Abstract
Oxidative stress exerts major role in the pathogenesis of side effects of many antineoplastic drugs, including ototoxicity of cisplatin. In particular, increased levels of reactive oxygen species (ROS) represent one of the molecular mechanisms underlying the apoptosis of different types of hearing cells. Antioxidants and ROS scavengers may thus represent potential therapeutic options to prevent platinum-associated ototoxicity.
The aim of this preliminary case-control study was to explore the efficacy of a dietary antioxidant supplement, in order to hamper the occurrences of ototoxicity in patients undergoing cisplatin chemotherapy.
As results, a significant protection against cochlear toxic damage was demonstrated in patients who took the antioxidant supplement, which furthermore prevented the occurrence of hearing disorders and tinnitus. These clinical evidences were corroborated by the oxidative status of patients. After cisplatin chemotherapy, the plasma derivatives of reactive oxygen metabolites (d-ROMs) content rapidly increased in control patients, but it was maintained in those under dietary supplementation, likely because of a higher anti-ROMs potential. Indeed, an increment in rapid anti-ROMs was detected in supplemented patients, though no differences were highlighted in terms of slow anti-ROMs.
In conclusion, in this preliminary report we demonstrated the feasibility of a dietary antioxidant supplementation in order to prevent the cisplatin induced hearing damage.
Keywords: Human toxicology, Food science, Oncology
1. Introduction
Oxidative stress exerts major role in both tumor growth and anticancer therapy [1]. Increased levels of reactive oxygen species (ROS) may contribute to tumor formation and are required for the aberrant proliferation of cancer cells. To counteract the accumulation of high ROS concentrations, which otherwise would induce severe cellular damage and death, cancer cells display enhanced antioxidant capacity. Nevertheless, they are still characterized by higher oxidative stress status than normal cells, making them more susceptible to agents that cause further ROS accumulation, such as taxanes, vinca alkaloids, anthracyclines, and platinum coordination complexes [2, 3, 4]. However, the ROS accumulation in normal tissues can be cause of toxicity and some side effects of these drugs. For example, the doxorubicin-mediated cardiotoxicity underlies the competition between the drug and coenzyme Q10 for the electron transport chain in the inner membrane of cardiac mitochondria [2].
In this context, cisplatin was associated with many severe dose-limiting side effects [5] that are related (e.g. nephrotoxicity, neurotoxicity, and ototoxicity) or not (bone-marrow suppression, and hepatotoxicity) to the production of ROS. Many of the reported side effects can be actively counteracted in the clinics, but no effective treatments to prevent ototoxicity have been up to now introduced. Cisplatin mediated ototoxicity is usually bilateral, sensorineural and considered permanent once established, and the most common symptoms are hearing loss and tinnitus. The ototoxicity is directly correlated with the cumulative dose of administered drug, having the etiology in the cisplatin dose-dependent and ROS-mediated apoptosis of cochlear hair cells, supporting cells, marginal cells of the stria vascularis, and spiral ganglion cells [6].
Antioxidants, ROS scavengers, or anti-inflammatory drugs may thus represent potential therapeutic options to prevent platinum-associated ototoxicity. Among these, the supplementation of water-soluble coenzyme Q10 (Q10 terclatrate − QTer®) was already able to prevent in animals [7, 8, 9, 10] and human volunteers [11, 12] both noise-induced hear loss (NIHL) and gentamycin mediated ototoxicity, which have a similar ROS mediated etiology. Moreover, the same strategy was reported to exert significant improvements of hearing thresholds at 1000 Hz, 2000 Hz, 4000 Hz, and 8000 Hz in patients with presbycusis [13, 14].
Based on these evidences, the aim of this preliminary case-control study was to explore the feasibility of a dietary supplementation of QTer® plus multivitamins and other ingredients in order to hamper the ototoxicity in patients undergoing cisplatin chemotherapy. In preparation to a larger scale efficacy study, here we focalized our attention on a limited number of patients, who were followed for the first three cycles of chemotherapy and monitored for the occurrences of otological symptoms as well as for their oxidative stress status.
2. Materials and methods
2.1. Patients and treatment
From January 2013 to June 2014, 26 adult patients belonging to the Micone Hospital (Genoa − Sestri Ponente, Italy) were enrolled in this randomized pilot study (Table 1). The inclusion criterion was the diagnosis of cancer eligible for the treatment with cisplatin as single agent or with radiotherapy.
Table 1.
