Abstract
Objective
Smoking as an epidemiological exposure can be quantified in many ways including duration, intensity, pack-years, recency, and age at initiation. However it is not clear which of these are most important for cardiovascular disease (CVD), and how they should be modeled.
Study Design and Setting
Using the Multi-Ethnic Study of Atherosclerosis, Cox models for time to incident CVD adjusted for age, sex, race/ethnicity, education category, and income category were compared which included various characterizations of smoking history.
Results
Duration, age at starting, time since quitting, and non-cigarette forms of smoking were not independently associated with CVD, while baseline current intensity was (e.g. hard CVD hazard ratio (HR) 1 pack/day of 1.85 95% CI [1.33, 2.57]). Former smokers, regardless of duration, intensity or recency, were not at increased risk, suggesting that risk may risk may drop precipitously from the time of quitting. For CVD events, representing smoking exposure as baseline smoking intensity produced better model fit as measured by AIC than models using smoking status or pack-years.
Conclusion
The association of smoking with incident CVD events was well captured by including a simple term for baseline smoking intensity.
Keywords: smoking, intensity, cardiovascular disease, Multi-Ethnic Study of Atherosclerosis, cohort
1. Introduction
In 2012 there were 17.5 million deaths from cardiovascular disease, and tobacco use is a modifiable cause of many of these deaths.[1] Smoking increases cardiovascular disease (CVD) risk, consequently it is of interest to know which components of smoking are most associated with CVD outcomes. Even if a study is not investigating smoking as an exposure directly, smoking is so strongly related to CVD that it is also important to adjust for this exposure to control confounding. However, there are many different ways to quantify smoking, including whether the person is currently smoking, how long the person has smoked, what type of tobacco product is used, how much the person smokes each day, and the period of life during which the tobacco exposure occurred. Consequently, how smoking should be modeled is not clear. That is, which components of smoking are associated with CVD outcomes, how these components should be modeled in order to increase our understanding of smoking-CVD relationships, and what may be the best way to adjust for smoking as a potential confounder.
One of the most common ways to model smoking is by dividing subjects into never, former, and current smoking categories. Compared to never smokers, current smoking was associated with increased CVD risk while the evidence was not as strong for past smoking.[2-4] However, one way that heterogeneity can enter into smoking status categories is via smoking intensity, specifically cigarettes/day. Previous studies suggest that there is increased risk of coronary heart disease (CHD) death with increased intensity.[2, 3, 5, 6] Pope et al studied the functional form of how CVD death risk varies over a range of intensities and concluded that a non-linear model of tobacco smoke intensity fit the data best.[7]
Pack-years is a cumulative measurement of smoking and is generally calculated by multiplying average packs smoked per day by the duration of smoking, in years.[8] However, with no definitive evidence that duration, in isolation, is significantly associated with CVD risk, the question of the utility of using pack-years is a valid one, especially since this is a common way of adjusting for smoking in studies.[8-11]
Forms of tobacco exposure other than active cigarette smoking, including second-hand smoke exposure, cigar use, and pipe use were also associated with CVD.[12-15] There was, however, conflicting evidence about whether age at starting smoking affected heart disease risk.[2, 6] Higher time since quitting is a widely acknowledged protective factor for CVD.[16, 17] In addition, age, age at starting, duration, and time since quitting are often additive combinations of the other variables, and so care must be taken not to adjust for all of these in the same model.[8]
In part due to the problem of collinear aspects of smoking and to reduce the number of adjustment covariates, single smoking indices have been proposed.[9, 18, 19] Two indices were discussed in Leffondré et al which both incorporated non-linear forms of time since quitting, duration, and intensity, and two parameters that can be based either on features of the dataset in use or on earlier scientific findings/hypotheses.[19] In this paper we evaluate which of the above quantitative aspects of smoking behavior are most associated with incident cardiovascular events. Our aim is to inform the future modeling of smoking behavior in the context of CVD research.
2. Methods
2.1 Study Population
MESA is a cohort of 6814 participants initially free of clinical heart disease at baseline in 2000-2002.[20, 21] MESA participants were recruited at six sites across the United States, had an age range of 45-84 years, and were 47% male. The ethnic proportions were 38% Caucasian, 12% Chinese-American, 28% African-American, and 22% Hispanic.[21] MESA collected questionnaire data on smoking status, and participants were considered to be smokers if they reported smoking at least 100 cigarettes in their lifetimes and then further classified into current or former smokers at baseline by whether they “smoked cigarettes during the last 30 days”.[21, 22] Baseline intensity (“On average about how many cigarettes a day do/did you smoke”) and ages at first starting and quitting smoking for cigarette, cigar, pipe, snuff and chewing tobacco were also collected.[20-22] Reported intensities of greater than 100 cigarettes/day were set to missing due to implausibility (4 instances). 20 cigarettes define a pack. Pack-years was calculated by multiplying intensity in packs/day by duration in years. MESA determined a number of adjudicated CVD endpoints including the primary outcomes: CHD hard (CHDH) (myocardial infarction, resuscitated cardiac arrest, CHD death), CHD all (CHDA) (CHDH, definite angina, probable angina if followed by revascularization), CVDH (CHDH, stroke death, stroke), and CVDA (CVDH, CHDH, atherosclerotic death, CVD death).[23] Our secondary endpoints (also adjudicated) are stroke, CVD death, and all death. Deep vein thrombosis or pulmonary embolism (DVT-PE) was collected using medical claims information. This endpoint was included since it is a clinically important outcome of smoking, but with a different mechanism relative to CVD.[24] Study of this endpoint provides evidence that our approach is sensitive to such differences.
