Abstract
A walnut supplement for a Western-style diet in men was shown to improve sperm motility, vitality, and morphology. To gain further insights into factors underlying this improvement, we administered a parallel walnut-enriched diet to mice [including those with a defect in sperm motility due to deletion of Plasma Membrane Ca2+-ATPase 4 (Pmca4−/−)] to determine if there is a similar improvement that is accompanied by reduced sperm membrane peroxidative damage. Although sperm vitality and acrosome reaction rate were unaffected, the diet led to a significant improvement in motility (P < 0.05) and morphology (P < 0.04) in wild-type sperm and in morphology (P < 0.01) in Pmca4−/−, confirming the diet’s efficacy, which appeared to be more modest in mice than in humans. In both strains of mice, the diet resulted in a significant decrease in sperm lipid peroxidation (oxidative stress) levels, but did not rescue the significantly increased apoptotic levels seen in the testis and epididymis of Pmca4 nulls. Our findings support the effectiveness of walnuts on sperm quality, associated with reduced peroxidative damage; and suggest that oxidative stress is involved in the mechanism(s) underlying male reproductive defects in Pmca4−/−.
Keywords: Physiology, Food science, Reproductive medicine, Health sciences
1. Introduction
The interaction between diet and reproductive health has been of growing concern in recent decades. A Western-style diet has been a primary focus of researchers, as it has been shown to be associated with reduced sperm function, particularly motility, vitality, and morphology in both humans and mice (Hu, 2002; Bakos et al., 2010; Palmer et al., 2012; Robbins et al., 2012). This type of diet, which is high in processed food, sugar, and refined grain, is known to lead to obesity and subsequently DNA damage in male germ cells; lowering rates of fertilization and impairing the health of the fetus and offspring (Bakos et al., 2010 and 2011; Palmer et al., 2012). Increased oxidative stress has been cited as the primary cause of reduced sperm function resulting from a Western-style diet (Bakos et al., 2010).
Sperm are susceptible to reactive oxygen species-induced damage due to high levels of polyunsaturated fatty acids (PUFAs) present in their membrane, and they rely on antioxidants found in the seminal fluid (Alvarez and Storey, 1995; Griveau et al., 1997). Dietary supplements of fish oil, containing n-3 fatty acids, were shown to have positive effects on sperm quality and parameters in rams (Alizadeh et al., 2014). While in a similar manner, the addition of oral antioxidants to men diagnosed with oligoasthenoteratozoospermia, a disorder characterized by low sperm count, motility, and morphology, also greatly increased motility and overall sperm quality (Wirleitner et al., 2012). As both of these measures, separately, exhibited positive effects on sperm health, natural supplements containing both PUFAs and antioxidants were studied. When administered to men on a Western-style diet, walnuts, a balanced whole food, significantly increased sperm motility, vitality, and normal morphology (Robbins et al., 2012). To date, a study of the effects of a walnut-enriched diet on sperm quality has not been conducted in an animal model and the mechanism by which sperm quality was improved in humans has not been investigated.
The mouse model provides the opportunity to test the diet in wild-type (WT) animals as well as a genetic mutant with defective sperm quality. Deletion of the murine Pmca4 gene which encodes PMCA4, the major Ca2+ efflux pump in mouse sperm (Wennemuth et al., 2003), leads to loss of sperm motility and consequently male infertility (Okunade et al., 2004; Schuh et al., 2004). The gene is expressed in both the testis and the epididymis where PMCA4 is secreted in the luminal fluid and acquired by sperm during epididymal maturation, via epididymosomes (extracellular vesicles) (Patel et al., 2013).
While epididymal sperm maturation has been recently shown to be under dietary regulation (Sharma et al., 2016), the underlying mechanism of the sperm defect and infertility in Pmca4−/− has not been fully established. However, it has been shown to be associated with oxidative stress resulting from elevated levels of nitric oxide (NO) and its highly reactive effector, peroxynitrite (OONO−), due to increased activity of nitric oxide synthases which are unregulated in PMCA4’s absence (Andrews et al., 2015).
Based on the above observations, we: 1) investigated the effect of a walnut-enriched diet parallel to that given to men on sperm characteristics of both wild-type and Pmca4−/− mice, 2) determined if lipid peroxidation levels, a key factor in loss of sperm motility (Hellstrom et al., 1994) may accompany the improvement in sperm characteristics resulting from the diet, and 3) examined the impact of the diet on testis and epididymal structure in Pmca4−/− males. Although the effects of the diet were found to be more modest than those in humans, our results confirm the diet’s efficacy on sperm characteristics in wild-type mouse sperm and show that this may be mediated by a reduction in sperm membrane lipid peroxidation. Thus, they suggest a potential mechanism for the findings in human sperm. Our results also reveal the impact of Pmca4 deletion on sperm membrane damage, consistent with increased lipid peroxidation, as well as apoptosis.
2. Results
2.1. The walnut-enriched diet has no impact on body weight
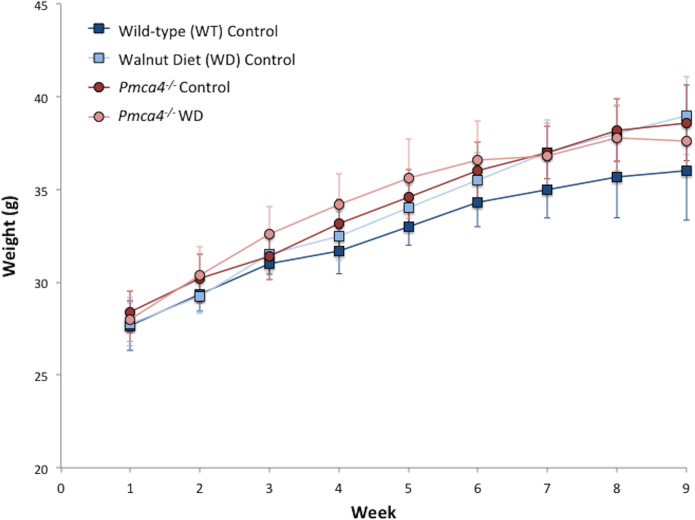
The body mass of WT mice fed a walnut-enriched diet (WD), parallel to that administered by Robbins et al. (2012), did not differ significantly, compared to mice fed the standard (control) diet over a 9-week period (Fig. 1). Pmca4−/− also showed no change (Fig. 1).
