Abstract
Purpose
Congenital muscular dystrophy (CMD) comprises a rare group of genetic muscle diseases that present at birth or early during infancy. Two common subtypes of CMD are collagen VI-related muscular dystrophy (COL6-RD) and laminin alpha 2-related dystrophy (LAMA2-RD). Traditional outcome measures in CMD include gross motor and mobility assessments, yet significant motor declines underscore the need for valid upper extremity (UE) motor assessments as a clinical endpoint. This study validated a battery of UE measures in these two CMD subtypes for future clinical trials.
Methods
For this cross-sectional study, 42 participants were assessed over the same 2–5 day period at the National Institutes of Health Clinical Center (CC). All UE measures were correlated with the Motor Function Measure 32 (MFM32). The battery of UE assessments included the Jebsen Taylor Hand Function Test, Quality of Upper Extremity Skills Test (QUEST), hand held dynamometry, goniometry, and MyoSet Tools. Spearman Rho was used for correlations to the MFM32. Pearson was performed to correlate the Jebsen, QUEST, hand-held dynamometry, goniometry and the MyoSet Tools. Correlations were considered significant at the 0.01 level (2-tailed).
Results
Significant correlations were found between both the MFM32 and MFM Dimension 3 only (Distal Motor function) and the Jebsen, QUEST, MyoGrip and MyoPinch, elbow flexion/extension ROM and myometry. Additional correlations between the assessments are reported.
Conclusions
The Jebsen, the Grasp and Dissociated Movements domains of the QUEST, the MyoGrip and the MyoPinch tools, as well as elbow ROM and myometry were determined to be valid and feasible in this population, provided variation in test items, and assessed a range of difficulty in CMD. To move forward, it will be of utmost importance to determine whether these UE measures are reproducible and sensitive to change over time.
Keywords: Congenital muscular dystrophy, Collagen VI-related muscular dystrophy, Laminin alpha 2-related dystrophy, Upper extremity measures, QUEST, Jebsen, Motor Function Measure
1. Introduction
Congenital muscular dystrophy (CMD) is a general term for a rare group of genetic muscle diseases that present at birth or early during infancy. Although there are many genetic subtypes within CMD, collagen VI-related muscular dystrophy and laminin alpha 2-related dystrophy are two of the most common subtypes. Both collagen VI and laminin alpha 2 are associated with the muscle extracellular matrix. Collagen VI is a microfibrillar collagen encoded by three genes (COL6A1-3) that is found in many extracellular matrices but is strongly associated with the extracellular matrix of skeletal muscle. The collagen VI-related muscular dystrophy (COL6-RD) phenotypic spectrum includes Bethlem myopathy, the mildest form, intermediate collagen IV-related CMD, and Ullrich CMD, clinically the most severe form. Laminin alpha 2-related dystrophy (LAMA2-RD) is caused by recessive mutations in LAMA2, the heavy chain of laminin 211, also known as merosin. Laminin 211 is a major laminin of muscle and nerve basement membranes. Even though they are typically congenital disorders, both subtypes may present at birth or in adulthood with a range of phenotypic and functional severity. Individuals with COL6-RD present with joint laxity, hypotonia, and develop contractures early in childhood. Individuals with LAMA2-RD present with weakness, atrophy of skeletal muscles, and joint deformities. Integral features of both pediatric onset subtypes are hypotonia at birth, early respiratory involvement, feeding problems, diffuse joint contractures, and notably progressive muscle weakness leading to functional and respiratory decline. Muscle weakness is often severe, and the majority of affected children experience delays in their motor milestones. Lower extremity functional limitations include difficulty in sitting, standing unassisted, or walking, and most children lose motor function as the disease progresses. Upper extremity functional limitations can result from both orthopedic deformity and underlying muscle weakness. Limitations in reaching and grasping develop rapidly during early childhood and limit independence in activities of daily living, play, and school-based participation [1–5].
Current research in CMD is focused on understanding the molecular processes that lead to muscle loss in these disorders and investigating therapeutic approaches to counteract or arrest these processes. In order to determine the effectiveness of new therapeutic strategies in clinical trials, measures of skeletal muscle structure and function that can quantify disease progression and are validated for patients with CMD are needed [6]. Traditional measures of strength and function in various neuromuscular disorders, including CMD, have incorporated muscle strength testing (myometry), motor performance (6-minute walk test, timed 10-m walk/run and ascend/descend stairs), range of motion (goniometry), forced vital capacity, and anthropometric measures [7,8]. Few studies have utilized upper extremity motor scales or measured upper limb function. What we do know is that upper extremity function in COL6 and LAMA2 CMD is determined not only by weakness, but also by prominent and progressive joint contractures and, in the case of COL6-RD, prominent distal laxity. Progressive lower extremity gross motor and mobility limitations eventually prevent the use of traditional measures and underscore the need for valid upper extremity motor assessments as a clinical endpoint.
Existing data on upper extremity outcome measures and the natural history of upper extremity muscle progression in CMD are lacking. In order to plan and perform clinical trials in these patient populations, there is a need to develop a consensus on diagnostic criteria, biomarkers, medical management and clinical endpoints. To address part of this need, the main goal of this study was to validate the use of a battery of upper extremity measures in two CMD subtypes for use as clinical endpoints in future clinical trials.