2.2 Intensity, Duration and Pack-years
Cox models were used to investigate the association of intensity and duration alone, in combination and expressed as pack-years with the risk of four incident CVD endpoints (CVDH, CVDA, CHDH, and CHDA). Another cox model included intensity, duration, and the multiplicative interaction of intensity and duration. Initial models were stratified by current versus former smoking status. A second set of models that pooled all participants were adjusted for smoking status. All Cox models in this paper were adjusted for age, sex, race/ethnicity, education category (highest completed education level), and income category (gross family income in last year).[21] Education and income were adjusted for in order to account for confounding by socioeconomic status. Traditional risk factors such as blood pressure and body mass index were not included in models since these are likely mediators of the smoking-CVD relationship and not confounders. In order to maximize sample size, participants were included in models for which they had complete data in all included covariates unless otherwise specified. Duration, intensity, and pack-years were all centered, after stratification by smoking status, and these centered variables were used for all modeling. Proportional hazards were tested using Schoenfeld residuals and none of the Cox models failed the proportional hazards assumption test. Collinearity between smoking measures was checked and the variance inflation factors were less than four. Cox models including a competing risk of death were also performed and results were similar to the non-competing risk models.[25] Model performance was summarized using the Akaike information criterion (AIC).
Generalized additive models (GAM), accounting for time elapsed to the CVD endpoints were then used to investigate the functional form of each aspect of smoking and test for non-linearity.
2.3 Age at Starting/Time since Quitting
The age at starting smoking was examined by running Cox models adjusted for intensity separately for current and former smokers. Similarly, Cox models using either continuous or categorical years since quitting and adjusted for intensity were run in former smokers. The outcomes for these models were the four incident CVD endpoints.
2.4 Non-Cigarette Forms of Tobacco Exposure and Secondhand Smoke
Cox models with the four CVD endpoints and adjusted for cigarette smoking status, intensity, duration, and the interaction of cigarette smoking status and intensity looked at pipe, cigar, snuff, chewing tobacco, and secondhand smoke exposure status, and secondhand smoke exposure intensity one at a time. These models were pooled, except for the secondhand smoke models which only included non-smokers.
2.5 Compound Smoking Indices
For convenience, the two considered indices will be named Compound Smoking Index X1 (CSI-X1) and Compound Smoking Index X2 (CSI-X2).[19] These indices include time since quitting (T), duration (D), intensity (I), delta (δ), which is “lag time” or time from exposure to disease occurrence, and tau (τ), which is “half-life” or a measure of how long it takes for a quitter’s risk to return to that of a never smoker.[19] CSI-X1 was formulated as ln[(1-0.5(max(D+T-δ,0)-max(T-δ,0))/τ)*0.5(max(T-δ,0)/τ)*I+1], and CSI-X2 as (1-0.5(max(D+T-δ,0)-max(T-δ,0))/τ)*(0.5max(T-δ,0)/τ)*ln(I+1).[19] CSI-X1 and CSI-X2 were calculated using each integer δ and τ combination from δ=0 to 9, and τ=1 to 20, as suggested previously.[19]
2.6 Comparing Models of Smoking
To directly compare performance of different ways of modeling smoking as an exposure, the AICs for eleven Cox models for all eight outcomes were calculated. We selected models to compare based on availability and significance of the smoking variables in the MESA data, as well as models which have appeared in prior publications. These models all included the same participants who had complete data and were adjusted for age, sex, race, income, education, and the following measures of smoking. First was smoking status (never/former/current). Second was an ever smoker indicator, pack-years, years since quitting, and the interaction of time since quitting and pack-years.[9] Third was smoking status, intensity, and the interaction of smoking status and intensity. Fourth and fifth were smoking status and intensity or pack-years respectively. Sixth was smoking status, intensity, and duration. Seventh was smoking status and current smoking intensity (reported average intensity for current smokers, zero for others). Eighth and ninth were current smoking intensity and current pack-years respectively. Tenth and eleventh were CSI-X1 and CSI-X2 respectively, using the δ and τ combinations that were calculated to give minimum AICs for each outcome. STATA 13 was used for all analyses [StataCorp, College Station, TX].
3. Results
3.1 Cohort Characteristics
There were 3418 never, 2487 former, and 887 current smokers at baseline. 22 participants were missing cigarette smoking status information and were excluded. Among former smokers, mean intensity was 17 cigarettes/day and mean duration was 23 years. Among current smokers, mean intensity was 13 cigarettes/day and mean duration was 40 years. Cohort characteristics including missing data are detailed in table 1. The median follow-up time was 10 years. Among all MESA participants, there were 450 CVDH events, 639 CVDA events, 284 CHDH events, 449 CHDA events, 180 strokes, 161 CVD deaths, 709 deaths, and 100 DVT-PE events.