Patient cohort characteristics.
| Gender | Age | Tumor localization | TNM | Treatment | ||
|---|---|---|---|---|---|---|
| Controls |
1 | M | 58 | Hypopharynx | rT4N0M1 | CT |
| 2 | M | 59 | Hypopharynx | T3N0M1 | CT | |
| 3 | M | 66 | Hypopharynx | T3N2bM0 | CT/RT | |
| 4 | M | 50 | Pharynx | T3N3M0 | CT/RT | |
| 5 | M | 59 | Larynx | T3N2bM0 | CT/RT | |
| 6 | M | 65 | Throat Cancer (Larynx and Pharynx) | T3N2bM0 | CT/RT | |
| 7 | M | 66 | Hypopharynx | T3N2bM0 | CT/RT | |
| 8 | M | 67 | Nasopharyngeal Cancer | T3N2bM0 | CT/RT | |
| Supplemented | 1 | F | 67 | Base of the Tongue | T1N2bM0 | CT/RT |
| 2 | M | 66 | Larynx | T4N2cM0 | CT/RT | |
| 3 | M | 66 | Oral Cavity | T4N3M0 | CT/RT | |
| 4 | M | 70 | Lung | T2N3M0 | CT | |
| 5 | M | 74 | Pancreas | T2N1M0 | CT | |
| 6 | M | 62 | Oropharynx | T1N2bM0 | CT/RT | |
| 7 | M | 49 | Oral Cavity | T1N2bM0 | CT/RT | |
| 8 | M | 67 | Hypopharynx | rT3N0M1 | CT | |
| 9 | M | 54 | Liver | T3N1M1 | CT | |
| 10 | M | 66 | Oropharynx | T2N2bM0 | CT/RT | |
| 11 | M | 68 | Diffuse Metastases | CT | ||
| 12 | F | 82 | Hypopharynx | T2N2aM0 | CT/RT | |
| 13 | F | 58 | Oropharynx | T3N1M0 | CT/RT | |
| 14 | M | 43 | Oropharynx | T2N1M0 | CT/RT | |
| 15 | M | 67 | Oropharynx | T2N1M0 | CT/RT | |
| 16 | M | 75 | Hypopharynx | T4N2bM0 | CT/RT | |
| 17 | M | 56 | Hypopharynx | T3N1M0 | CT/RT | |
| 18 | M | 69 | Diffuse Metastases | CT | ||
The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2002 and was approved by the local Ethical Committee (Protocol n° 17–12 approved on September 13, 2012). Informed consent was obtained from all individual participants included in the study. Thus, patients underwent unequal randomization [15] in two groups: i) Controls (N = 8), who received 100 mg/m2 cisplatin every 21 days; ii) Supplemented (N = 18), who received 100 mg/m2 cisplatin every 21 days plus oral supplementation with 1 sachet/day of Acuval® Audio (QTer®, vitamins B1, B2, B6, B12, and E, choline, melatonin, Ginkgo biloba extract, and Lactium® milk protein hydrolysate) from 7th day before the beginning of cisplatin administration, until the 21st day after the completion of the chemotherapy schedule (Table 1). All Acuval® Audio sachets were from the Lot No. 0915 (date of manufacture: July 1, 2013). The dosage of provided supplement was the recommended one in accord with the manufacturer leaflet.
2.2. Hearing function assessment
Before and thirty days after the end of the cisplatin chemotherapy schedule, all patients underwent conventional pure-tone audiometry (ORBITER 922, Version 2, Madsen Electronic) in a soundproof room. The pure-tone thresholds for each ear were measured at frequencies of 250, 500, 1000, 2000, 4000, and 8000 Hz.
Diagnosis of otological disorders (e.g. hearing loss) after cisplatin treatment was performed by comparing the recorded thresholds for each patient. The presence of tinnitus was detected on the basis of patient perceptions.
2.3. Reactive oxygen metabolites and antioxidants in blood
Blood samples were collected before the beginning of the chemotherapy schedule and before every cisplatin administration, in order to measure the status of oxidative stress. After collection, plasma samples were stored at −80 °C until their analysis.
The oxidative stress was evaluated by means of d-ROMs test [16, 17, 18], which spectrophotometrically quantifies in the samples the presence of hydroperoxide derivatives (e.g. alkoxy and hydroperoxyl radicals). The results were expressed in arbitrary unit known as Carratelli unit (U-CARR), defined as the oxidative potential of 0.08 mg H2O2/dL [16, 19].