2. Methods
This cross-sectional study is a component of a larger longitudinal study focusing on the natural history and outcome measure validation in two subtypes of CMD, as well as assessing the feasibility, reliability, and validity of a battery of muscle strength, motor function, and patient reported outcome measures for this patient population [6]. The study received approval from the National Institute of Neurological Disorders and Stroke (NINDS) Institutional Review Board (IRB). Participants were recruited through IRB-approved advertisements in regional, national, and international neurology, neuromuscular, and genetics centers, and through the Congenital Muscle Disease International Registry. Diagnosis was confirmed prior to study enrollment. Written consent was obtained from at least one parent or guardian and both parents when possible. Written assent was obtained from minor participants whenever possible, and verbal assent was obtained from children who were able to understand and agree. Dissent from all minors was respected. Data were collected from all participants over the same 2–5 day period at the National Institutes of Health Clinical Center. The focus of this study was for UE instrument validation with the CMD population and did not provide any treatment.
Cross-sectional data to assess the validity of UE measures in CMD were obtained on a cohort of 42 participants. Although validation studies typically require between 50 and 100 participants [9], our cohort was limited due to the rarity of CMD. The cohort used for this study was selected from 35 participants who received the administration of the Jebsen Taylor Hand Function Test (Jebsen) in 2013. Subsequent to that administration, 7 additional participants were added in 2014. Outcomes from all additional assessments analyzed in this UE validation study were taken in the same year as the Jebsen. No participant is presented more than once in our data set. All upper extremity measures used in this study were correlated with the Motor Function Measure 32 (MFM32). The MFM32 evaluates head, trunk, upper and lower limb function in both ambulatory and non-ambulatory individuals, is reliable, and has been validated in individuals with CMD [6,7,10]. In addition to the Jebsen, the battery of upper extremity assessments included the Quality of Upper Extremity Skills Test (QUEST), hand held dynamometry, goniometry, and MyoSet Tools. The order of the testing was randomized due to the number of assessments completed in the larger longitudinal study, including gross motor tests, pulmonary function test, and quality of life measures [6]. In an attempt to limit fatigue, we did not schedule back to back active testing. Breaks with snacks and water were provided throughout the testing sessions.
Each of these upper extremity assessments have been used in clinical trials and longitudinal studies, validated in diverse populations, and has a developed protocol for administration. As a team, we met and reviewed each measure to ensure full understanding and agreement of administration procedures. Notebooks were provided to all clinicians who were administering these assessments. The team of assessors also met each evening to discuss issues that may have evolved during administration of assessments.
SPSS Version 22 was used for statistical analysis. Spearman Rho correlations were performed for correlations to the MFM32. Spearman Rho is a nonparametric measure of statistical dependence between two variables and is appropriate for both continuous and discrete variables, including ordinal variables. Pearson correlations were performed to correlate the Jebsen, QUEST, hand-held dynamometry, goniometry and the MyoSet Tools. We used Pearson correlations to determine the strength of the linear association between the variables. As these two CMD subtypes present with common phenotypes yet different genotypes, we present demographic and descriptive information for the total cohort and by diagnosis. Due to the multiple assessments, correlations were considered significant at the 0.01 level (2-tailed).
3. Outcome measures
The Motor Function Measure (MFM32) [7] is a 32-item test that assesses overall motor function and has been validated in individuals with COL6 and LAMA2-RD [6]. It includes three dimensions: (1) Standing and Transfers (13 items), (2) Proximal and Axial Motor Function (12 items) and (3) Distal Motor Function (7 items). For the purpose of this study, the total MFM32 scores and the Distal Motor Function scores (Dimension-3) were used for the correlations.
The Jebsen Taylor Hand Function Test [11] is a performance measure which assesses unilateral hand function. The Jebsen is easy to administer and score, and includes tasks that are functional and reflect real-life skills, such as writing, feeding, and picking up objects of various weights and sizes. The Jebsen tests both dominant (D) and non-dominant (ND) hands separately and does not include bimanual tasks. The Jebsen uses time as the only measurement variable. For our study, if a participant took over 120 seconds or did not attempt the task due to perceived inability, the subject was given a score of 120 seconds. For each hand, the times were summated, resulting in a non-dominant (ND) Jebsen sum and a dominant (D) Jebsen sum.
Quality of Upper Extremity Skills Test (QUEST) [12] is an outcome measure designed to evaluate movement patterns and hand function in children with cerebral palsy (CP). The QUEST is a 34 item, criterion-referenced observation test within four domains: Dissociated Movement, Grasp, Protective Extension, and Weight Bearing. The QUEST has demonstrated strong correlations with the Gross Motor Performance Measure [13], the Peabody Developmental Fine Motor Scales [14], as well as the Melbourne Assessment of Unilateral Upper Limb Function [15]. The QUEST has been determined to be both reliable and valid in CP. The QUEST was chosen because the upper extremity contractures observed in children with CMD often resemble similar contractures in children with CP, although important differences should be noted. In CP the contractures are a combination of dynamic and myostatic, which contrasts with CMD and other muscle diseases where the contractures are myostatic. Individuals with CP may also have coexisting motor control impairments. These differences between CP and CMD do not preclude the potential for using the QUEST in our CMD population. The four domain scores of the QUEST were used in this study in lieu of the total QUEST score [16].