Table 1.
Description of cohort by cigarette smoking status.
| Covariates-Mean (SD) [# missing] |
Never N=3418 |
Former N=2487 |
Current N=887 |
|---|---|---|---|
| Age | 62 (11) | 63 (10) | 58 (9) |
| Gender - Male (%) | 38% | 58% | 53% |
| Race/Ethnicity (%) | |||
| Caucasian | 34% | 47% | 34% |
| Chinese-American | 18% | 6% | 5% |
| African-American | 25% | 28% | 38% |
| Hispanic | 24% | 20% | 23% |
| Education (%) | |||
| Less than high school | 20% | 14% | 19% |
| High school | 43% | 48% | 57% |
| College | 18% | 18% | 13% |
| Graduate school | 19% | 19% | 11% |
| Missing | 0% | 0% | 0% |
| Income (dollars) (%) | |||
| <25,000 | 33% | 27% | 31% |
| 25,000-49,000 | 27% | 27% | 31% |
| 50,000-99,000 | 24% | 27% | 25% |
| 100,000+ | 13% | 15% | 9% |
| Missing | 3% | 4% | 5% |
| Intensity (cigarettes/day) | NA | 17 (14) [25] | 13 (11) [19] |
| Duration (years) | NA | 23 (14) [50] | 40 (10) [3] |
| Pack-Years | NA | 22 (24) [59] | 27 (24) [22] |
| Age at Starting | NA | 18 (5) [10] | 19 (6) [3] |
| Years since Quitting | NA | 22 (13) [47] | NA |
| Median Time to Event (Years) [N] |
|||
| CVDH | 5.2 [208] | 5.0 [157] | 5.0 [84] |
| CVDA | 4.8 [277] | 4.5 [257] | 4.9 [104] |
| CHDH | 5.6 [127] | 4.7 [104] | 4.9 [53] |
| CHDA | 4.8 [186] | 4.2 [194] | 4.4 [69] |
| Stroke | 4.2 [88] | 5.6 [60] | 5.5 [31] |
| CVD Death | 6.5 [80] | 5.5 [55] | 6.4 [25] |
| Death | 6.8 [296] | 6.0 [286] | 6.6 [125] |
| DVT-PE | 5.9 [44] | 5.1 [41] | 5.5 [15] |
3.2 Never/Former/Current
In Cox models with all participants, the hazard ratios for current smoking status were significant for all four endpoints, and ranged from 1.66 for CHDA to 1.98 for CVDH (Table 2). Compared to never smokers, the hazard ratios for former smokers were all non-significant, and the hazard ratios ranged from 0.89 for CVDH to 1.13 for CHDA.
Table 2.
Cox models adjusted for age, gender, race, education, income and cigarette smoking status (never smokers are the comparison group).
| Hazard Ratio 95% CI |
CVDH | CVDA | CHDH | CHDA |
|---|---|---|---|---|
| P-value | N=6514 | N=6515 | N=6514 | N=6514 |
|
| ||||
| Current Smoker | 1.98 | 1.80 | 1.94 | 1.66 |
| 1.51,2.60 | 1.42,2.29 | 1.38,2.74 | 1.23,2.22 | |
| <0.0005 | <0.0005 | <0.0005 | 0.001 | |
|
| ||||
| Former Smoker | 0.89 | 1.06 | 0.91 | 1.13 |
| 0.72,1.11 | 0.89,1.27 | 0.69,1.20 | 0.92,1.40 | |
| 0.308 | 0.496 | 0.507 | 0.251 | |
3.3 Intensity/Duration/Pack-Years
In the smoking status stratified Cox models, duration was not significantly associated with any of the four CVD endpoints examined in either the current or former smokers except for CHDA in the former smokers (HR per 20 year increase of 1.26, p=0.03). (Table 3). Higher intensity was associated with higher hazard of all four endpoints in the current smokers (HRs 1 pack/day increase: CVDH 1.92, CVDA 1.69, CHDH 2.00, CHDA 1.83), but intensity was not associated with any of the four endpoints among the former smokers (HRs ranged from 1.06 for CVDA to 1.17 for CHDA). Pack-years was also associated with higher hazard of all four endpoints in the current smokers (HRs 20 pack-year increase: CVDH 1.29, CVDA 1.22, CHDH 1.34, CHDA 1.28), but not in the former smokers. There was no evidence of any interaction between duration and intensity. Pooled models yielded similar results (data not shown). Comparing time-updated versus baseline measurements of current smoking status and intensity, the HRs were quite similar. There was no significant non-linearity detected via the GAM plots for any aspect of smoking.
Table 3.