Fig. 1.
The walnut-enriched diet had no effect on body mass. Body mass (weight) is expressed as a function of the number of weeks on the diet. Data expressed as mean (±SEM) of WT control (N = 3), walnut diet (N = 5), Pmca4−/− control (N = 4) and Pmca4−/− walnut diet (N = 5) mice. No significant difference is evident between genotype or diet treatment.
2.2. Sperm motility and morphology were positively impacted by the diet
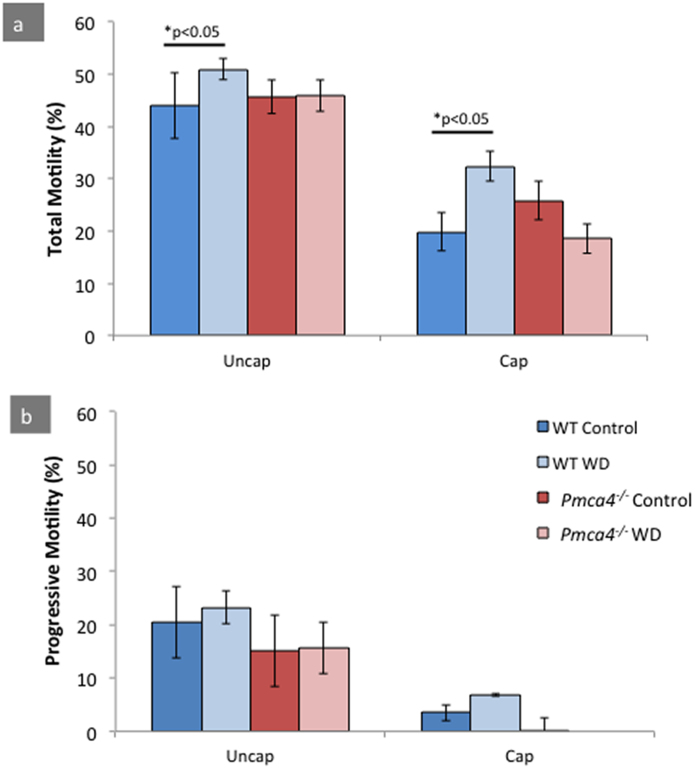
When WT males were treated for 9–11 weeks with a walnut-enriched diet, there was a significant increase in total sperm motility under both uncapacitating (uncap, 44.39% vs. 50.93%) and capacitating (cap, 19.8% vs. 32.34%) conditions (Fig. 2a), compared to control sperm from animals without the diet (P < 0.05). While total motility was positively impacted in WT sperm, there was no significant increase seen in progressive motility (Fig. 2b). For Pmca4−/− and their control sperm, the diet resulted in no significant difference in total or progressive motility, under both conditions (Fig. 2). The data also revealed sperm motility levels that are consistent with that reported earlier for Pmca4−/− (Okunade et al., 2004; Schuh et al., 2004).
Fig. 2.
The rate of total sperm motility (non-progressive and progressive) is significantly improved with the diet in WT, but unchanged in Pmca4−/−. (a) Average percentage of total motility in uncap (uncapacitating) and cap (capacitating) conditions. (b) Average percentage of progressive motility in uncap and cap conditions. (Two-way ANOVA: *p < 0.05, N = 5, mean ± SEM).
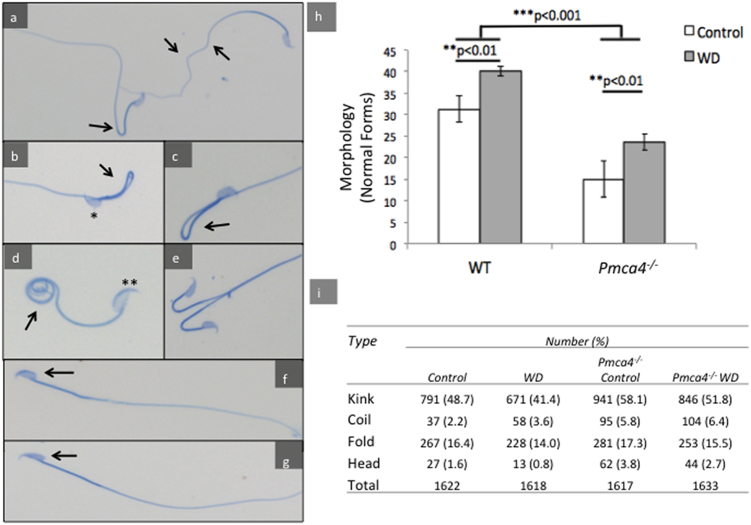
Sperm vitality and the ability to acrosome react were not different between diet treatments (Table 1). However sperm morphology, studied blindly after Coomassie Blue staining on coded slides, revealed differences with diet treatment. A population of >1600 sperm (>400/animal) with 4 animals in each group was analyzed from control and diet-treated males for the presence of structural abnormalities. Structural abnormalities that were detected included flagellar kinked (Fig. 3a), folded (Fig. 3b-c), and coiled (Fig. 3d) tails, as well as head deformities (Fig. 3e), with tail abnormalities being the most common. The diet significantly (P < 0.01) increased the numbers of morphologically normal WT sperm (Fig. 3h). A similar improvement with diet was seen for Pmca4 null sperm (P < 0.01) that had significantly (P < 0.001) less normal sperm than WT, with similar types of abnormalities. Two-way ANOVA showed an overall significant (P < 0.01) relationship between normal sperm morphology and the diet (Fig. 3).
Table 1.
