Abstract
Introduction
The prevalence and characteristics of Agrin and LRP4 antibody positive amyotrophic lateral sclerosis (ALS) patients were studied.
Methods
We tested 82 ALS patients and 59 controls for Agrin and LRP4 antibodies using ELISA.
Results
We found that 13.8% of ALS patients had Agrin antibodies, and 9.8% had LRP4 antibodies. Women ALS patients are twice as likely as men to have antibodies. Agrin-positive ALS patients are younger than Agrin-negative ALS patients.
Discussion
Antibodies to Agrin and LRP4 are found in ALS patients. It must be determined if these antibodies are pathogenic. Since antibody positive patients have upper as well as lower motor neuron findings, the antibodies’ effects cannot be explained solely by their actions at the neuromuscular junction. Perhaps a breakdown in inter-neuronal signaling might cause ALS. Further research is needed to resolve this question.
Keywords: Agrin, Amyotrophic Lateral Sclerosis, Antibodies, LRP4, Low Density Lipoprotein Related Receptor Protein 4, ALS
Introduction
Amyotrophic lateral sclerosis (ALS) is a syndrome characterized by progressive motor neuron degeneration1. The causes of sporadic ALS may be multifactorial, similar to familial ALS which is associated with multiple gene defects2. However, clinical characteristics and biomarkers are lacking for different forms of ALS, which limits our ability to develop therapeutic strategies. Agrin is released by the motor neuron and binds to muscle membrane LRP4 (Low Density Lipoprotein Related Receptor Protein 4)3,4. We, along with others, recently identified Agrin and LRP4 antibodies in myasthenia gravis (MG) 5–9 and demonstrated that LRP4 antibodies are causal for MG10. Tzartos et al reported LRP4 antibodies in 23.1 % of ALS patients11. The aim of our study was to determine if ALS patients have antibodies to both LRP4 and Agrin.
Methods
Fifty-nine healthy controls and 82 ALS patients gave informed consent and participated in this IRB approved study. Patients underwent a comprehensive neurological exam and met El-Escorial criteria12 for possible, probable, probable laboratory-supported, or definite ALS. Their blood samples were assayed by ELISA for Agrin and LPR4 antibodies as previously described 7,9. Statistical analyses were performed using Excel (Microsoft, Redmond, WA) and QI Macros (KnowWare International, Denver, CO).
Results
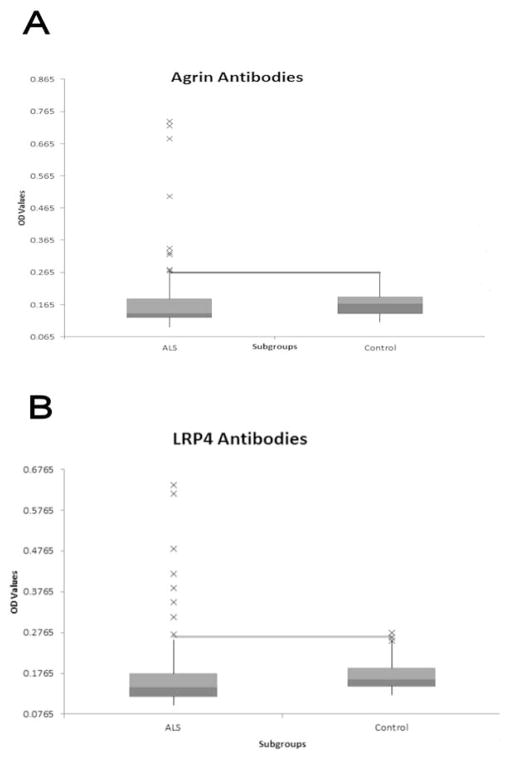
Agrin and LRP4 antibody levels were not significantly different between ALS patients and controls (Agrin t=1.289, p=0.200, LRP4 t=0.192, p=0.869). The variances of Agrin and LRP4 antibody levels were significantly higher for ALS patients than for controls (Agrin F= 12.11, p< 0.001, LRP4 F= 7.04, p<0.001). A small number of ALS patients accounted for the increased variance of both Agrin and LRP4 values (Figure 1), thus identifying a subgroup of ALS patients with increased Agrin and/or LRP4 antibody levels. The normal values for Agrin and LRP4 were set at 0.265 and 0.267, respectively, representing the mean plus 2.5 standard deviations of our control population. Only 1 control subject had elevated LRP4 antibody levels and none had elevated Agrin levels. No ALS patient’s antibody level was between 2.0 and 2.5 standard deviations above the mean.