Cox models separately investigating duration, intensity, and pack-years. All models are adjusted for age, gender, race, education, and income.
| Hazard Ratio 95% CI |
CVDH | CVDA | CHDH | CHDA | ||||
|---|---|---|---|---|---|---|---|---|
| P-value | Current | Former | Current | Former | Current | Former | Current | Former |
|
| ||||||||
| Intensity | 1.92 | 1.11 | 1.69 | 1.06 | 2.00 | 1.17 | 1.83 | 1.12 |
| (Packs/day) | 1.33,2.76 | 0.90,1.38 | 1.20,2.38 | 0.90,1.27 | 1.29,3.09 | 0.90,1.52 | 1.23,2.73 | 0.92,1.36 |
| <.0005 | 0.328 | 0.003 | 0.477 | 0.002 | 0.235 | 0.003 | 0.260 | |
|
| ||||||||
| Duration | 0.93 | 1.03 | 1.09 | 1.07 | 1.86 | 1.25 | 2.19 | 1.26 |
| (20 years) | 0.43,2.00 | 0.81,1.30 | 0.53,2.23 | 0.89,1.29 | 0.59,5.85 | 0.93,1.66 | 0.77,6.21 | 1.02,1.57 |
| 0.849 | 0.827 | 0.816 | 0.463 | 0.289 | 0.135 | 0.140 | 0.034 | |
|
| ||||||||
| Pack-Years | 1.29 | 1.03 | 1.22 | 1.05 | 1.34 | 1.08 | 1.28 | 1.09 |
| (20 pack-years) | 1.11,1.50 | 0.92,1.16 | 1.06,1.41 | 0.96,1.15 | 1.12,1.60 | 0.94,1.24 | 1.09,1.51 | 0.99,1.21 |
| 0.001 | 0.597 | 0.007 | 0.299 | 0.001 | 0.260 | 0.003 | 0.089 | |
Comparing model fits of intensity alone, intensity and duration, and pack-years alone, among the current smokers yielded similar patterns across the four endpoints (data not shown). In the CVDH endpoint the model including intensity alone had an AIC of 1020. The model adding duration (AIC 1022) and the model with pack-years (AIC 1021) had worse performance than the model with intensity alone. This relationship among the three models generally held across endpoints, with the intensity only model having the lowest AIC. The exception was CHDH (pack-years AIC 670, intensity AIC 670, intensity and duration AIC 671).
3.4 Age at Starting Smoking/Time since Quitting Smoking/Other Forms of Smoking
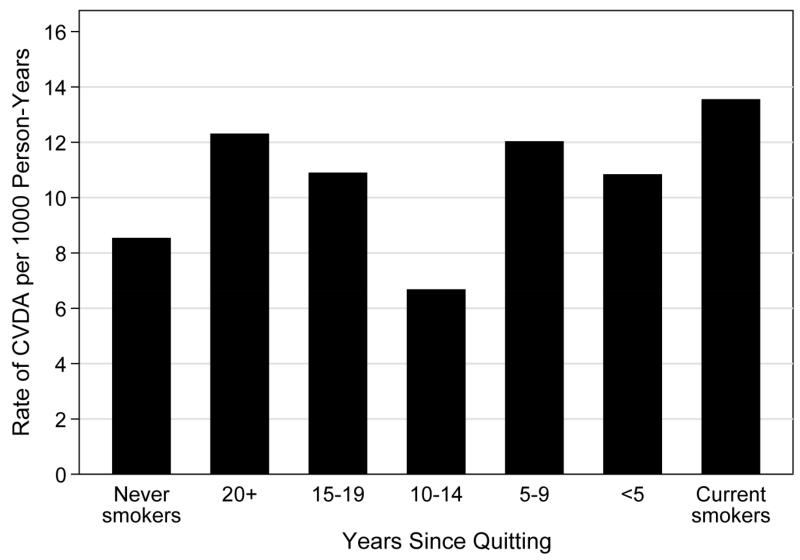
Age at starting smoking for both current and former smokers (HRs per 1 year increase in age at starting smoking 0.97 to 1.00), years since quitting (HRs per 1 year increase in time since quitting smoking 0.99 to 1.00), and all of the non-cigarette tobacco exposures were not significantly associated with any of the four outcomes (Table 4). In addition, the hazard ratios did not follow the expected pattern of increasing risk as time since quitting decreased (Figure 1).
Table 4.
Other forms of smoking: Pipe, cigar, chewing tobacco, secondhand smoke. Adjusted for age, gender, race, cigarette smoking status, cigarette intensity, cigarette duration, interaction of cigarette status and intensity, education and income.
| Hazard Ratio 95% CI P-value |
CVDH | CVDA | CHDH | CHDA |
|---|---|---|---|---|
| Cigar use former N=476 |
0.77 0.53,1.12 0.174 |
0.82 0.61,1.11 0.195 |
0.71 0.45,1.13 0.146 |
0.82 0.58,1.14 0.233 |
| Cigar use current N=123 |
1.42 0.79,2.56 0.241 |
1.05 0.61,1.80 0.858 |
1.09 0.51,2.34 0.831 |
0.71 0.35,1.45 0.350 |
| Pipe use former N=497 |
0.93 0.65,1.32 0.670 |
0.90 0.68,1.20 0.466 |
0.86 0.56,1.32 0.485 |
0.84 0.60,1.16 0.283 |
| Pipe use current N=37 |
1.42 0.52,3.84 0.495 |
1.25 0.55,2.82 0.595 |
1.57 0.49,4.98 0.445 |
0.81 0.26,2.55 0.722 |
| Chewing tobacco use former N=70 |
1.54 0.79,3.03 0.208 |
1.51 0.86,2.64 0.149 |
1.40 0.61,3.20 0.427 |
1.15 0.56,2.33 0.709 |
| Chewing tobacco use current N=18 |
0.63 0.09,4.51 0.645 |
0.37 0.05,2.66 0.325 |
Cannot be estimated |
Cannot be estimated |
| Secondhand smoke exposed N=1166 |
0.78 0.57,1.06 0.115 |
0.80 0.61,1.05 0.104 |
0.67 0.44,1.00 0.052 |
0.77 0.56,1.07 0.120 |
| Secondhand smoke Intensity (hours per week) |
0.99 0.97,1.00 0.111 |
1.00 0.98,1.01 0.425 |
0.98 0.96,1.01 0.137 |
0.99 0.98,1.01 0.342 |
Figure 1.