Membrane quality rates for sperm from wild-type and Pmca4−/− males on the walnut-enriched diet.
|
Mean Percentage (± SEM) |
|||||
|---|---|---|---|---|---|
| WT Control | WT WD | Pmca4−/− Control | Pmca4−/− WD | p-Value | |
| Vitality | 88.75 (0.38) | 90 (3.84) | 83.58 (2.79) | 87.83 (4.10) | NS |
| Acrosome reaction* | 20.5 (6.76) | 18.5 (2.96) | 17.5 (4.17) | 18.5 (3.81) | NS |
N = 4 males per treatment group.
NS, not significant, using two-way ANOVA.
Intact and acrosome reacted sperm are seen in Fig. 3f, g.
Fig. 3.
Normal sperm morphology, assessed via Coomassie Blue staining, is significantly increased with the diet in WT and Pmca4−/− sperm where it is significantly lower than in WT in untreated animals (a-e) Arrows indicate kinks (a), folds (b-c), coils (d), and head deformities (e). (f) A sperm with an intact acrosomal cap (arrowed), also seen in d (**) and (g) with the cap missing after AR, also seen in b (*). (h) Average percent of morphologically normal sperm (n > 400/animal) show significantly lower levels in Pmca4−/− compared to WT and a significant increase with the diet. (Two-way ANOVA: **p < 0.01, ***p < 0.001, N = 4, mean ± SEM). (i) Frequency of morphological abnormalities per genotype and diet-type. Highest occurring abnormality was sperm tail kinks.
2.3. The walnut-enriched diet significantly reduces sperm lipid peroxidation levels
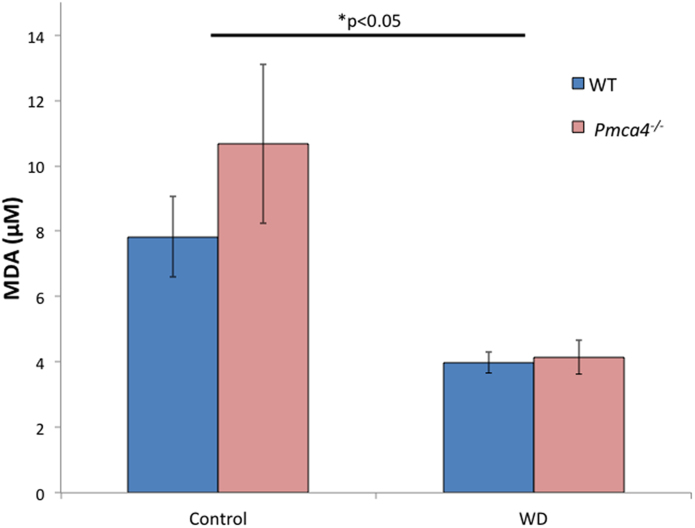
To determine if a reduction of lipid peroxidation levels is involved in the improvement of normal sperm morphology and total motility in diet-treated animals, lipid peroxidation levels were assessed by performing a Thiobarbituric Acid Reactive Substances (TBARS) Assay. The results in Fig. 4 reveal that following treatment with the diet there was a ∼2-fold decrease in lipid peroxidation levels in WT sperm. When compared to the WT, the untreated control mutants showed an increase in lipid peroxidation levels, but this was insignificant. However, lipid peroxidation levels were decreased, ∼2.5-fold, in sperm of Pmca4−/− diet-treated compared to untreated males (Fig. 4). A two-way ANOVA found that together there was a significant decrease (P < 0.05) is sperm from diet −treated animals.
Fig. 4.
Lipid peroxidation levels are significantly decreased in WT as well as Pmca4−/− sperm following administration of the walnut-enriched diet. Lipid peroxidation levels were assessed via a Thiobarbituric Acid Reactive Substances Assay (TBARS) assay. Mean malondialdehyde [MDA (μM)] levels in WT and Pmca4−/− sperm from control and animals treated with a diet (enriched in PUFAs and antioxidants) for 9–11 weeks are shown. Lipid peroxidation levels decrease in both WT and nulls following diet implementation. (Two-way ANOVA: *p < 0.05, N = 6, mean ± SEM).
2.4. The walnut-enriched diet did not impact the elevated testicular apoptotic rate or the altered epididymal integrity detected in Pmca4−/−
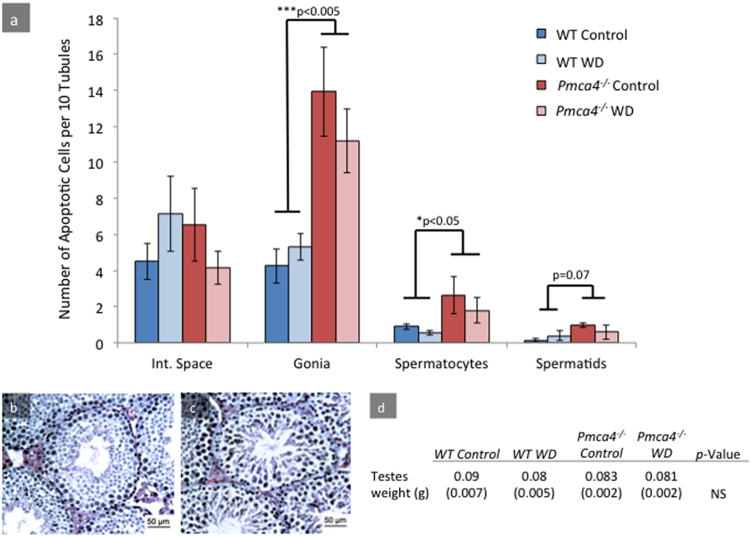
In order to determine if diet had an effect on the testicular histology and architecture, a TUNEL assay was performed on testicular sections from WT and Pmca4−/− males, diet-treated, and untreated (Fig. 5). Two-way ANOVAs were conducted separately on each testicular cell type. Although this revealed a significant increase in TUNEL positive cells in Pmca4−/− testes compared to WT, specifically in spermatogonia (P < 0.005) and spermatocytes (P < 0.05) (Fig. 5a), there was no significant effect of diet on the WT or the number of apoptotic cells in Pmca4 nulls. (Fig. 5a).