The antioxidant capacity of plasma to reduce ferric iron to ferrous iron were evaluated spectrophotometrically by means of anti-ROMs [20, 21] test. It quantifies the concentration of fast-acting antioxidants, e.g. Vitamin C and Vitamin E (rapid anti-ROMs) and then the amount of slow antioxidants, such as thiol (-SH) groups and uric acid (slow anti-ROMs). The results were expressed in μEqFe2+/L using ascorbic acid as standard [22].
Both tests were performed according to the manufacturer instruction (Diacron Labs S.r.l., Grosseto, Italy) and using a free radical elective evaluator (F.R.E.E.; Diacron Labs S.r.l.).
2.4. Statistical analysis
The differences in hearing threshold were compared between groups by means of Independent Sample t-Test whereas incidences of hearing disorders and tinnitus were compared by means of Fisher’s Exact Test. Differences among groups in d-ROMs, rapid anti-ROMs, and slow anti-ROMs throughout chemotherapy were detected by means of linear regression comparison (slope and elevation tests). All analyses were carried out with GraphPad Prism version 6.0e (GraphPad Software, San Diego, CA), with P < 0.05 as the significant cut-off.
3. Results
3.1. Patients
Amongst the enrolled twenty-six patients, eighteen received concomitant radiotherapy. All patients were classified according to the TNM classification system and their respective main characteristics were reported in Table 1. After randomization, patients were followed for a mean period of 4 months. Patients underwent to 3 cycles of chemotherapy with an average of 462 mg cisplatin for each administration. No statistical differences were detected between the two group populations (Table 2).
Table 2.
Group cohorts’ comparison.
| Controls | Supplemented | P | |
|---|---|---|---|
| Gender | |||
| Male | 8/8 (100.0%) | 15/18 (83.3%) | 0.529 |
| Female | 0/8 (0.0%) | 3/18 (16.7%) | |
| Age (years ± SD) | 62.0 ± 6.2 | 65.8 ± 10.1 | 0.337 |
| Chemotherapy cycles | 3.0 ± 1.6 | 3.6 ± 1.5 | 0.365 |
| Cisplatin dose (mg) | 456.8 ± 195.5 | 464.6 ± 110.7 | 0.897 |
| Metastases | 2/8 (25.0%) | 2/18 (11.1%) | 0.563 |
| Concomitant radiotherapy | 6/8 (75.0%) | 12/18 (66.7%) | 1.000 |
| Previous otologic pathologies | 1/8 (12.5%) | 3/18 (16.7%) | 1.000 |
| Previous tinnitus | 1/8 (12.5%) | 2/18 (11.1%) | 1.000 |
3.2. Hearing disorders
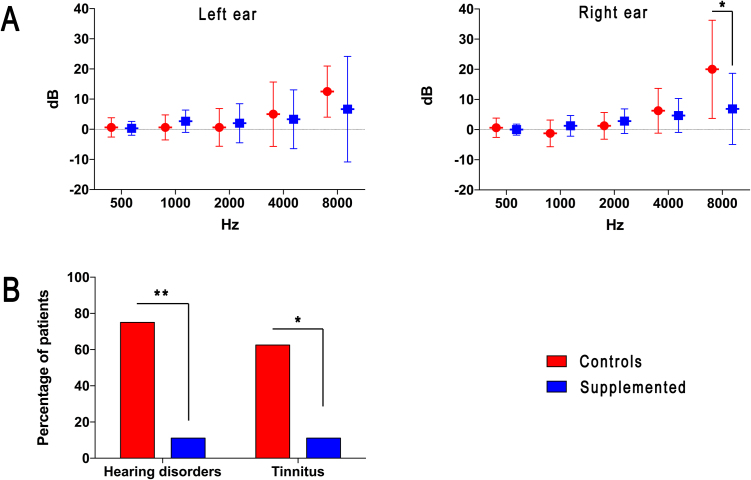
The analysis of the tonal audiometry demonstrated a higher hearing impairment in control patients if compared to those undergoing dietary antioxidant supplementation (Fig. 1A). A statistically significant difference was detectable analyzing the functionality of the right ear at 8000 Hz frequency: in Controls, the hearing threshold level was increased of 20.0 ± 16.2 dB, while in Supplemented group the threshold augment of 6.9 ± 11.8 dB (P < 0.05). A similar trend was also detected for the left ear, though the differences did not reach the statistical significance.
Fig. 1.
Hearing impairments and disorders in patients. A) Variations in hearing threshold between pre and post-chemotherapy. Data were expressed as mean ± standard deviation. B) Incidences of hearing disorders and tinnitus in the patient cohort as consequence of cisplatin administration. * = P < 0.05, ** = P < 0.01.