Rate of CVDA per 1000 person-years by smoking status and years since quitting.
3.5 Additional Endpoints
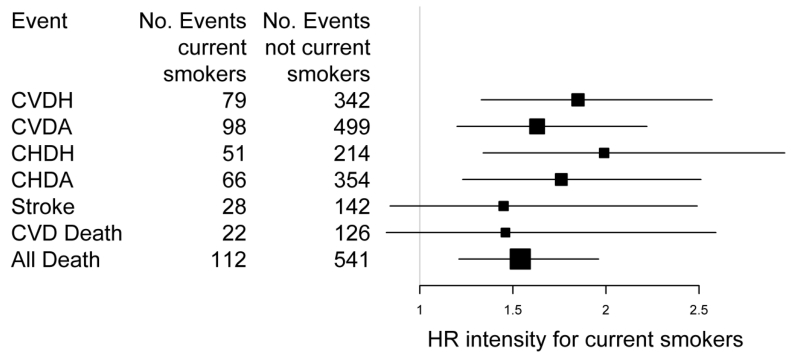
In the pooled models which adjusted for smoking status, duration, intensity and status/intensity interactions, former smoking and duration were not significantly associated with the additional four endpoints of stroke, CVD death, all death, and DVT-PE. Current smoking status was significantly associated with stroke, CVD death, and all death but not DVT-PE, though the HR was in the same direction (DVT-PE HR 1.37, p=0.4). Intensity for current smokers was significantly associated with all death but not stroke, CVD death, and DVT-PE though the HRs were in the same direction (Figure 2).
Figure 2.
Forest plot of the HRs and 95% confidence intervals of intensity for current smokers across endpoints from the pooled models including smoking status, intensity, duration, and the interaction of intensity and smoking status.
3.6 Compound Smoking Indices
The δs (lag-time) and τs (half-life) associated with the minimum AIC were the minimum tested δ (0) and τ (1) for most of the outcomes (Supplemental Table 1). The best τs for CVDA, CHDA, and DVT-PE were larger and the best δs for death and DVT-PE were larger.
3.7 Comparing Models of Smoking as an Exposure
The two compound indices had similar performance in terms of AIC, and were never more than 2 units apart (Table 5). For the death outcome, the best fitting models by more than 10 units were the compound index models. For DVT-PE the most complex model, model 2, fit best. For all other outcomes, the current intensity only models and the compound index models fit best and their AICs were within 3 units of each other.
Table 5.
Comparing models of smoking in terms of AIC. All models were also adjusted for age, sex, race, income, and education, and only subjects with complete smoking status, intensity, pack-years, and time since quitting information were included. Lower values are better, and values are only comparable within a column.
| Model # |
AIC | CVDH N=6435 |
CVDA N=6436 |
CHDH N=6435 |
CHDA N=6435 |
Stroke N=6433 |
CVD Death N=6457 |
Death N=6457 |
DVT-PE N=6433 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Never/Former/ Current |
7018 | 9973 | 4412 | 7040 | 2865 | 2390 | 10684 | 1574 |
| 2 | Ever, Pack-years, Years since quitting, Packyears*Years since quitting |
7035 | 9980 | 4418 | 7037 | 2874 | 2397 | 10683 | 1565 |
| 3 | Never/Former/ Current, Intensity, Intensity*Former |
7010 | 9968 | 4405 | 7035 | 2867 | 2392 | 10672 | 1569 |
| 4 | Never/Former/ Current, Intensity |
7014 | 9972 | 4408 | 7039 | 2866 | 2390 | 10672 | 1567 |
| 5 | Never/Former/ Current, Pack-years |
7016 | 9971 | 4408 | 7036 | 2866 | 2390 | 10666 | 1566 |
| 6 | Never/Former/ Current, Intensity, Duration |
7015 | 9974 | 4410 | 7038 | 2866 | 2392 | 10671 | 1569 |
| 7 | Never/Former/ Current, Current intensity |
7009 | 9966 | 4404 | 7034 | 2865 | 2390 | 10676 | 1575 |
| 8 | Current intensity | 7009 | 9964 | 4402 | 7031 | 2863 | 2388 | 10683 | 1571 |
| 9 | Current pack-years | 7016 | 9970 | 4407 | 7034 | 2865 | 2389 | 10684 | 1570 |
| 10 | CSI-X1 | 7009 | 9963 | 4404 | 7032 | 2862 | 2388 | 10659 | 1567 |
| 11 | CSI-X2 | 7007 | 9963 | 4404 | 7031 | 2860 | 2388 | 10659 | 1568 |
4. Discussion
Evaluating the different ways to measure the association between baseline assessment of smoking and incident cardiovascular disease indicates that both current smoking and smoking intensity tended to result in the strongest associations. Once model fit was considered, baseline current smoking intensity ended up being a better measure of cardiovascular risk than baseline current smoking status. Evaluation of smoking indices supported this finding, by showing that the best parameterization of a compound smoking index involved no lag time and a half-life of smoking risk for cardiovascular outcomes of one year – making the current level of smoking intensity seem to be the most pertinent measure as opposed to past exposure. In summary, these findings point towards the possibility that baseline current smoking intensity could be a better summary measure of smoking-related cardiovascular risk, relative to other simple measures, such as current smoking status or total pack-years of smoking.