Fig. 5.
TUNEL assay reveals increased levels of apoptosis in testes with Pmca4 deletion, compared to WT, with no effect of the diet. (a) Quantification of TUNEL results in WT and Pmca4−/− testes from control and walnut-diet. No significant difference is evident between genotype or diet-type in the interstitial space; however, significant differences are seen between genotypes for spermatogonia (Gonia) and spermatocytes. The increase in spermatids did not reach significance. Implementation of the walnut-enriched diet reduced the number of apoptotic cells in Pmca4−/− but not significantly. (Two-way ANOVA: N = 3, ±SEM). (b-c) Histological staining of testis tissue is consistent with apoptotic activity in Pmca4−/−, evident through increased tubule disorganization with spaces between germ cells. Scale bar = 50 μm. (d) No statistically significant difference was detected in testicular weight between genotypes or treatment.
H&E stained testis sections revealed increased disorganization in the structure as well as enlarged spaces between germ cells of Pmca4−/− nulls (Fig. 5c) which, compared to controls (Fig. 5b), were still evident in testes from Pmca4−/− treated with the diet (data not shown). However, the weights of the testes were not significantly different amongst treatment groups (Fig. 5d).
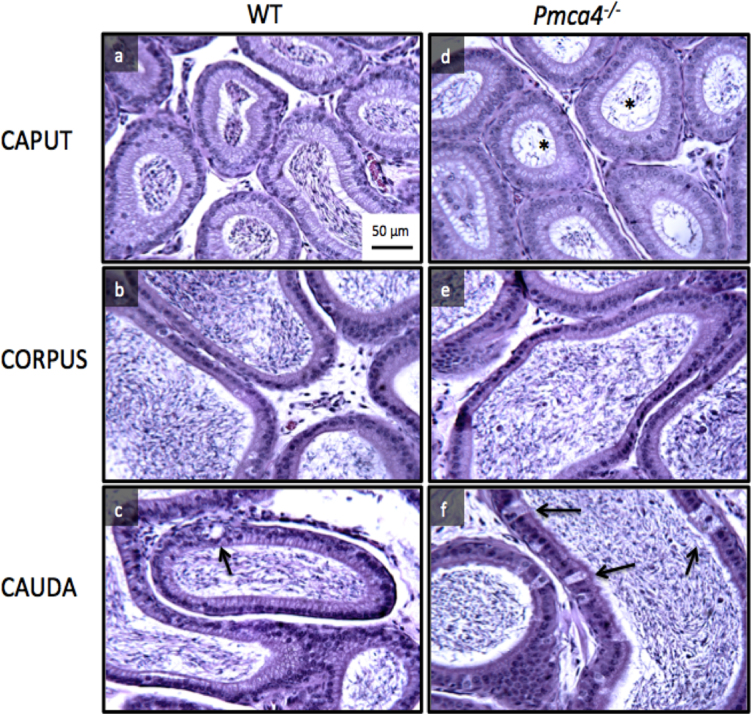
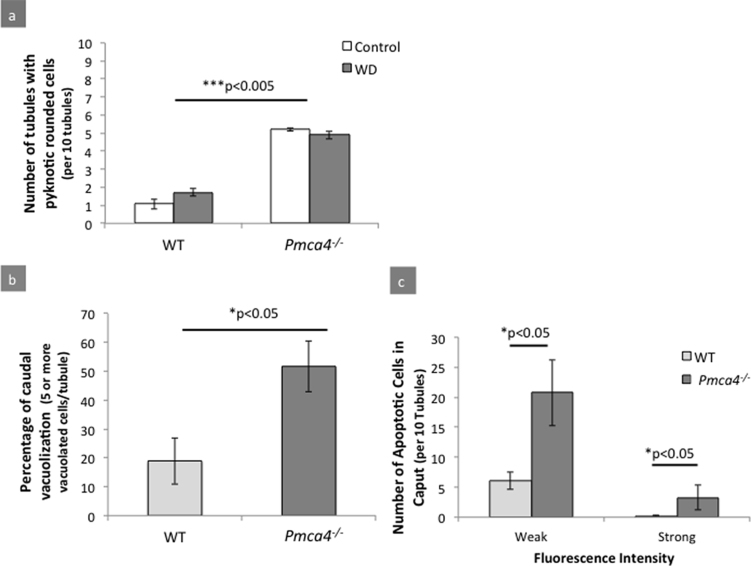
Analysis of H&E staining of the three regions of the epididymis, where sperm mature, revealed differences in untreated WT and Pmca4−/−, with the caput of the latter showing a paucity of germ cells and increased numbers of pyknotic-rounded cells in the tubules (Figs. 6 and 7a-c). However, a two-way ANOVA showed that there was no difference in the number of tubules with pyknotic-rounded cells between diet and control groups, although the increase of these cells in Pmca4−/− compared to WT controls was significant (P < 0.005) (Fig. 7a). Similarly in the caput, TUNEL-positive cells and the number of apoptotic cells were significantly increased in Pmca4 nulls for both fluorescence intensity thresholds (P < 0.05) (Fig. 7c). Finally, no difference was noted between caudal epididymal sections of control and diet-treated groups (data not shown), although those from Pmca4 nulls showed a significant (P < 0.05) increase in intraepithelial vacuolization (Figs. 6 and 7b).
Fig. 6.
Histological analyses reveal variations in epididymal structure and luminal content between WT and Pmca4−/− males, with no effect of diet. Sections were prepared from caput (a, d), corpus (b, e), and cauda (c, f) and stained with hematoxylin and eosin. The germ cell population in the caput epididymis of Pmca4−/− has a paucity of germ cells and contain pyknotic-rounded cells (asterisks). In the caudal epididymis of Pmca4−/− the presence of intraepithelial vacuolization (arrowed) increased, as quantified in Fig. 7b. Scale bar = 50 μm.