Hand-Held Dynamometry (HHD) is a method of measuring maximal force exerted against a hand-held device (Microfet, Hoggan Health, Inc.). To measure strength, the child is prompted to exert maximal effort while the examiner keeps the device stationary. Muscle groups tested included the elbow flexors and extensors with standardized positioning and instructions provided. Three trials were performed bilaterally and additional trials were performed if the trials were inconsistent. To reflect best performance, the maximum values were used for analyses. These maximum values were normalized for age, weight and gender.
Goniometry was used to assess range of motion (ROM) of elbow extension bilaterally using a standard goniometer while the patient was in supine. Full extension was measured as 0 degrees. If a patient had less than full extension (due to contractures) the measurement was subtracted from the 0 degree position, resulting in negative values.
MyoSet Tools [17] were developed by the Institut de Myologie to assess upper extremity strength and function in non-ambulatory patients; specifically patients with severe contractures. The MyoSet includes the MyoGrip, MyoPinch and the MoviPlate. The MyoGrip dynamometer is an electronic device specifically developed for measuring isometric grip strength in weak patients with measures up to 0.01 kg. The MyoPinch dynamometer measures key pinch using a high precision load cell, with measures up to 0.001 kg. The MoviPlate measures the ability to produce repeated movements of the fingers and wrist for 30 seconds between two cylindrical target keys aligned in the sagittal plane. The device is made of an adaptable platform on which the subject places his forearm. The subject alternately flexes and extends the wrist and fingers to press two targets as many times as possible during the 30-second time period. For the MyoGrip and MyoPinch, three trials were attempted for each hand; for the MoviPlate, two trials were attempted. To reflect best performance, the maximum values were used for analyses. These tools have demonstrated sensitivity and reliability in a cohort of non-ambulant patients with Duchenne muscular dystrophy [17].
Pediatric occupational and physical therapists collected all upper extremity (UE) data. Inter-rater reliability among the therapists was assessed in the first year of our data collection. For inter-rater reliability (IRR), two raters administered the upper extremity assessments on three different participants. Statisticians were independent of the individual who organized the IRR testing and were blinded to the raters. Interclass correlation coefficients (ICCs) were determined between raters for the MFM32 (ICC −.92), Jebsen dominant (ICC −.76) and non-dominant (ICC −.89), QUEST (ICC −.99), myometry (ICC −.89), goniometry (ICC −.91), and the Myotools (ICC −.99).
4. Results
Forty-two individuals with CMD participated in the UE validation study. Of these participants, 22 were diagnosed with COL6-RD and 20 with LAMA2-RD, with an equal distribution of males and females. Participants ranged in age from 5 to 19 years, and significantly more COL6-RD participants were ambulatory. Study participant demographics (Table 1) and descriptive statistics for UE outcome measures performed (Table 2) are provided by total cohort and by diagnostic type. Analyses results are provided for the total cohort (N = 42).
Table 1.
Study participant demographics (N = 42).
| Total | COL6-RD | LAMA2-RD | |
|---|---|---|---|
| Participants | 42 (100%) | 22 (52%) | 20 (48%) |
| Age range-years (SD) | 5–19 (5.7) | 7–19 (6.9) | 5–15 (2.7) |
| Females | 20 (48%) | 9 (45%) | 11 (55%) |
| Ambulatory | 14 (33%) | 11 (79%) | 3 (21%) |
Table 2.
Descriptive statistics for all upper extremity outcome measures: by total and by diagnosis type.
| Test | Measure | N | Mean | SD | SE | |
|---|---|---|---|---|---|---|
| MFM32 | DISTAL-D3 | TOTAL | 42 | 15.4 | 4.6 | .7 |
| COL6 | 22 | 17.50 | 3.57 | .76 | ||
| LAMA2 | 20 | 13.1 | 4.51 | 1.01 | ||
| MFMTOTAL | TOTAL | 42 | 46.6 | 22.9 | 3.5 | |
| COL6 | 22 | 55.27 | 23.18 | 4.94 | ||
| LAMA2 | 20 | 37.15 | 19.01 | 4.25 | ||
| Jebsen | Non-dominant (ND) | TOTAL | 42 | 228.2 | 209.4 | 32.3 |
| COL6 | 22 | 161.83 | 201.54 | 42.97 | ||
| LAMA2 | 20 | 301.25 | 197.42 | 44.14 | ||
| Dominant (D) | TOTAL | 42 | 191.6 | 209.6 | 32.3 | |
| COL6 | 22 | 128.34 | 200.30 | 42.70 | ||
| LAMA2 | 20 | 261.10 | 201.76 | 45.11 | ||
| Quest | Dissociated movements | TOTAL | 42 | 86.66 | 14.01 | 2.16 |
| COL6 | 22 | 92.00 | 11.17 | 2.38 | ||
| LAMA2 | 20 | 80.78 | 14.72 | 3.29 | ||
| Grasps | TOTAL | 42 | 88.8 | 9.82 | 1.52 | |