4.1 Never/Former/Current
Baseline smoking status is an important factor for CVD risk in this study as well as in previous studies.[4] Current smokers are at a significantly higher risk for CVDA, CHDA, CVDH, and CHDH events compared to never smokers, but former smokers are not. This pattern was also seen in Huxley et al.[2] These results imply that the main group that experiences increased CVD risk from smoking is the current smokers. One obvious implication of this is that using “ever smoker” is not a good strategy. This would group the former smokers with the dissimilar current smokers.
4.2 Intensity/Duration/Pack-Years
Average intensity of smoking at baseline is significantly associated with CVD outcomes in current smokers but not in former smokers. The Nurses’ Health Study (NHS) also found that the adjusted HR of CHD death increased with increasing intensity.[6] However, there is the possibility of recall bias since current smokers may more accurately report their intensity than smokers who quit years ago. Current smoking intensity is not significant for stroke, CVD death, or DVT-PE, which may be due to decreased power since there are fewer events for these endpoints. Alternatively there may be differences in biological pathways. Higher smoking intensity increases the risk of death for both former and current smokers.
Duration of smoking is not associated with CVD outcomes in either current or former smokers. Similarly in the Cancer Protection Study II, though rates of CHD death were higher with higher duration in many groups, this pattern was not consistent in all strata.[11] Taken together with the intensity and status findings, this suggests that former smokers in MESA, regardless of how long or how much they smoked, may no longer be at increased CVD risk.
Pack-years of smoking follows the same pattern as intensity. Mannan et al also showed that pack-years of smoking was associated with CVD risk.[26] Since duration is not associated with outcomes, the pack-years association is primarily due to the intensity component. In fact, compared to models with intensity alone, using pack-years or duration and intensity does not improve the AIC. These results suggest that duration does not add information and that only current smoking intensity is significant for CVD risk.
4.3 Age at Starting Smoking
There was no effect of age at starting smoking in any smoking status group on risk of any CVD outcome. Though there was a significant trend of higher risk of CVD the younger the age at starting smoking in Huxley et al, the NHS did not find such a trend.[2, 6] This result implies that smoking at an earlier age does not pose an independent risk for CVD later in life compared to those who start later.[6]
4.4 Time Since Quitting Smoking
Whether it was modeled as a continuous or categorical variable, time since quitting smoking was not associated with CVD. Though this agrees with Huxley et al, this contrasts with the NHS, which reached the intuitive conclusion that risk of CVD is higher for people who quit more recently.[2, 6] This could be because the NHS only included women.[6] Additionally, the impact of smoking may vary significantly within the first year of quitting and this cannot be explored in MESA. Finally, only 241 former smokers with less than 5 years of time since quitting are in this study, and there are only 9 CHDH events among them. Though the point estimates of the hazard ratios for this group are greater than one, the risk is much less than the risk of current smokers.
4.5 Other Forms of Tobacco Exposure
Secondhand smoke, pipe, cigar, and chewing tobacco exposure were not significantly associated with CVD risk. This differs from previous studies which found such associations, but these results did not include cigarettes smokers.[12-15] Additionally, there were also very few current cigar (123), pipe (37), and chewing tobacco (18) users, so there was limited power to detect these effects and the models may possibly be overfit. Finally, one weakness of the study is that all of the smoking information was self-reported and secondhand smoking may be particularly hard to estimate accurately.
4.6 Compound Smoking Indices
In the compound index analysis, the best fitting δ parameter for all endpoints except for death and DVT-PE was zero. This suggests there is no lag time between the exposure to smoking and the outcome, implying that smoking is immediately increasing the risk for these outcomes. For death, the lag time is a bit longer at two years, which might be due to cancer or other longer-term disease processes.
For CVDH, CHDH, stroke, CVD death, and death the best fitting τ was one, the minimum tested value. As τ is the “half-life” parameter, this implies that the risk of these endpoints drops very quickly after the exposure to smoking.[9, 19] This agrees with the previously stated conclusion that only current smoking intensity is important, not duration of exposure. The best fitting τ is larger for the endpoints which include angina (CHDA and CVDA). For these endpoints, the risk decreases more slowly, or lingers longer, after the last exposure to smoking. It may be valuable in future studies to fit the compound indices for angina endpoints separately. DVT-PE has a long lag time and half-life compared to the other endpoints, and this may be due to the difference in etiology. This difference in etiology was the reason for including DVT-PE, and so the different results were expected.