Fig. 7.
Pmca4−/− males have significantly more rounded and pyknotic cells in the caput and vacuolized caudal cells than WT males. Histological and functional abnormalities in the epididymis were quantified in WT and Pmca4−/− mice and showed: (a) a significant increase in pyknotic-rounded cells in Pmca4−/− caput (5.21 ± 0.16/10 tubules) compared to WT (1.07 ± 0.47/10 tubules) (Two-way ANOVA: ***p < 0.005, [WT-C (N = 3), WT-D (N = 4), KO-C (N = 3), KO-D (N = 5)], mean ± SEM). (b) Significantly increased numbers of vacuolized cells were seen in Pmca4−/− caudal epididymis (Two-sample T-test: *p < 0.05, N = 4, mean ± SEM). (c) Significant increase in TUNEL positive cells of the caput Pmca4−/− epididymis at weak and strong fluorescence intensities (Two-way ANOVA: *p < 0.05, N = 3, mean ± SEM).
3. Discussion
3.1. A walnut dietary supplement positively impacts murine sperm motility and morphology but did not rescue the defective motility of Pmca4 null sperm
The effects of a walnut-enriched diet that is high in PUFAs and antioxidants have been documented in human sperm, however, to our knowledge, a similar study has not been conducted in a model organism. In the present study, a nutritional intervention in a controlled murine model was performed where the effects of a walnut supplement were directly studied in relation to semen quality parameters. Administration of the diet where walnuts composed 19.6% of caloric intake for a 9–11 week period, in parallel to that in the study in humans (Robbins et al., 2012), caused no change in body weight in the mice. The stability of body weight confirms that the design of the diet accurately parallels that in men who were given a supplement of 75 g of walnuts daily without weight gain, but with significant improvements in serum fatty acid profiles (Robbins et al., 2012).
The diet significantly improved total sperm motility and morphology in WT mouse sperm. The finding of an improvement in these murine sperm parameters with the diet is similar to that reported in humans and supports a role of a walnut dietary supplement in positively impacting sperm function and morphology. It should be noted that previous work has shown the importance of fatty acid profile changes in sperm maturation and differentiation (Rettersøl et al., 2001; Oresti et al., 2010) and a positive relationship between n-3 from diet and normal sperm morphology in humans (Attaman et al., 2012).
With respect to morphology, increased rates of abnormal morphology, particularly flagellar defects, which were largely found in this study, have been shown to be associated with increased ROS and oxidative stress in murine sperm (Smith et al., 2015), as well as DNA damage, although the exact mechanism is unclear (Aziz et al., 2004; Aitken and De luliis, 2010). The significantly increased rates of normal morphology in WT sperm with administration of the walnut supplement suggests that the diet may have the ability to restore damage inflicted by oxidative stress.
While our findings for sperm morphology and motility are consistent with those in the human study, a species difference was detected with respect to sperm vitality, which did not show an improvement in the mouse. This suggests that murine sperm membrane integrity, as detected with eosin-nigrosin staining, is not significantly impacted by the diet. The finding that the rate of acrosome reaction, which is dependent on sperm membrane fluidity, was also unchanged with the diet, supports this conclusion. These observations for WT sperm are consistent with those seen in equine sperm incubated in a ROS generating system where motility was impacted but membrane-related parameters were not (Baumber et al., 2000).
With respect to the species difference on the impact of the diet, it is possible that the control lab-diet fed to mice induces less oxidative stress than the Western-style diet consumed by men, and therefore the walnut supplement has less space for improvement. It is also possible that the population sizes in the two studies may contribute to the difference, as in the human investigation a much larger population was studied.
It was proposed that a walnut supplement, enriched in PUFAs and antioxidants might work to positively impact Pmca4 null sperm which have a loss of progressive and hyperactivated sperm motility (Okunade et al., 2004; Schuh et al., 2004) associated with oxidative stress, resulting from elevated levels of NO and OONO− [unpublished observations]. Our results show that sperm motility was not rescued or even improved in Pmca4 nulls administered the diet, similar to vitality and acrosome reaction rates. It should be noted that the data showed similar rates for total motility in WT and Pmca4−/− uncapacitated control sperm, as reported by Okunade et al. (2004); while the ∼15% progressive motility detected for Pmca4−/− sperm is similar to the 14% reported by Schuh et al. (2004). Loss of progressive motility for Pmca4−/− sperm under capacitating conditions of high intracellular Ca2+ concentration ([Ca2+]i), as reported earlier (Okunade et al., 2004; Schuh et al., 2004) and more recently (Navarrete et al., 2016), was confirmed in the present study.
While the motility defect in Pmca4 null sperm was not impacted by the diet, normal sperm morphology was significantly improved, similar to WT sperm. Our data also showed that the rate of normal morphology in the untreated nulls, studied for the first time, is significantly lower than in the untreated WT. The differential response of motility and normal morphology in Pmca4 null sperm to the diet regimen suggests that different mechanisms are involved in the defects. While oxidative stress may be involved in both, motility could be impacted by other factors such as reduced ATP levels, as seen when PMCA4 activity is reduced in Jam-A null sperm (Aravindan et al., 2012) and altered pH levels. As PMCA4 regulates intracellular pH (pHi) (Di Leva et al., 2008) and this impacts motility (Nishigaki et al., 2014), PMCA4’s absence could implicate altered pH regulation. Motility defects in the nulls could also result from flagellar defects, but this is unlikely to play a major role since the improvement in diet with morphology was not seen with motility. Thus, while motility in Pmca4−/− may arise from one or more of several factors, abnormal morphology may be due mostly to increased ROS and therefore was responsive to the diet.