The result was confirmed by the different incidences in terms of cisplatin-related hearing disorders and tinnitus (Fig. 1B). In fact, within the third cycle of chemotherapy, cisplatin-related hearing disorders occurred in 6 out of 8 patients (75.0%) of the Control group. Otherwise, only 2 out of 18 patients (11.1%) who took daily the supplement were affected in the same time period (P < 0.01). Similarly, incidence of tinnitus was higher (P < 0.05) in Control group (5 out of 8, 62.5%) than in Supplemented cohort (2 out of 18, 11.1%). Tinnitus has always been referred as intense and bilateral, with varying tones more frequently acute. Only in one case belonging to the Control group, tinnitus regressed after 48 h.
3.3. Evaluation of reactive oxygen metabolite derivatives
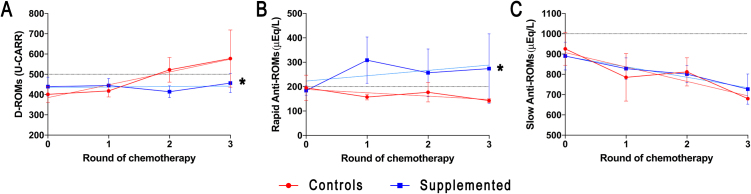
D-ROMs were elevated in both groups before chemotherapy (400–440 U-CARR corresponding to high oxidative stress), but demonstrated a statistically different trend between groups (regression slope test, P < 0.05) throughout the chemotherapy schedule. In fact, in control cases, d-ROMs rapidly grew-up from the second cycle of chemotherapy, overtaking the value of 500 U-CARR, which corresponds to a very high oxidative stress. Otherwise, supplemented patients maintained a stable concentration of d-ROMs until the third chemotherapy cycle (Fig. 2A).
Fig. 2.
Oxidative status in plasma samples during the chemotherapy courses. Data were expressed as mean ± standard deviation and the corresponding linear regressions were reported. Horizontal lines represent the limit for high oxidative stress (A) and the normality threshold for Rapid (B) and Slow (C) Anti-ROMs. * = P < 0.05.
3.4. Evaluation of anti-ROMs
Before cisplatin treatment, the amounts of rapid anti-ROMs were just below of reference range (≥200 μEq/L) in both groups. In control cases, rapid anti-ROMs slowly decreased with the increment of cisplatin chemotherapy cycles. In supplemented patients, rapid anti-ROMs were recovered to the reference range (up to ∼300 μEq/L) since the first cycle of chemotherapy (regression elevation’s test, P < 0.05, Fig. 2B).
Otherwise, no differences between groups were found in slow anti-ROMs concentrations, which constantly decreased with the progression of the chemotherapic treatment (Fig. 2C).
4. Discussion
Cisplatin is one of the most effective and widely used chemotherapeutic agents for the treatment of several solid malignant neoplasms including testicular, ovarian, bladder, cervix uteri, lung, head and neck cancers [23, 24]. Nevertheless, it exerts serious dose-limiting adverse effects, among which the ototoxicity is nowadays far to be addressed. The underlying molecular mechanism is mainly due to the enhancement of lipid peroxidation and the depletion of antioxidant systems [25]. In more detail, cisplatin induces the enhancement of NOX3 [26] and iNOS [27] expression and the consequent production of superoxides [28]. These can lead to the generation of hydrogen peroxide that, catalyzed by iron, produces hydroxyl radicals, which in turn interact with lipids in cell membranes (poly-unsaturated fatty acids) to form the highly toxic 4-hydroxynonenal [27, 29]. At the same time, superoxides can react with nitric oxides generating peroxynitrites, which form tyrosine adducts (nitrotyrosine) interacting with proteins [27]. All these events induce the reduction of both glutathione content and activity of enzymes like glutathione peroxidase, glutathione reductase, and catalase, which in turn lead to the apoptotic death of outer hair cells, tissues of lateral wall, and spiral ganglion cells [25].
Here, we evaluated the feasibility of an antioxidant treatment to hamper the hearing impairment, frequently developed by oncological patients undergoing cisplatin chemotherapy. In particular, we administered an already commercially available dietary supplement (Acuval® Audio), whose efficacy in preventing cisplatin ototoxicity was recently demonstrated in rats [30].