Figure 1. Distribution of Agrin and LRP4 antibodies<.
br>Bar and whisker graphs show the median, minimum, and maximum values and upper and lower quartiles of the study population. Outliers are indicated by x on the graph. The line indicates the upper limit of normal. A: Distribution of Agrin antibodies in the ALS population compared to normal controls. B: Distribution of LRP4 antibodies in the ALS population compared to normal controls. OD: Optical Density.
Nine of 65 ALS subjects (13.8%) were positive for Agrin antibodies (Figure 1). Eight of 82 ALS subjects (9.8%) were positive for LRP4 antibodies (Figure 1). Agrin-positive ALS patients’ mean value was 0.464 which was 7.71 standard deviations above the control mean. LRP4-positive ALS patients’ mean value was 0.435 which was 6.99 standard deviations above the control mean. Agrin and LRP4 values were strongly correlated in ALS patients (r=0.791, r2=0.626). One subject was positive for Agrin and not LRP4.
Antibody positive ALS patients were slightly younger than negative patients. This was significant for Agrin (48.4 vs 59.7 p=0.021). Antibody positive patients had upper and lower motor neuron findings. The location of the first symptom varied among antibody positive patients; there was initial upper extremity involvement in 55.6 % of antibody-positive patients compared to 37.0% of antibody negative patients. Women with ALS were twice as likely to have antibodies as men. Approximately 15% of the women with ALS were antibody positive compared to only 8% of the men. There was no difference in race or ALS Functional Rating Scale score between antibody positive and negative ALS patients.
Discussion
In our population of ALS patients 13.8% had Agrin antibodies and 9.8% had LRP4 antibodies, which indicates that there is a significant subgroup of patients who are positive for these antibodies. Tzartos et al. described LRP4 antibodies in 23.1% of an Italian and Greek population of ALS patients11. Our values may differ from theirs for several reasons. First, our assay was a quantitative ELISA assay with purified LRP4 while Tzartos et al. used a qualitative cell-based assay. When a radio-immunoprecipitation assay was used on the same population, only 11.5% were positive for LRP4 11. Interestingly 4 of the antibody-positive subjects were not positive using the cell-based assay. Second, the prevalence might depend on patient demographics. In a later study of 87 Israeli subjects, Tzartos et al. found 14.9 % to be positive using the cell based assay 13. The prevalence of LRP4 antibodies might be higher in Europe compared to that of the Southeastern US.
The question remains whether Agrin and LRP4 antibodies present in a significant portion of our ALS patients are pathogenic. Future studies are warranted to determine whether these antibodies will produce disease in experimental animals. Previous studies, including ours, have shown antibodies to Agrin and LRP4 in patients with MG 9,5–8. The positive ALS patients in this study do not show clinical symptoms of MG. The actions of Agrin and LRP4 antibodies at the neuromuscular junction cannot explain the significant upper motor neuron findings seen in our ALS patients. Therefore, some actions of these antibodies probably occur elsewhere in the motor system. LRP4 has been shown to play a role in hippocampal neuronal plasticity14 and astrocyte ATP release15, and LRP4 antibodies are seen in the cerebrospinal fluid of ALS patients13,11. It is likely that Agrin and LRP4 antibodies play a role in neuronal function elsewhere in the nervous system. At the neuromuscular junction, Agrin acts as a motor neuron-derived signal. When Agrin binds to muscle LRP4, it triggers a complex sequence of events which lead to neuromuscular junction development16. Likewise, elsewhere in the nervous system, Agrin might act as a messenger in inter-neuronal communication by binding to LRP4. Perhaps the breakdown in intercellular communication leads to degeneration of the motor system as seen in ALS. This might explain the contiguous spread of symptoms in ALS and why degeneration is largely confined to motor neurons.
Why antibodies to Agrin and LRP4 produce MG in some patients and possibly produce ALS in others is not yet known. Perhaps different serotypes of these antibodies might be an explanation. It is hoped that LRP4 and Agrin antibodies might be a biomarker that identifies a subpopulation of ALS patients who can be studied further. Even if they do not contribute to the pathogenesis of ALS, the presence of these antibodies is of interest and warrants further study.