3.2. Decreased levels of lipid peroxidation accompany the improvement of sperm parameters associated with a walnut-enriched diet
Because of the large numbers of PUFAs present in sperm membrane (Wathes et al., 2007), sperm are particularly vulnerable to lipid peroxidative damage and destabilization of the membrane. The damage to the membrane results in a decrease in the number of functioning PUFAs and an increase in radical by-products such as malondialdehyde (MDA) and 4-hydroxynonenol (Aitken, 1995; Ayala et al., 2014). When MDA levels were assessed in sperm, they indicated that the walnut-enriched diet significantly lowered lipid peroxidation in both WT and Pmca4−/− sperm, as it did for morphological abnormalities. Thus our findings reveal an association between reduced lipid peroxidation and improvement in a sperm characteristic, morphology, which is impacted by elevated ROS levels. Whether or not the dietary supplement interrupts the lipid peroxidation cascade by providing added antioxidant benefits, or replaces damaged PUFAs, or does both, remains to be seen by further studies.
3.3. The walnut–enriched diet did not impact the increased levels of apoptotic activity detected in the testis and epididymis of Pmca4−/−
The importance of diet on testis organization has been seen by the atrophy and tubule degeneration in rats given a diet lacking essential fatty acids or a supplement of linolenic acid (omega-3) only (Leath et al., 1983). In the present study the significant increases in apoptosis in Pmca4−/− spermatogonia and spermatocytes, corroborated by H&E staining, were not impacted by the diet. Histological sections of the testes revealed a lack of organization in the Pmca4−/− tubules and increased space between the germ cells. This finding, which is indicative of a disruption by ROS in Sertoli cell integrity and therefore the Sertoli-Sertoli junctional complexes responsible for tubule shape (Krishnamoorthy et al., 2013), is consistent with oxidative stress seen in the sperm from these animals.
When epididymal histology was analyzed in WT and Pmca4−/− mice the increased damage in Pmca4−/− associated with apoptosis, corroborated via TUNEL assay, and observed for the first time, was not impacted by diet. The damage is however consistent with oxidative stress known to cause peroxidative damage in sperm plasma membranes (Doreswamy et al., 2004; Baker and Aitken, 2005).
4. Conclusions
Our study demonstrates the positive effects of a walnut-enriched diet, accounting for 19.6% of caloric intake and administered for 9–11 weeks, on murine sperm motility and morphology. Although the study confirmed one previously done in humans, there was a species difference as sperm vitality, which was improved in humans, did not improve in the murine model. When administered to Pmca4 null mice, which have sperm motility defects and were shown for the first time to have decreased levels of morphologically normal sperm compared to WT, the diet significantly improved only morphology, suggesting that different contributing factors may be involved in the motility and morphological defects. Lipid peroxidation, an indicator of oxidative stress, was significantly decreased in both WT and Pmca4 null sperm of diet-treated mice, suggesting, for the first time, that a walnut supplement may function to reduce elevated levels of oxidative stress associated with abnormal sperm morphology. The diet did not impact the increased apoptotic activity that was found in both the testes and the epididymis of Pmca4 null mice. To determine if the beneficial effects of the diet can ultimately impact individuals affected with subfertility, further studies of the fertilizing ability in vivo/in vitro of sperm from diet-treated males are required.
5. Materials and methods
5.1. Animals and dietary intervention
Sexually mature male mice (>3 months old) of the FVBN outbred strain were used in the investigations. Pmca4−/− males on the FVBN background were generated from matings of Pmca4+/− animals or Pmca4+/− males and Pmca4−/− females (which are fertile) and were donated by the Shull laboratory (Okunade et al., 2004). The breeding and genotyping of these mice were described previously (Okunade et al., 2004). Studies were approved by the Institutional Animal Care and Use Committee at the University of Delaware (AUP Number: 1181-2015-2) and agree with the Guide for the Care and Use of Laboratory Animals published by the National Research Council of the National Academies, 8th ed., Washington, D.C. All chemicals were purchased from Fisher Scientific Co. (Malvern, PA), Sigma (St. Louis, MO) or Invitrogen (Carlsbad, CA), unless otherwise specified.
Groups of WT and Pmca4−/− males were randomly assigned a control (LabDiet #5015) diet or a parallel diet in which walnuts consisted of 19.6% caloric intake (Purina Animal Nutrition Center, Gray Summit, MO) over a 9–11 week period. This diet is proportional to 75 g/day walnut supplement in humans provided previously (Robbins et al., 2012).
5.2. Sperm recovery and evaluation
Following the 9–11 week period, males were sacrificed via CO2 asphyxiation and the caudal epididymides were removed and placed in 400 μl pre-warmed (to 37 °C) human tubal fluid (HTF with HEPES, Cat. # 2002, Invitrocare, Frederick, MD), a capacitating medium used previously (Aravindan et al., 2012; Shao et al., 2008; Andrews et al., 2015). The tissue was minced and sperm were separated by gravity settling for 10 min. An aliquot of the sperm suspension was immediately assessed blindly for motility by visual estimation using AmScope with ∼200 sperm per sample and a minimum of five fields per specimen videographed. To assess motility under capacitating conditions, sperm were incubated for 90 min at 37 °C in HTF medium and analyzed similarly as before. The mean number of total motility (progressive + no progression) as well as progressively motile sperm were recorded and expressed as a percentage of the total number of sperm.
Vitality of caudal sperm was determined using eosin-nigrosin dye exclusion test as described (World Health Organization, 2010). A 50 μl sperm suspension was mixed with an equal volume of eosin-nigrosin staining solution (0.67 g eosin Y and 0.9 g NaCl were mixed in 100 ml of purified water with gentle heating, and 10 g of nigrosin added, mixed, boiled, cooled and filtered) and allowed to stand for 30 s. The suspension was quickly smeared onto a clean glass slide and allowed to air-dry before examination using brightfield optics on a Zeiss microscope (Carl Zeiss). The number of stained (dead) and unstained (live) sperm of 200 cells were counted on duplicate slides per sample and the percent vitality calculated.