Despite the patient numerosity allowed us to follow patients for only 3 chemotherapy courses, a significant protection against cochlear toxic damage was demonstrated in the group of supplemented patients. If compared to their respective pre-chemotherapy hearing profile, these patients suffered indeed of a limited variation (up to 6.9 dB) in their hearing threshold at acute frequencies (8 KHz). Otherwise, patients belonging to the Control group had a more pronounced worsening of hearing threshold (up to 20.0 dB; P < 0.05), which can be considered as clue of outer hair cell cytotoxicity. Anyhow, in our series, we detected significant threshold differences between the two groups in the right ear only (Fig. 1). This is likely due to a worsen 8 KHz baseline hearing threshold in the left ear of Control group, which is higher (65.6 ± 23.0 dB) than the right ear (58.8 ± 26.6 dB), although the left and right thresholds become superimposable (left ear: 78.1 ± 19.8 dB; right ear: 78.8 ± 17.7 dB) after 3 cisplatin courses. Moreover, the daily consumption of the studied supplement greatly prevents the occurrence of hearing disorders and tinnitus (both 11.1%) if compared to those detected in control patients (75.0% and 62.5%, respectively; P < 0.01 and P < 0.05). Such results were likely achieved by hampering the typical ROS and free radical enhancements detectable in patients treated with cisplatin, whereas the prevention of tinnitus occurrences could be related to a protection against cisplatin-mediated spiral ganglion neurotoxicity. In order to validate this hypothesis, we assessed the amount of alkoxy and hydroperoxyl radicals as “derivatives of reactive oxygen metabolites” (d-ROMs) in patients’ blood. Direct ROS and free radical measurement is generally difficult in plasma sample due to their high biochemical instability. Anyhow, the spectrophotometric d-ROMs test [16, 17, 18], though challenged, is reliable and reproducible when caution is given to the sample handling (e.g. storage at −20 °C). Consistently with their physiopathological condition, in our patient cohorts the average oxidative status was elevated (≥400 U-CARR). In control patients, the plasma content of d-ROMs rapidly increased to overtake the 500 U-CARR limit (meaning of very high oxidative stress) following cisplatin chemotherapy. Otherwise, patients undergoing dietary antioxidant supplementation were able to maintain stable, though elevated, their d-ROM level.
According to the assumption that decreased levels of natural antioxidants and diminished scavenging enzyme capacity may be responsible for the excess of ROS observed in cisplatin-induced toxicity [31, 32], an inverse correlation between plasma concentrations of reactive oxygen metabolites and antioxidant compounds is likely. Thus, we assessed in patients’ plasma samples the antioxidant potential in terms of iron-reducing capabilities (anti-ROMs test) [20, 21]. At the enrollment, patients were accounted of low levels of both rapid and slow anti-ROMs that can be referred to an imbalance of antioxidant defenses. As the slow anti-ROMs are mainly referable to uric acid and thiols contents, our results demonstrated that the endogenous antioxidant system was impaired in both patient groups throughout the cisplatin-based chemotherapy, without any sign of activity mediated by the supplementation. On the other hand, levels of rapid anti-ROMs are generally linked to the exogenous antioxidant system (e.g. dietary intake of vitamins) and are sensitive to the assumption of formulations intended to increase the efficiency of defense systems against oxidative insult. Consistently, a significant increment (p < 0.05) in rapid anti-ROMs content (≥200 μEq/L) was detected in patients supplemented with Acuval® Audio. This formulation is indeed based on water-soluble coenzyme Q10 (Qter®) and vitamins (B1, B2, B6, B12, E), along with choline, melatonin, Ginkgo biloba extract, and Lactium®.
As it is a mixture of several different compounds, it is difficult to discriminate the components most involved in hearing loss prevention. However, several components have already demonstrated activities against oxidative damages leading to hearing impairment. Coenzyme Q10 is a potent antioxidant by either directly scavenging free radicals or recycling and regenerating other antioxidants [33, 34]. Noteworthy, Qter® showed enhanced bioavailability with respect to the native form [35] and demonstrated a protective effect in preventing damage to the outer hair cells of the cochlea following noise exposure [8, 10, 12]. Likewise, Ginkgo biloba extract has demonstrated to prevent gentamicin and cisplatin-induced ototoxicity in both in-vitro and animal models [36, 37], as well as to protect cochlear hair cells against ototoxicity induced by gentamicin through the reduction of ROS and nitric oxide-related apoptosis [37]. By means of both direct free radical scavenging and indirect antioxidant activity, melatonin has been shown to increase the efficacy and reduce the toxicity of a large number of drugs [38], including the ototoxicity caused by cisplatin [39]. Also, vitamin E has been proved to exert otoprotection against drug and noise related hearing damage in several animal models [40, 41, 42, 43, 44].