| COL6 | 22 | 91.25 | 7.98 | 1.70 | ||
| LAMA2 | 20 | 86.10 | 11.10 | 2.48 | ||
| Weightbearing | TOTAL | 38 | 71.09 | 16.78 | 2.72 | |
| COL6 | 19 | 65.04 | 30.58 | 6.52 | ||
| LAMA2 | 19 | 63.54 | 21.83 | 4.88 | ||
| Protective extension | TOTAL | 37 | 63.06 | 15.17 | 2.49 | |
| COL6 | 20 | 57.51 | 22.92 | 4.89 | ||
| LAMA2 | 17 | 53.40 | 27.72 | 6.20 | ||
| Myometry | Elbow flex ND | TOTAL | 42 | 20.59 | 12.30 | 1.90 |
| COL6 | 22 | 29.33 | 14.54 | 3.10 | ||
| LAMA2 | 20 | 16.40 | 14.94 | 3.34 | ||
| Elbowflex D | TOTAL | 42 | 19.77 | 12.48 | 1.93 | |
| COL6 | 22 | 30.59 | 16.10 | 3.43 | ||
| LAMA2 | 20 | 15.52 | 13.87 | 3.10 | ||
| Elbowext ND | TOTAL | 42 | 17.09 | 15.25 | 2.36 | |
| COL6 | 22 | 18.85 | 10.14 | 2.16 | ||
| LAMA2 | 20 | 9.68 | 15.66 | 3.50 | ||
| Elbowext D | TOTAL | 42 | 17.60 | 17.03 | 2.63 | |
| COL6 | 22 | 18.73 | 11.18 | 2.38 | ||
| LAMA2 | 20 | 10.00 | 13.99 | 3.13 | ||
| Goniometry | Range of motion | |||||
| Elbowext ND | TOTAL | 42 | −44.84 | 34.23 | 5.63 | |
| COL6 | 22 | −38.68 | 37.09 | 7.90 | ||
| LAMA2 | 20 | −54.00 | 29.72 | 6.65 | ||
| Elbowext D | TOTAL | 42 | −47.14 | 35.70 | 5.87 | |
| COL6 | 22 | −40.27 | 38.62 | 8.23 | ||
| LAMA2 | 20 | −55.60 | 31.30 | 7.00 | ||
| Myoset tools | Myogrip | TOTAL | 42 | 4.30 | 4.57 | .70 |
| COL6 | 22 | 5.95 | 4.98 | 1.06 | ||
| LAMA2 | 20 | 2.48 | 3.31 | .74 | ||
| Myopinch | TOTAL | 40 | 1.89 | 1.83 | .29 | |
| COL6 | 21 | 2.55 | 2.06 | .44 | ||
| LAMA2 | 19 | .97 | 1.07 | .24 | ||
| Moviplate | TOTAL | 39 | 52 | 15 | .29 | |
| COL6 | 21 | 56.95 | 17.58 | 3.75 | ||
| LAMA2 | 18 | 38.56 | 17.75 | 3.96 |
MFM32 = Motor Function Measure; Distal-D3 = MFM Dimension 3; Jebsen = Jebsen Taylor Hand Function Test; QUEST = Quality of Upper Extremity Skills Test; Myometry = Hand Held Dynamometry-muscle strength; Goniometry = range of motion; Myoset Tools = upper extremity strength and function; Total = all participants; COL6 = collagen Vl-related muscular dystrophy; LAMA2 = laminin alpha 2-related dystrophy; N = number of participants; Mean of measures; SD = standard deviation; SE = standard error.
The Jebsen (dominant (D) and non-dominant (ND)) (N = 42) correlated strongly with the MFM32 total and MFM Dimension-3 – Distal Motor Function (p < 0.001). Correlations were observed with myometry elbow extension/flexion, goniometry elbow extension, MyoGrip, MyoPinch and goniometry elbow extension. The MoviPlate correlated with the Jebsen at p = 0.03 (Table 3).
Table 3.
Jebsen correlations with upper extremity outcome measures for total participants (N = 42).
| Jebsen non-dominant | Jebsen dominant | |
|---|---|---|
| MFM32 total | −.723** | −.723** |
| MFM distal-D3 | −.814** | −.805** |
| Quest | ||
| Dissociated movements | −.740** | −.693** |
| Grasps | −.586** | −.525** |
| Weight bearing | −.475* | −.427* |
| Protective extension | −.381 | −.326 |
| Myometry elbow extension | −.453** | −.419** |
| Myometry elbow flexion | −.558** | −.581** |
| Goniometry elbow extension | −.535** | −.421** |
| MyoGrip | −.503** | −.431** |
| MyoPinch | −.465** | −.498** |
| MoviPlate | −.356 | −.349 |
MFM32 = Motor Function Measure; MFM Distal-D-3 = Dimension 3; QUEST = Quality of Upper Extremity Skills Test.
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.001 level (2-tailed).
Of our 42 participants, all were able to complete the QUEST Dissociated Movements and Grasp domains, 4 participants were unable to complete the Weight Bearing domain (1 COL6 and 3 LAMA2), and 5 participants were unable to complete the Protective Extension domain (all LAMA2) (Table 4). QUEST Dissociated Movements domain correlated strongly with total MFM32, MFM Dimension-3, Jebsen (D and ND), myometry elbow extension/flexion, Goniometry elbow extension, MyoGrip Max, and MyoPinch Max, but did not correlate with the MoviPlate Max (p = 0.044). QUEST Grasp domain correlated strongly with total MFM32, MFM Dimension-3, Jebsen (D and ND), myometry elbow flexion (D and ND), Goniometry elbow extension (D and ND). Moderate correlations were found with the myometry elbow extension (D) and flexion (ND), and the MyoGrip and MyoPinch Max. The QUEST Weight Bearing domain correlated strongly with total MFM32, MFM Dimension-3, and myometry elbow extension (D), Goniometry Elbow extension (D and ND), and MyoGrip Max. Weight Bearing also correlated moderately with myometry elbow extension (ND) and the Jebsen (D and ND). QUEST Protective Extension correlated strongly with the MFM32 Total and D-3, and the Goniometry elbow extension (D and ND). Moderate correlations would be found with the myometry elbow extension (ND).