5.3. Hyaluronic acid-enhanced progesterone-induced acrosome reactions and structural sperm defects
Acrosome reaction was induced under physiological conditions as previously described (Morales et al., 2004). Briefly, following separation of the tissues from the sperm suspension by gravity settling, the suspension was incubated at 37 °C in a water bath for 1 h and treated with 100 μg ml−1 hyaluronic acid for 30 min followed by progesterone (3.18 μmol l−1 for 5 min). Treated sperm were pelleted (500 x g, 15 min) and re-suspended in 4% paraformaldehyde and stored overnight at 4 °C.
Sperm were re-suspended in PBS (1,370 mM NaCl, 27 mM KCl, 100 mM Na2HPO4, 20 mM KH2PO4, pH 7.4) and 200 μl of sperm suspension was dragged across a clean glass slide and allowed to air-dry. Slides then were stained with 0.44% Coomassie Brilliant Blue G-250 in 60% methanol-acetic acid for 10 min. Slides were examined microscopically (blindly) to determine presence/absence of the acrosomal cap, as distinguished in Fig. 3b, d, f, and g, in ∼400 sperm/sample.
To assess sperm morphology, an aliquot of sperm suspension was fixed (4% paraformaldehyde), mounted on slides and stained with Coomassie Brilliant Blue G-250 in 60% methanol-acetic acid for 10 min. Sperm (∼400 sperm/sample) were analyzed blindly for morphological abnormalities according to World Health Organization (2010) standards.
5.4. Thiobarbituric acid reactive substances (TBARs)
Lipid peroxidation levels were assessed using a TBARS Assay Kit (Cayman Chemical Company) and fluorometry. Caudal sperm was pelleted (500 x g, 15 min) and homogenized in 1X RIPA buffer (1% Triton X–100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris HCl, 150 mM NaCl) overnight at 4 °C. The suspension was centrifuged (500 g, 15 min) and the supernatant collected for analysis. The protein concentration was determined using a bicinchoninic acid (BCA) assay kit (Pierce).
Malondialydehyde (MDA) levels, indicative of lipid peroxidation, were analyzed according to the manufacturer’s protocol and the fluorescence intensities at an excitation wavelength of 530 nm and an emission wavelength of 550 nm recorded. A standard curve was generated using MDA and protein levels were normalized to 100 μg for analysis. Lipid levels were assessed as a function of protein levels in the lysate.
5.5. Tissue recovery
Caput epididymal tissue and testes from WT and Pmca4−/−, control and diet-treated mice were embedded in optimum cutting temperature (OCT) medium (Tissue Tek, Torrance, CA), and placed on dry ice for 15 min before freezing at −80 °C. TUNEL assays were conducted on prepared frozen sections with the ApopTag Fluorescein In situ Apoptosis Detection Kit (Millipore) according to the manufacturer’s instructions. Briefly, prepared slides were fixed in 1% paraformaldehyde for 10 min at RT before postfixing in pre-cooled ethanol: acetic acid (2:1) for 5 min at −20 °C in a coplin jar followed by two washes in PBS for 5 min each. Following processing, slides were incubated in Tdt (terminal deoxynucleotidyl transferase) enzyme in a humidified chamber at 37 °C for 1 h. After washing in stop/wash buffer, slides were incubated in anti-digoxigenic conjugate for 30 min, washed in PBS, counter-stained with fluorescein and mounted with fluoro-Gel II with DAPI. Slides were stored at −20 °C until analyzed and imaged using a Zeiss LSM 780 confocal microscope (Carl Zeiss). For testes, the number of apoptotic cells in ∼100 tubules total and their location were recorded blindly for each group. For caput tissue, the number of apoptotic cells in ∼100 tubules was determined and separated by fluorescence signal strength, using Image J software (Rasband, 2016).
Regions of the epididymis (caput, corpus, cauda) and testes were fixed in 10% Buffered Formalin solution. Samples were dehydrated, and embedded in paraffin. After processing, sections were stained with hematoxylin and eosin (H & E) for histological analysis.
5.6. Statistical analysis
Two-way ANOVA and Student’s t-tests were performed on the means (± SEM) for replicates [SAS (SAS Institute)]. Significant differences were recorded for P-values <0.05 or less. At least three separate experiments were conducted for each study.
Declarations
Author contributions statement
Lauren S. Coffua: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Patricia A. Martin-DeLeon: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the following grants: NIH-5RO3HD073523 (to P.A.M-D), C.O.B.R.E. (to P.A.M-D), and I.N.B.R.E. (to P.A.M-D.).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would graciously like to thank the California Walnut Commission for donation of walnuts for the study. Thanks are extended to Michael Moore and the Delaware Bioimaging Center for assistance with confocal microscopy and Dr. John McDonald with statistics. We also appreciate the assistance of Joanne Cooper and Dr. Roger Wagner with histological sample preparation and analysis and Drs. Randall Duncan and Gary Laverty for critical analysis of the work. This work was submitted by L.S.C. in partial fulfillment for the requirements of the Master’s degree.