In conclusion, in this preliminary report we demonstrated the feasibility of a dietary antioxidant administration to patients undergoing cisplatin chemotherapy in order to prevent the hearing damage. Antioxidants that act as reducing agents (such as coenzyme Q10 or vitamins) do not appear to interfere with the antineoplastic activity of cisplatin [45]. However, some promising nucleophilic antioxidants (e.g. sodium thiosulfate and N-acetylcysteine) have already demonstrated to directly interact with the chemotherapy drug, making cisplatin not able to exert its therapeutic activity [46, 47], whereas others (e.g. D − and L −methionine) still retain the cisplatin tumor cytotoxicity, though chelating it [48]. Although the use of dietary supplements is generally free from severe side effects, a greater cohort of patients will be required to fully unravel the molecular interactions and the antioxidant activities that underlie the so far demonstrated otoprotection.
Declarations
Author contribution statement
Felice Scasso: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Andrea Elio Sprio, Giovanni Nicolao Berta: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Luciano Canobbio: Conceived and designed the experiments; Performed the experiments.
Chiara Scanarotti: Performed the experiments; Analyzed and interpreted the data.
Giorgio Manini: Contributed reagents, materials, analysis tools or data.
Anna Maria Bassi: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
Giorgio Manini is an employee of Scharper S.P.A, which provided the Acuval® Audio batch for experimentation.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Dr. F. Di Scipio for editing the manuscript.
References
- 1.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug. Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 2.Conklin K.A. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004;3(4):294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 3.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012 doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann S.H., Earnshaw W.C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000;256(1):42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Schilsky R., Reichert C.M., Reddick R.L., Rozencweig M., Young R.C., Muggia F.M. Toxic effects of cis-dichlorodiammineplatinum(II) in man. Cancer Treat. Rep. 1979;63(9-10):1527–1531. [PubMed] [Google Scholar]
- 6.Rybak L.P., Whitworth C.A., Mukherjea D., Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear. Res. 2007;226(1-2):157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Cascella V., Giordano P., Hatzopoulos S., Petruccelli J., Prosser S., Simoni E., Astolfi L., Fetoni A.R., Skarzynski H., Martini A. A new oral otoprotective agent: Part 1: Electrophysiology data from protection against noise-induced hearing loss. Med. Sci. Monit. 2012;18(1):BR1–8. doi: 10.12659/MSM.882180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fetoni A.R., Piacentini R., Fiorita A., Paludetti G., Troiani D. Water-soluble Coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–116. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Fetoni A.R., Eramo S.L., Rolesi R., Troiani D., Paludetti G. Antioxidant treatment with coenzyme Q-ter in prevention of gentamycin ototoxicity in an animal model. Acta Otorhinolaryngol. Ital. 2012;32(2):103–110. [PMC free article] [PubMed] [Google Scholar]
- 10.Fetoni A.R., De Bartolo P., Eramo S.L., Rolesi R., Paciello F., Bergamini C., Fato R., Paludetti G., Petrosini L., Troiani D. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci. 2013;33(9):4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fetoni A.R., Garzaro M., Ralli M., Landolfo V., Sensini M., Pecorari G., Mordente A., Paludetti G., Giordano C. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: a pilot study. Med. Sci. Monit. 2009;15(11):PR1–8. [PubMed] [Google Scholar]
- 12.Staffa P., Cambi J., Mezzedimi C., Passali D., Bellussi L. Activity of coenzyme Q 10 (Q-Ter multicomposite) on recovery time in noise-induced hearing loss. Noise Health. 2014;16(72):265–269. doi: 10.4103/1463-1741.140499. [DOI] [PubMed] [Google Scholar]
- 13.Salami A., Mora R., Dellepiane M., Manini G., Santomauro V., Barettini L., Guastini L. Water-soluble coenzyme Q10 formulation (Q-TER((R))) in the treatment of presbycusis. Acta Otolaryngol. 2010;130(10):1154–1162. doi: 10.3109/00016481003727590. [DOI] [PubMed] [Google Scholar]