Table 4.
QUEST correlations with upper extremity outcome measures for total participants (N = 42).
| Dissociated movements (n = 42) | Grasp (n = 42) | Weight-bearing (n = 38) | Protective extension (n = 37) | |
|---|---|---|---|---|
| MFM 32 Total | .861** | .739** | .776** | .575** |
| MFM Distal D-3 | .848** | .702** | .651** | .534** |
| Jebsen | ||||
| Dominant | −.693** | −.525** | −.427* | −.326 |
| Non-dominant | −.740** | −.586** | −.475* | −.381 |
| Myometry elbow extension | ||||
| Dominant | .521** | .407* | .506** | .411 |
| Non-dominant | .499* | .335 | .443* | .440* |
| Myometry elbow flexion | ||||
| Dominant | .530** | .537** | .309 | .257 |
| Non-dominant | .518** | .511* | .250 | .162 |
| Goniometry elbow extension | ||||
| Dominant | .739** | .704** | .705** | .510** |
| Non-dominant | .779** | .706** | .761** | .528** |
| MyoGrip | .558** | .497* | .506** | .371 |
| MyoPinch | .520** | .447* | .364 | .143 |
| MoviPlate | .325 | .131 | .218 | .108 |
MFM32 = Motor Function Measure; MFM Distal-D-3 = Dimension 3.
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.001 level (2-tailed).
MyoSet correlations for the total cohort can be found in Table 5. Both the MyoGrip and the MyoPinch strongly correlated with the total MFM32, MFM Dimension-3, myometry elbow extension/flexion (D and ND), and Goniometry elbow extension (D and ND) and the Jebsen. The MoviPlate strongly correlated with the MFM Dimension-3 and the myometry elbow flexion (D and ND). Correlations with the MoviPlate were at the 0.05 significance level for the MFM32 total, and myometry (D and ND elbow extension).
Table 5.
MyoSet Tools Correlations (ICC) with upper extremity outcome measures for total participants (N = 42).
| Correlations | |
|---|---|
| MyoGrip Max vs MFM Total | .814** |
| MyoGrip Max vs MFM Distal D-3 | .863** |
| ND MyoGrip vs ND Myometry Elbow Extension | .640** |
| D MyoGrip vs D Myometry Elbow Extension | .667** |
| ND MyoGrip vs ND Myometry Elbow Flexion | .734** |
| D MyoGrip vs D Myometry Elbow Flexion | .722** |
| ND MyoGrip vs ND Goniometry Elbow Extension | .530** |
| D MyoGrip vs D Goniometry Elbow Extension | .463** |
| MyoPinch Max vs MFM Total | .775** |
| MyoPinch Max vs MFM D3 | .847** |
| ND MyoPinch vs ND Myometry Elbow Extension | .481** |
| D MyoPinch vs D Myometry Elbow Extension | .604** |
| ND MyoPinch vs ND Myometry Elbow Flexion | .733** |
| D MyoPinch vs D Myometry Elbow Flexion | .667** |
| ND MyoPinch vs ND Goniometry Elbow Extension | .450** |
| D MyoPinch vs D Goniometry Elbow Extension | .443** |
| MoviPlate Max vs MFM Total | .389* |
| MoviPlate Max vs MFM D3 | .569** |
| ND MoviPlate vs ND Myometry Elbow Extension | .339* |
| D MoviPlate vs D Myometry Elbow Extension | .336* |
| ND MoviPlate vs ND Myometry Elbow Flexion | .500** |
| D MoviPlate vs D Myometry Elbow Flexion | .531** |
| ND MoviPlate vs ND Goniometry Elbow Extension | .099 |
| D MoviPlate vs D Goniometry Elbow Extension | .079 |
MFM = Motor Functional Assessment, MFM Distal D-3 = Dimension 3; ND = Non-dominant, D = Dominant.
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.001 level (2-tailed).
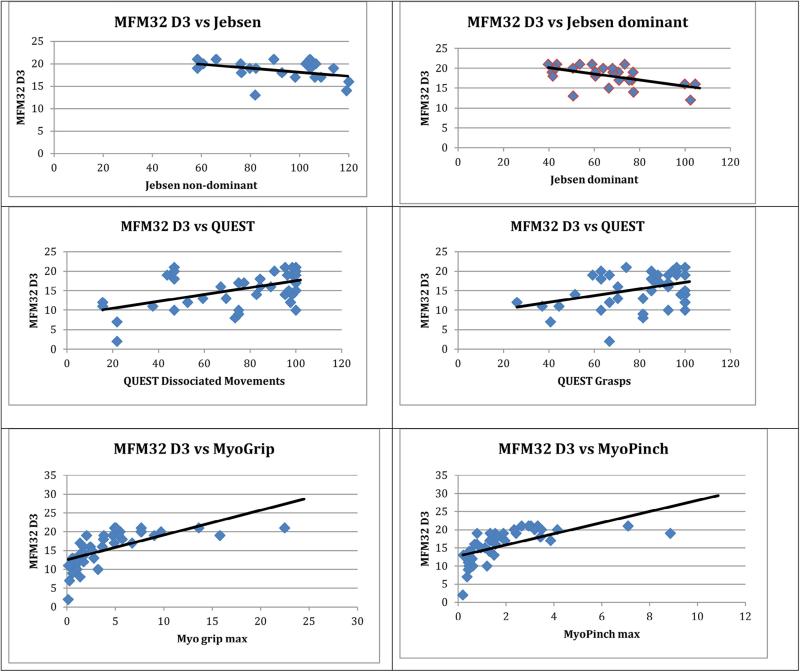
Scatterplots are presented for the MFM32 Dimension-3 and Jebsen (D and ND), QUEST Dissociated Movements and Grasps, and the MyoGrip and MyoPinch (Fig. 1). To aid in the interpretation of the correlations, trend lines were used to visually represent the relationship and general course of tendency, but do not represent the actual linear correlation.