4.7 Comparing Models of Smoking as an Exposure
It is important to know the best way to model smoking, both to understand what aspects of smoking impact CVD risk, as well as to inform proper adjustment for smoking as a confounder in studies that use CVD as an outcome. For CVDH, CVDA, CHDH, CHDA, stroke, and CVD death the best model fit came from adjusting for only current smoking intensity or a compound index and the model fit for these were too similar to definitively prefer one over the other.[9, 27] This again supports the idea that current smoking intensity alone is the most important element of CVD risk. In addition, the model involving only smoking status (never/former/current) often had much worse model fit; indicating that using smoking status alone is a less effective way to adjust for smoking. Since it requires no extra calculation, it may be best to use current smoking intensity alone as the measurement of smoking for these outcomes.
For death, the compound smoking indices had by far the best model fit, suggesting that these measures better capture the importance of former and current smoking intensity, and the lag time and half-life parameters for the many causes of death, including CVD and cancer. For DVT-PE, the compound index models fit very well, but the best fitting model was the rather complex model 2. Again, this suggests that DVT-PE involves different biological pathways than the other CVD outcomes since factors such as time since quitting that are not significant for other CVD outcomes improve model fit. For death and DVT-PE, it is probably best to fit the compound index and use this to adjust for smoking.
4.8 Limitations
MESA participants are aged 45-84 and relatively healthy at study entry, therefore these results may not generalize to a younger population or to those with pre-existing heart disease symptoms. MESA is an observational study, and though adjustment factors were carefully considered there is the possibility of residual confounding in the Cox models. All smoking behaviors were self-reported and subject to recall errors and social desirability biases. Additionally, the examined smoking behaviors were from the baseline exam only. Finally, this study had limited power to assess the association of CVD with rarer smoking exposures in MESA, such as pipe use or very recent cigarette use cessation.
4.9 Conclusions
In MESA, the best way to model smoking for CVD outcomes is either baseline current smoking intensity, CSI-X1, or CSI-X2.[9] The compound smoking indices seem to be more appropriate for all-cause mortality. Using smoking status alone (never/former/current) alone is insufficient to fully adjust for the effect of smoking on CVD. Duration was not associated with CVD, while average baseline current intensity was, and so pack-years appears to be a suboptimal way to model smoking as an exposure for CVD endpoints.
Supplementary Material
Key Findings.
This paper demonstrates that smoking intensity is the primary risk factor for associations between cardiovascular disease events and tobacco cigarette use and provides the best fit for statistical models.
Duration of smoking was not associated with increased risk among former smokers, who showed a rapid (within a few years at most) return to baseline cardiovascular disease risk.
Deep vein thrombosis and death follow different patterns, and the association between smoking and events for these outcomes remains elevated much longer than for cardiovascular disease events.
What this adds to what was known
Adjusting for smoking intensity is preferable to adjusting for pack-years of smoking or smoking status (never/former/current) in models with cardiovascular disease outcomes.
Time since quitting and duration of smoking, to the precision we could measure these parameters, were not associated with increased risk of cardiovascular disease for former smokers.
What is the implication, what should change now
The optimal adjustment for the association of smoking in cardiovascular disease studies, smoking intensity, should be used instead of the more commonly used pack-years.
Smoking cessation programs aimed at cardiovascular disease prevention may be encouraged by a relatively quick drop in this particular association.
Acknowledgements
This research was supported by R01 HL 103729-01A1 and contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
Contributor Information
Robin Nance, University of Washington, Collaborative Health Studies Coordinating Center, Building 29, Suite 210, 6200 NE 74th Street, Box 354922, Seattle, WA USA 98115.
Joseph Delaney, University of Washington, Collaborative Health Studies Coordinating Center, Box 354922, Building 29, Suite 310, 6200 NE 74th Street, Seattle, WA USA 98115-8160, jacd@u.washington.edu, 1-206-897-1918.
John W McEvoy, Johns Hopkins, 600 N. Wolfe Street, Baltimore, MD USA 21287, USA, jmcevoy1@jhmi.edu 1-410-955-7376.
Michael J Blaha, Johns Hopkins Hospital, Johns Hopkins Hospital 600 N. Wolfe Street Blalock 524C, Baltimore, MD USA 21287, mblaha1@jhmi.edu 1-410-955-7911.
Gregory Burke, Wake Forest University Health Sciences, Department of Public Health Sciences, Medical Center Blvd. Winston-Salem, NC USA 27157-1063, gburke@wakehealth.edu 1-336-716-2930.
Ana Navas-Acien, Johns Hopkins Department of Medicine and Johns Hopkins Bloomberg School of Public Health, 2024 E. Monument Street, Suite 2-607, W7033B, Baltimore, MD USA 21205-2217, anavasa1@jhu.edu 1-410-502-4267.
Joel D Kaufman, University of Washington, Environmental & Occupational Health Sciences, Medicine, and Epidemiology, 4225 Roosevelt Way NE, Suite 100, Seattle, WA USA 98105, joelk@u.washington.edu, 1-206-616-3501.