- 14.Guastini L., Mora R., Dellepiane M., Santomauro V., Giorgio M., Salami A. Water-soluble coenzyme Q10 formulation in presbycusis: long-term effects. Acta Otolaryngol. 2011;131(5):512–517. doi: 10.3109/00016489.2010.539261. [DOI] [PubMed] [Google Scholar]
- 15.Dumville J.C., Hahn S., Miles J.N., Torgerson D.J. The use of unequal randomisation ratios in clinical trials: a review. Contemp. Clin. Trials. 2006;27(1):1–12. doi: 10.1016/j.cct.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Alberti A., Bolognini L., Macciantelli D., Caratelli M. The radical cation of N,N-diethyl-para-phenylendiamine: A possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 2000;26(3):253–267. [Google Scholar]
- 17.Cesarone M.R., Belcaro G., Carratelli M., Cornelli U., De Sanctis M.T., Incandela L., Barsotti A., Terranova R., Nicolaides A. A simple test to monitor oxidative stress. Int. Angiol. 1999;18(2):127–130. [PubMed] [Google Scholar]
- 18.Gerardi G., Usberti M., Martini G., Albertini A., Sugherini L., Pompella A., Di L.D. Plasma total antioxidant capacity in hemodialyzed patients and its relationships to other biomarkers of oxidative stress and lipid peroxidation. Clin. Chem. Lab. Med. 2002;40(2):104–110. doi: 10.1515/CCLM.2002.019. [DOI] [PubMed] [Google Scholar]
- 19.Cornelli U., Terranova R., Luca S., Cornelli M., Alberti A. Bioavailability and antioxidant activity of some food supplements in men and women using the D-Roms test as a marker of oxidative stress. J. Nutr. 2001;131(12):3208–3211. doi: 10.1093/jn/131.12.3208. [DOI] [PubMed] [Google Scholar]
- 20.Maruoka H., Nakagawa K., Miyagi J., Matsubara M. Licorice flavonoid oil reduces oxidative stress and total body fat in overweight subjects: a randomized, double-blind, placebo-controlled study. Med. J. Nutrition Metab. 2013;6(3):239–246. [Google Scholar]
- 21.Dentone C., Fenoglio D., Signori A., Cenderello G., Parodi A., Bozzano F., Guerra M., De Leo P., Bartolacci V., Mantia E., Orofino G., Kalli F., Marras F., Fraccaro P., Giacomini M., Cassola G., Bruzzone B., Ferrea G., Viscoli C., Filaci G., De Maria A., Di Biagio A. Relationship between innate immunity, soluble markers and metabolic-clinical parameters in HIV+ patients ART treated with HIV-RNA <50 cp/mL. J. Int. AIDS Soc. 2014;17(4 Suppl. 3) doi: 10.7448/IAS.17.4.19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meineri G., Giacobini M., Forneris G. Evaluation of physiological parameters of the plasma oxidative status in rabbits. J. Appl. Anim. Res. 2017;45(1):315–319. [Google Scholar]
- 23.Jordan J.A., Schwade N.D., Truelson J.M. Fosfomycin does not inhibit the tumoricidal efficacy of cisplatinum. Laryngoscope. 1999;109(8):1259–1262. doi: 10.1097/00005537-199908000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Fram R.J. Cisplatin and platinum analogues: recent advances. Curr. Opin. Oncol. 1992;4(6):1073–1079. doi: 10.1097/00001622-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Rybak L.P., Husain K., Morris C., Whitworth C., Somani S. Effect of protective agents against cisplatin ototoxicity. Am. J. Otol. 2000;21(4):513–520. [PubMed] [Google Scholar]
- 26.Mukherjea D., Whitworth C.A., Nandish S., Dunaway G.A., Rybak L.P., Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139(2):733–740. doi: 10.1016/j.neuroscience.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.E., Nakagawa T., Kita T., Kim T.S., Iguchi F., Endo T., Shiga A., Lee S.H., Ito J. Mechanisms of apoptosis induced by cisplatin in marginal cells in mouse stria vascularis. ORL J. Otorhinolaryngol. Relat. Spec. 2004;66(3):111–118. doi: 10.1159/000079329. [DOI] [PubMed] [Google Scholar]
- 28.Dehne N., Lautermann J., Petrat F., Rauen U., de Groot H. Cisplatin ototoxicity: involvement of iron and enhanced formation of superoxide anion radicals. Toxicol. Appl. Pharmacol. 2001;174(1):27–34. doi: 10.1006/taap.2001.9171. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.E., Nakagawa T., Kim T.S., Endo T., Shiga A., Iguchi F., Lee S.H., Ito J. Role of reactive radicals in degeneration of the auditory system of mice following cisplatin treatment. Acta Otolaryngol. 2004;124(10):1131–1135. doi: 10.1080/00016480410017521. [DOI] [PubMed] [Google Scholar]