Fig. 1.
MFM32 D3 = Motor Functional Assessment Dimension 3; Jebsen = Jebsen Taylor Hand Function Test; QUEST = Quality of Upper Extremity Skills Test. Trend lines are used as a visual representation indicating the general course or tendency.
5. Discussion
Due to the relative rarity of CMD, there is limited knowledge on the natural history and the impact of these diseases on children and their families, even though when combined they are one of the most important contributors to early childhood neuromuscular morbidity. Given that children with CMD (COL6-RD and LAMA2-RD) often progress to a non-ambulatory status or never achieve ambulation, the ability to fully evaluate the natural history of disease progression via motor deficits in the upper extremities is essential to determine response to treatment in upcoming clinical trials in CMD. The Motor Function Measure 32 (MFM32) has been validated in CMD subtypes of COL6-RD and LAMA2-RD [6]. Although the MFM32 is commonly used for testing motor function in CMD, there are limitations. Dimension-2 of the MFM32 is focused on axial and proximal motor function, such as the child's ability to hold head in midline or bring their hand to their shoulder while in supine and sitting. Dimension-3, the Distal Motor Function Dimension of the MFM32 (used in our study), consists of functional items that demonstrate important distal upper limb abilities and are applicable to individuals who are non-ambulatory. Importantly, the items are limited in their breadth and ability to provide us with a broad understanding of more proximal upper extremity motor deficits in this population. This motivated us to explore additional assessments to ensure increased understanding and evaluation of a full range of hand and upper extremity motor functions in CMD and attempt to capture a broader spectrum of disease progression.
This study used a battery of UE assessments to determine which measures were reliable and valid in this patient population. We compared the Jebsen, QUEST, Hand Held Dynamometry, Goniometry, and MyoSet Tools to the Motor Function Measure (MFM32) total score and Dimension 3 – Distal Motor Function in a cohort of 42 patients with CMD. In this cross-sectional study, all comparative assessments, with the exception of the MoviPlate, correlated with the MFM32 total score and the Dimension-3 score.
The Jebsen Taylor Hand Function Test was initially validated as an objective and standard test of hand function in cerebral palsy [11], and allows those who are non-ambulatory to participate in the assessment. The Jebsen has been used to test hand function and dexterity in boys with Duchenne muscular dystrophy, but valid and reliable results have been inconsistent [5,18,19]. As was found in Wagner et al. scores for our cohort were higher (reflecting more time) for those with severe contractures, whereby the subjects with fewer contractures, and therefore fewer limitations in their range of movement, scored better (less time) on the Jebsen [19]. Yet based on pediatric norms for the Jebsen, even our best cohort timed scores were more than six times slower than unaffected youth. Of interest, the Jebsen allows for accommodative strategies if the outcome reflects attainment (or not) of the intended task. This leads us to consider that such accommodations that reflect muscle strength changes may be evaluated as part of study results during longitudinal clinical trials.
The QUEST was developed to evaluate upper extremity quality of movement in children with CP. The QUEST was determined to be an appropriate measure for our CMD population due to the similar upper extremity contractures and functional limitations observed in this population. The four domains of the QUEST (Dissociated Movement, Grasp, Weight Bearing, and Protective Extension) do not focus completely on the hand, but incorporate components of joint movement in the shoulder, elbow, forearm and wrist. We found limitations in the use of the QUEST with the CMD population due to disease progression and inability to complete all four domains. As many of our participants were non-ambulatory and had significant contractures, the ability to lie prone on the floor and weight bear on an extended elbow while reaching with the opposite hand was rarely accomplished. The fact that the Jebsen correlated strongly with the QUEST Dissociated Movements and Grasp domains, mildly with the QUEST Weight-Bearing and not at all with the QUEST Protective Extension is not surprising, as the Jebsen assesses hand dexterity and strength, and not whole arm protective reactions and upper body strength as do the QUEST Weight-Bearing and Protective Extension domains. These two domains correlated the least with the other assessments, as they were the most difficult, if not impossible, for our CMD cohort to achieve. Due to limitations in data collection for the domains of Weight Bearing and Protective Extensions, we did not use QUEST total scores and considered all domains independently when scoring. This was also reported by Thorley et al. [16] who determined that the domains of the QUEST are not unidimensional, do not measure the same concept, and should be considered and scored separately. Although the QUEST domains of Dissociated Movement and Grasp were highly correlated with most of our UE battery of assessments, as a whole this assessment was difficult for participants to perform and would best serve to evaluate those whose weakness or contractures are not significant. In addition, the QUEST is a difficult and timely assessment to score. Altogether, these concerns limit the use of the QUEST with the CMD population. It may be more useful to focus on UE assessments that are more feasible and have less variability for this population.