Elizabeth C Oelsner, Columbia University, 630 West 168th Street, Presbyterian Hospital, 9-105E, Division of General Medicine, New York, NY USA 10032, Eco7@cumc.columbia.edu, 1-917-880-7099.
Robyn L McClelland, University of Washington, Collaborative Health Studies Coordinating Center, Box 354922, Building 29, Suite 310, 6200 NE 74th Street, Seattle, WA USA 98115-8160, rmcclell@u.washington.edu, 1-206-897-1956.
References
- [1].Organization WH . Global status report on noncommunicable diseases 2014. 2014. [Google Scholar]
- [2].Huxley RR, Yatsuya H, Lutsey PL, Woodward M, Alonso A, Folsom AR. Impact of age at smoking initiation, dosage, and time since quitting on cardiovascular disease in african americans and whites: the atherosclerosis risk in communities study. Am J Epidemiol. 2012;175:816–26. doi: 10.1093/aje/kwr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ehteshami-Afshar S, Momenan A, Hajshekholeslami F, Azizi F, Hadaegh F. The impact of smoking status on 9.3 years incidence of cardiovascular and all-cause mortality among Iranian men. Ann Hum Biol. 2014;41:249–54. doi: 10.3109/03014460.2013.853834. [DOI] [PubMed] [Google Scholar]
- [4].McEvoy JW, Blaha MJ, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, et al. Cigarette smoking and cardiovascular events: role of inflammation and subclinical atherosclerosis from the MultiEthnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:700–9. doi: 10.1161/ATVBAHA.114.304562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299:2037–47. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pope CA, 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–8. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- [8].Leffondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156:813–23. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- [9].Hudson M, Lo E, Baron M, Steele R, Canadian Scleroderma Research G Modeling smoking in systemic sclerosis: a comparison of different statistical approaches. Arthritis Care Res (Hoboken) 2011;63:570–8. doi: 10.1002/acr.20416. [DOI] [PubMed] [Google Scholar]
- [10].Peto J. That the effects of smoking should be measured in pack-years: misconceptions 4. Br J Cancer. 2012;107:406–7. doi: 10.1038/bjc.2012.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thun M, Myers DG, Day-lally C, Namboodiri MM, Calle EE, Flanders WD, et al. Age and the exposure-response relationships between cigarette smoking and premature death in Cancer Prevention Study II. In: Smoking and Tobacco Control Program NCI, editor. Monograph 8: Changes in Cigarette-Related Disease Risks and Their Implementation for Prevention and Control. 1997. pp. 383–415. [Google Scholar]
- [12].Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–98. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- [13].Henley SJ, Thun MJ, Chao A, Calle EE. Association between exclusive pipe smoking and mortality from cancer and other diseases. J Natl Cancer Inst. 2004;96:853–61. doi: 10.1093/jnci/djh144. [DOI] [PubMed] [Google Scholar]
- [14].Jacobs EJ, Thun MJ, Apicella LF. Cigar smoking and death from coronary heart disease in a prospective study of US men. Arch Intern Med. 1999;159:2413–8. doi: 10.1001/archinte.159.20.2413. [DOI] [PubMed] [Google Scholar]
- [15].Whincup PH, Gilg JA, Emberson JR, Jarvis MJ, Feyerabend C, Bryant A, et al. Passive smoking and risk of coronary heart disease and stroke: prospective study with cotinine measurement. BMJ. 2004;329:200–5. doi: 10.1136/bmj.38146.427188.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].The Real Rewards of Quitting. Institute TCRBotNC; [Google Scholar]
- [17].McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, et al. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–10. doi: 10.1161/ATVBAHA.114.304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Indrayan A, Kumar R, Dwivedi S. Collection of Biostatistics Research Archive; COBRA Preprint Series. Berkeley Electronic Press; 2008. A Simple Index of Smoking. [Google Scholar]
- [19].Leffondre K, Abrahamowicz M, Xiao Y, Siemiatycki J. Modelling smoking history using a comprehensive smoking index: application to lung cancer. Stat Med. 2006;25:4132–46. doi: 10.1002/sim.2680. [DOI] [PubMed] [Google Scholar]
- [20].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [21].Center MC. Multi-Ethnic Study of Atherosclerosis (MESA) Protocol. Collaborative Health Studies Coordinating Center, University of Washington; Unpublished results. [Google Scholar]
- [22].MESA Personal History. Collaborative Health Studies Coordinating Center, University of Washington; Atherosclerosis TM-ESo. Unpublished results. [Google Scholar]
- [23].MESA Events Data Set Variable Guide. Collaborative Health Studies Coordinating Center, University of Washington; Atherosclerosis M-ESo. Unpublished results. [Google Scholar]
- [24].Hirsh J, Hoak J, American Heart Association Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consultation with the Council on Cardiovascular Radiology) Circulation. 1996;93:2212–45. doi: 10.1161/01.cir.93.12.2212. [DOI] [PubMed] [Google Scholar]
- [25].Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- [26].Mannan H, Stevenson C, Peeters A, Walls H, McNeil J. Framingham risk prediction equations for incidence of cardiovascular disease using detailed measures for smoking. Heart Int. 2010;5:e11. doi: 10.4081/hi.2010.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. Springer; New York: 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.