Acknowledgments
Study funding by Georgia Health Science Foundation ALS Fund and in part by grants from NIH
The authors would like to thank Nicole Smalley who provided technical assistance and Dr Thomas Swift M.D. who reviewed the paper
Abbreviations
- ALS
Amyotrophic Lateral Sclerosis
- ALSFRS
ALS Functional Rating Scale
- ELISA
Enzyme-Linked Immunosorbent Assay
- LMN
Lower Motor Neuron
- LRP4
Low Density Lipoprotein Related Receptor Protein 4
- MG
Myasthenia Gravis
- OD
Optical Density
- UMN
Upper Motor Neuron
Footnotes
The Authors have no conflicts of interest to disclose.
References
- 1.Mehta P, Antao V, Kaye W, Sanchez M, Williamson D, Bryan L, Muravov O, Horton K Division of T; Human Health Sciences AfTS, Disease Registry AG, Centers for Disease C and Prevention. Prevalence of amyotrophic lateral sclerosis - United States, 2010–2011. Morbidity and mortality weekly report. Surveillance summaries. 2014;63(Suppl 7):1–14. [Google Scholar]
- 2.Sreedharan J, Brown RH., Jr Amyotrophic lateral sclerosis: Problems and prospects. Annals of neurology. 2013;74(3):309–316. doi: 10.1002/ana.24012. [DOI] [PubMed] [Google Scholar]
- 3.Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60(2):285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135(2):334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Annals of neurology. 2011;69(2):418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- 6.Pevzner A, Schoser B, Peters K, Cosma NC, Karakatsani A, Schalke B, Melms A, Kroger S. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. Journal of neurology. 2012;259(3):427–435. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B, Tzartos JS, Belimezi M, Ragheb S, Bealmear B, Lewis RA, Xiong WC, Lisak RP, Tzartos SJ, Mei L. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Archives of neurology. 2012;69(4):445–451. doi: 10.1001/archneurol.2011.2393. [DOI] [PubMed] [Google Scholar]
- 8.Gasperi C, Melms A, Schoser B, Zhang Y, Meltoranta J, Risson V, Schaeffer L, Schalke B, Kroger S. Anti-agrin autoantibodies in myasthenia gravis. Neurology. 2014;82(22):1976–1983. doi: 10.1212/WNL.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Shen C, Bealmear B, Ragheb S, Xiong WC, Lewis RA, Lisak RP, Mei L. Autoantibodies to agrin in myasthenia gravis patients. PloS one. 2014;9(3):e91816. doi: 10.1371/journal.pone.0091816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen C, Lu Y, Zhang B, Figueiredo D, Bean J, Jung J, Wu H, Barik A, Yin DM, Xiong WC, Mei L. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. The Journal of clinical investigation. 2013;123(12):5190–5202. doi: 10.1172/JCI66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzartos JS, Zisimopoulou P, Rentzos M, Karandreas N, Zouvelou V, Evangelakou P, Tsonis A, Thomaidis T, Lauria G, Andreetta F, Mantegazza R, Tzartos SJ. LRP4 antibodies in serum and CSF from amyotrophic lateral sclerosis patients. Ann Clin Transl Neurol. 2014;1(2):80–87. doi: 10.1002/acn3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders : official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 13.Tzartos J, Zisimopoulou P, Tsonis A, Evangelakou P, Rentzos M, Karandreas N, Zouvelou V, Thomaidis T, Lauria G, Andreetta F, Mantegazza R, Brenner T, Petrou P, Karusis D, Tzartos S. LRP4 antibodies are frequent in serum and CSF from amyotrophic lateral sclerosis patients (S34.004) Neurology. 2015;84(14 Supplement) doi: 10.1002/acn3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez AM, Froemke RC, Burden SJ. Synaptic plasticity and cognitive function are disrupted in the absence of Lrp4. eLife. 2014;3:e04287. doi: 10.7554/eLife.04287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun XD, Li L, Liu F, Huang ZH, Bean JC, Jiao HF, Barik A, Kim SM, Wu H, Shen C, Tian Y, Lin TW, Bates R, Sathyamurthy A, Chen YJ, Yin DM, Xiong L, Lin HP, Hu JX, Li BM, Gao TM, Xiong WC, Mei L. Lrp4 in astrocytes modulates glutamatergic transmission. Nature neuroscience. 2016 doi: 10.1038/nn.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barik A, Lu Y, Sathyamurthy A, Bowman A, Shen C, Li L, Xiong WC, Mei L. LRP4 Is Critical for Neuromuscular Junction Maintenance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(42):13892–13905. doi: 10.1523/JNEUROSCI.1733-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]