The MyoSet Tools include three devices developed to assess distal motor function of the upper extremity [17]. A number of MyoSet test results were excluded due to the severity of our participant's contractures. The MyoGrip and MyoPinch tools were very useful for those with severe weakness of the upper extremities, and scores did not present with a floor effect. However, for our participants with severe contractures at the shoulder, elbow or wrist, we were often required to adapt the positioning of the devices. For example, devices were placed higher and closer for those individuals who were in wheelchairs as opposed to those who could sit in a chair at the table. Another highlight of the MyoGrip and MyoPinch is that, unlike myometry, these tools were more sensitive to weak patients, as they are able to measure up to .001 kg. We consider this as a strength of these tools in this population, despite the fact that the test is less standardized due to accommodating positioning. In contrast, for several participants, the MoviPlate was turned horizontally as opposed to vertically to accommodate proximal UE contractures. Horizontal placement was done to allow the participant a “successful experience”, but altered position scores for the MoviPlate are considered invalid and were not included in the data collections for this study. In addition, some of the participants had difficulty maintaining their attention for the full 30 seconds required to complete the repetitive and alternating finger tapping associated with the MoviPlate. Attentional issues are a confounding variable that needs to be addressed when administering the MoviPlate. Based on our results, we recommend the use of the MyoGrip and MyoPinch tools, but would not recommend the MoviPlate for individuals with severe contractures or severe weakness of the upper extremity.
Within our study, only elbow ROM for extension and elbow myometry for flexion and extension were assessed. We found the elbow to be the most reliable joint to measure, with less variability than other joints, such as the shoulder and wrist. This is because the two bony landmarks for elbow ROM measurement (lateral epicondyle and the tip of the acromion process) were easier to locate on the elbow and shoulder, even when contractures were present. Upper extremity contractures are a significant concern in this population and limit the ability to perform many required arm, wrist and hand tasks. In our 2-year pilot study, we included shoulder and wrist goniometry measurements, but the variability of these testing positions was so wide, due to contractures, it was difficult to ensure standardization [6]. Of our cohort of 42, 41 subjects had documented contractures in shoulders (58%), elbows (85%), wrists (58%), long finger flexors (78%) and fingers (15%). The elbow contractures were the most common and the most severe of the reported contractures. This contracture report was part of the medical intake where the physician noted the presence or absence of contractures of shoulder, elbow, wrist and fingers. We included elbow goniometry in this validation study so that in the future, with pharmacological interventions, changes could be documented, since it is the joint with prominent landmarks for repeatability. Interestingly, even with the severity of contractures, our participants were able to attempt most of the components of each assessment and correlations were strong, most notably with the Jebsen, QUEST Dissociated Movements and Grasp, and the MyoGrip and MyoPinch.
There are a few limitations that are important to discuss. As CMD is a rare disease, our sample size was small in relation to standard validation studies. Sample sizes that are typically used for a validation include 50–100 or more participants [9].
Furthermore, as the sample size was small, it would be useful for future larger sample sized studies to analyze COL6-RD and LAMA2-RD separately for the purpose of using these assessments in a diagnostic-specific natural history or longitudinal clinical trial setting. Although we did attempt to analyze by diagnostic type, correlations for the small sample sizes were not stable and were reduced by the effect of restricting the range of scores on the correlation between variables [20]. We took under consideration the fact that the main goal of this study was to validate the use of a battery of upper extremity measures in two CMD subtypes for use as clinical endpoints in future clinical trials. Although there are subtleties and intricacies between these two subtypes, they are similar in their presentations of upper extremity weakness and contractures.
As a cross-sectional study, our ability to interpret these assessments and their clinical usefulness is limited. Utility, feasibility, ease of administration and learning the limitations and strengths of these specific assessments will help us move forward. Understanding which assessments provide the most useful information is also critical to help limit participant burden (redundant tasks on some of the assessments). This study is a component of a larger natural history study; as we begin to analyze change over time, we will able to consider clinical usefulness with more certainty.
6. Conclusion
This study validated the use of a battery of upper extremity measures in two CMD subtypes, COL6-RD and LAMA2-RD, for use as clinical endpoints in future clinical trials. Assessing upper limb function in the two subtypes of CMD remains challenging due to a variety of factors, such as weakness, contractures and compensatory factors, which increase variability and make it difficult to consistently and accurately obtain measures. Overall, many of the UE assessments in this battery were found to be feasible, as well as valid in the CMD population and correlated with formerly recognized instruments: the MFM32 and the Distal Motor Function scores (Dimension-3). Based on the UE assessments used in this study, we found the Jebsen, the Grasp and Dissociated Movements domains of the QUEST, the MyoGrip and the MyoPinch tools, as well as elbow range of motion and myometry, to be valid, to provide variation in test items, and to assess a range of difficulty in CMD. This information is vital in our attempt to measure improvement or progression over time. To move forward, it will be of utmost importance to determine whether these UE measures are reproducible and sensitive to change over time. The inclusion of UE assessments is essential in our attempt to capture the entire spectrum of disease progression in CMD, but these results must be inherently repeatable to reinforce our findings and ensure that the wider scientific community has the best information to move forward with better outcomes in CMD.
