbu.cam.ac.uk/impi.
- 34.Uhlen M, Oksvold P, Algenas C, et al. Antibody-based protein profiling of the human chromosome 21. Mol Cell Proteomics. 2012;11:M111.013458. doi: 10.1074/mcp.M111.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson LR, von Borstel RC. Induction of the cytoplasmic “petite” mutation by chemical and physical agents in Saccharomyces cerevisiae. Mutat Res. 1992;265:103–48. doi: 10.1016/0027-5107(92)90042-z. [DOI] [PubMed] [Google Scholar]
- 36.Parrella E, Longo VD. The chronological life span of Saccharomyces cerevisiae to study mitochondrial dysfunction and disease. Methods. 2008;46:256–262. doi: 10.1016/j.ymeth.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Human Protein Atlas. [accssed 12.14.2016];2016 http://www.proteinatlas.org/ENSG00000182362-YBEY/cell.
- 38.Bhattacharyya A, McMillan E, Chen SI, et al. A critical period in cortical interneuron neurogenesis in down syndrome revealed by human neural progenitor cells. Dev Neurosci. 2009;31:497–510. doi: 10.1159/000236899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.