- 30.Astolfi L., Simoni E., Valente F., Ghiselli S., Hatzopoulos S., Chicca M., Martini A. Coenzyme Q10 plus Multivitamin Treatment Prevents Cisplatin Ototoxicity in Rats. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weijl N.I., Cleton F.J., Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat. Rev. 1997;23(4):209–240. doi: 10.1016/s0305-7372(97)90012-8. [DOI] [PubMed] [Google Scholar]
- 32.Antunes L.M., Darin J.D., Bianchi Nde L. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol. Res. 2001;43(2):145–150. doi: 10.1006/phrs.2000.0724. [DOI] [PubMed] [Google Scholar]
- 33.Bhagavan H.N., Chopra R.K. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006;40(5):445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 34.Lenaz G., Fato R., Formiggini G., Genova M.L. The role of Coenzyme Q in mitochondrial electron transport. Mitochondrion. 2007;7(Suppl):S8–33. doi: 10.1016/j.mito.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Bergamini C., Moruzzi N., Sblendido A., Lenaz G., Fato R. A water soluble CoQ10 formulation improves intracellular distribution and promotes mitochondrial respiration in cultured cells. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cakil B., Basar F.S., Atmaca S., Cengel S.K., Tekat A., Tanyeri Y. The protective effect of Ginkgo biloba extract against experimental cisplatin ototoxicity: animal research using distortion product otoacoustic emissions. J. Laryngol. Otol. 2012;126(11):1097–1101. doi: 10.1017/S0022215112002046. [DOI] [PubMed] [Google Scholar]
- 37.Yang T.H., Young Y.H., Liu S.H. EGb 761 (Ginkgo biloba) protects cochlear hair cells against ototoxicity induced by gentamicin via reducing reactive oxygen species and nitric oxide-related apoptosis. J. Nutr. Biochem. 2011;22(9):886–894. doi: 10.1016/j.jnutbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Reiter R.J., Tan D.X., Sainz R.M., Mayo J.C., Lopez-Burillo S. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J. Pharm. Pharmacol. 2002;54(10):1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Gonzalez M.A., Guerrero J.M., Rojas F., Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J. Pineal Res. 2000;28(2):73–80. doi: 10.1034/j.1600-079x.2001.280202.x. [DOI] [PubMed] [Google Scholar]
- 40.Joachims H.Z., Segal J., Golz A., Netzer A., Goldenberg D. Antioxidants in treatment of idiopathic sudden hearing loss. Otol. Neurotol. 2003;24(4):572–575. doi: 10.1097/00129492-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Hou F., Wang S., Zhai S., Hu Y., Yang W., He L. Effects of alpha-tocopherol on noise-induced hearing loss in guinea pigs. Hear. Res. 2003;179(1-2):1–8. doi: 10.1016/s0378-5955(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 42.Scholik A.R., Lee U.S., Chow C.K., Yan H.Y. Dietary vitamin E protects the fathead minnow, Pimephales promelas, against noise exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2004;137(4):313–323. doi: 10.1016/j.cca.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Sergi B., Fetoni A.R., Ferraresi A., Troiani D., Azzena G.B., Paludetti G., Maurizi M. The role of antioxidants in protection from ototoxic drugs. Acta Otolaryngol. Suppl. 2004;552:42–45. [PubMed] [Google Scholar]
- 44.Fetoni A.R., Sergi B., Ferraresi A., Paludetti G., Troiani D. alpha-Tocopherol protective effects on gentamicin ototoxicity: an experimental study. Int. J. Audiol. 2004;43(3):166–171. doi: 10.1080/14992020400050023. [DOI] [PubMed] [Google Scholar]
- 45.Conklin K.A. Dietary antioxidants during cancer chemotherapy: impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer. 2000;37(1):1–18. doi: 10.1207/S15327914NC3701_1. [DOI] [PubMed] [Google Scholar]
- 46.Boven E., Verschraagen M., Hulscher T.M., Erkelens C.A., Hausheer F.H., Pinedo H.M., van der Vijgh W.J. BNP7787, a novel protector against platinum-related toxicities, does not affect the efficacy of cisplatin or carboplatin in human tumour xenografts. Eur. J. Cancer. 2002;38(8):1148–1156. doi: 10.1016/s0959-8049(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 47.Dickey D.T., Wu Y.J., Muldoon L.L., Neuwelt E.A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J. Pharmacol. Exp. Ther. 2005;314(3):1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 48.Deegan P.M., Pratt I.S., Ryan M.P. The nephrotoxicity, cytotoxicity and renal handling of a cisplatin-methionine complex in male Wistar rats. Toxicology. 1994;89(1):1–14. doi: 10.1016/0300-483x(94)90128-7. [DOI] [PubMed] [Google Scholar]