References
- 1.Zupan A. Assessment of the functional abilities of the upper limbs in patients with neuromuscular diseases. Disabil Rehabil. 1996;18:69–75. doi: 10.3109/09638289609166020. [DOI] [PubMed] [Google Scholar]
- 2.Mercuri E, Sewry C, Brown SC, Muntoni F. Congenital muscular dystrophies. Semin Pediatr Neurol. 2002;9(2):120–31. doi: 10.1053/spen.2002.33802. [DOI] [PubMed] [Google Scholar]
- 3.Mercuri E, McDonald C, Mayhew A, et al. International workshop on assessment of upper limb function in Duchenne muscular dystrophy. Neuromuscul Disord. 2012;22:1025–8. doi: 10.1016/j.nmd.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang CH, Bonnemann CG, Rutkowski A, et al. Consensus statement on standard of care for congenital muscular dystrophies. J Child Neurol. 2010;25(12):1559–81. doi: 10.1177/0883073810381924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzone ES, Vasco G, Palermo C, et al. A critical review of functional assessment tools for upper limbs in Duchenne muscular dystrophy. Dev Med Child Neurol. 2012;54:879–85. doi: 10.1111/j.1469-8749.2012.04345.x. [DOI] [PubMed] [Google Scholar]
- 6.Meilleur KG, Jain MS, Hynan LS, et al. Results of a two-year pilot study of clinical outcome measures in collagen VI- and laminin alpha2-related congenital muscular dystrophies. Neuromuscul Disord. 2015;25:43–54. doi: 10.1016/j.nmd.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berard C, Payan C, Hodgkinson I, Fermanian J, The MFM Collaborative Study Group A Motor Function Measure scale for neuromuscular diseases. Construction and validation study. Neuromuscul Disord. 2005;15:463–70. doi: 10.1016/j.nmd.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Bonnemann CG, Rutkowski A, Mercuri E, Muntoni F, for the CMD Outcomes Consortium 173rd ENMC International Workshop: congenital muscular dystrophy outcome measures 5–7 March 2010, Naarden, the Netherlands. Neuromuscul Disord. 2011;21:513–22. doi: 10.1016/j.nmd.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anthoine E, Moret L, Regnault A, Ronique V, Hardouin J-B. Sample size used to validate a scale: a review of publications on newly-developed patient reported outcomes measures. Health Qual Life Outcomes. 2014;12(176):1–10. doi: 10.1186/s12955-014-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuillerot C, Rippert P, Kinet V, et al. Rasch analysis of the Motor Function Measure in patients with congenital muscle dystrophy and congenital myopathy. Arch Phys Med Rehabil. 2014;95:2086–95. doi: 10.1016/j.apmr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jebsen RH, Taylor N, Treischmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–19. [PubMed] [Google Scholar]
- 12.DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. The reliability and validity of the Quality of Upper Extremity Skills Test. Phys Occup Ther Pediatr. 1993;13:1–18. [Google Scholar]
- 13.Sorsdahl AB, Moe-Nilssen R, Strand LI. Observer reliability of the gross motor performance measure and the Quality of Upper Extremity Skills Test, based on video recordings. Dev Med Child Neurol. 2008;50(2):146–51. doi: 10.1111/j.1469-8749.2007.02023.x. [DOI] [PubMed] [Google Scholar]
- 14.Klingels K, De Cock P, Desloovere K, et al. Comparison of the melbourne assessment of unilateral upper limb function and the Quality of Upper Extremity Skills Test in hemiplegic CP. Dev Med Child Neurol. 2008;50(12):904–9. doi: 10.1111/j.1469-8749.2008.03123.x. [DOI] [PubMed] [Google Scholar]
- 15.DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. The reliability and validity of the Quality of Upper Extremity Skills Test. Phys Occup Ther Pediatr. 1993;13(2):1–18. [Google Scholar]
- 16.Thorley M, Lannin N, Cusick A, Novak I, Boyd R. Reliability of the Quality of Upper Extremity Skills Test for children with cerebral palsy aged 2 to 12 years. Phys Occup Ther Pediatr. 2012;32(1):4–21. doi: 10.3109/01942638.2011.602389. [DOI] [PubMed] [Google Scholar]
- 17.Servais L, Deconinck N, Moraux A, et al. Innovative methods to assess upper limb strength and function in non-ambulant Duchenne patients. Neuromuscul Disord. 2013;23:139–48. doi: 10.1016/j.nmd.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Hiller LB, Wade CK. Upper extremity functional assessment scales in children with Duchenne muscular dystrophy: a comparison. Arch Phys Med Rehabil. 1992;73:527–34. [PubMed] [Google Scholar]
- 19.Wagner MB, Vignos PJ, Jr, Carlozzi C, Hull AL. Assessment of hand function in Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1993;74:801–4. doi: 10.1016/0003-9993(93)90004-t. [DOI] [PubMed] [Google Scholar]
- 20.Bobko P. Correlation and regression: applications for industrial organizational psychology and management. 2nd ed. Sage Publications; Thousand Oaks (CA): 2001. Range restriction. pp. 97–117. [Google Scholar]