References
- Aitken R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., De luliis G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- Alizadeh A., Esmaeili V., Shahverdi A., Rashidi L. Dietary fish oil can change sperm parameters and fatty acid profiles of ram sperm during oil consumption period and after removal of oil source. Cell J. 2014;16:289–298. [PMC free article] [PubMed] [Google Scholar]
- Alvarez J.G., Storey B.T. Differential incorporation of fatty acids into and loss of fatty acids from phospholipids of human sperm. Mol. Reprod. Dev. 1995;42:334–346. doi: 10.1002/mrd.1080420311. [DOI] [PubMed] [Google Scholar]
- Andrews R.E., Galileo D.S., Martin-DeLeon P.A. Plasma membrane Ca2+ATPase 4 (PMCA4): interaction with constitutive nitric oxide synthases in human sperm and prostasomes which carry Ca2+/CaM-dependent serine kinase (CASK) Mol. Hum. Reprod. 2015;21:832–843. doi: 10.1093/molehr/gav049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindan R.G., Fomin V.P., Naik U.P., Modelski M.J., Naik M.U., Galileo D.S., Duncan R.L., Martin-DeLeon P.A. CASK interacts with PMCA4b and JAM-A on the mouse sperm flagellum to regulate Ca2+ homeostasis and motility. J. Cell. Physiol. 2012;227:3138–3150. doi: 10.1002/jcp.24000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaman J.A., Toth T.L., Furtado J., Campos H., Hauser R., Chavarro J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012;27:1466–1474. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2nonenal. Oxid. Med. Cell. Longev. 2014;2014:1–31. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Saleh R.A., Sharma R.K., Lewis-Jones I., Esfandiari N., Thomas A.J., Agarwal A. Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil. Steril. 2004;81:349–354. doi: 10.1016/j.fertnstert.2003.06.026. [DOI] [PubMed] [Google Scholar]
- Baker M.A., Aitken R.J. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod. Biol. Endocrinol. 2005;3:67. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakos H.W., Henshaw R.C., Mitcherll M., Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil. Steril. 2011;95:1700–1704. doi: 10.1016/j.fertnstert.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Bakos H.W., Mitchell M., Setchell B.P., Lane M. The effect of paternal dietinduced obesity on sperm function and fertilization in mouse model. Int. J. Androl. 2010;34:402–410. doi: 10.1111/j.1365-2605.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- Baumber J., Ball B.A., Gravance C.G., Medina V., Davies-Morel M.C. The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J. Androl. 2000;21:895–902. [PubMed] [Google Scholar]
- Di Leva F., Domi T., Fedrizzi L., Lim D., Carafoli E. The plasma membrane Ca2+ ATPase of animal cells: structure, function and regulation. Arch Biochem. Biophys. 2008;476:65–74. doi: 10.1016/j.abb.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Doreswamy K., Shrilatha B., Rajeshkumar T., Muralidhara Nickel-induced oxidative stress in testis of mice: Evidence of DNA damage and genotoxic effects. J. Androl. 2004;25:996–1003. doi: 10.1002/j.1939-4640.2004.tb03173.x. [DOI] [PubMed] [Google Scholar]
- Griveau J.F., Griveau J.F., LeLannou D. Reactive oxygen species and human spermatozoa physiology and pathology. Int. J. Androl. 1997;20:61–69. doi: 10.1046/j.1365-2605.1997.00044.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom W.J.G., Bell M., Wang R., Sikka S.C. Effect of sodium nitroprusside on sperm motility, viability, and lipid peroxidation. Fertil. Steril. 1994;6:1117–1122. doi: 10.1016/s0015-0282(16)56766-1. [DOI] [PubMed] [Google Scholar]
- Hu F.B. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy G., Selvakumar K., Venkataraman P., Elumalai P., Arunakaran J. Lycopene supplementation prevents reactive oxygen species mediated apoptosis in Sertoli cells of adult albino rats exposed to polychlorinated biphenyls. Interdisp. Toxico. 2013;6:83–92. doi: 10.2478/intox-2013-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leath W.M.F., Northrop C.A., Harrison F.A., Cox R.W. Effect of dietary linoleic and linolenic acids on testicular development in the rat. Quart. J. Exp. Physiol. 1983;68:221–231. doi: 10.1113/expphysiol.1983.sp002714. [DOI] [PubMed] [Google Scholar]
- Morales C.R., Badran H., El-Alfy M., Zhang H., Men H., Martin-DeLeon P.A. Cytoplasmic Localization during Testicular Biogenesis of mRNA for Spam1 (PH-20), a protein involved in Acrosomal Exocytosis. Mol. Reprod. Develop. 2004;69:475–482. doi: 10.1002/mrd.20177. [DOI] [PubMed] [Google Scholar]
- Navarrete F.A., Alvau A., Lee H.C., Levin L.R., Buck J., Leon P.M., Santi C.M., Krapf D., Mager J., Fissore R.A., Salicioni A.M., Darszon A., Visconti P.E. Transient exposure to calcium ionophore enables in vitro fertilization in sterile mouse models. Sci. Rep. 2016;6 doi: 10.1038/srep33589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki T., Jose O., Gonzalez-Cota A.L., Romero F., Trevino C.I., Darszon A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Comm. 2014;450:1149–1158. doi: 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunade G.W., Miller M.L., Pyne G.J., Sutliff R.L., O’Connor K.T., Neumann J.C., Andringa A., Miller D.A., Prasad V., Doetschman T. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 2004;279:33742–33750. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- Oresti G.M., Reyes J.G., Luquez J.M., Osses N., Furland N.E., Aveldano M.I. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J. Lipid Res. 2010;51:2090–2121. doi: 10.1194/jlr.M006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer N.O., Bakos H.W., Owens J.A., Setchell B.P., Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am. J. Physiol. Endocrinol. Metab. 2012;302:E768–E780. doi: 10.1152/ajpendo.00401.2011. [DOI] [PubMed] [Google Scholar]
- Patel R., Al-Dossary A.A., Stabley D.L., Barone C., Galileo D.S., Strehler E.E., Martin-DeLeon P.A. Plasma membrane Ca2+-ATPase 4 in Murine Epididymis: Secretion of Splice Variants in the Luminal Fluid and a Role in Sperm Maturation. Biol. Reprod. 2013;89:1–11. doi: 10.1095/biolreprod.113.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W.S. U.S. National Institutes of Health; Bethesda, Maryland, USA: 2016. ImageJ.http://imagej.nih.gov/ij/ 1997-2016. [Google Scholar]
- Rettersøl K., Haugen T.B., Tran T.N., Christophersen B.O. Studies on the metabolism of essential fatty acids in isolated human testicular cells. Reprod. 2001;121:881–887. doi: 10.1530/rep.0.1210881. [DOI] [PubMed] [Google Scholar]
- Robbins W.A., Xun L., FitzGerald L.Z., Esguerra S., Carpenter C.L. Walnuts improve semen quality in men consuming a Western-style Diet: Randomized Control Dietary Intervention Trial. Bio. Reprod. 2012;87:1–8. doi: 10.1095/biolreprod.112.101634. [DOI] [PubMed] [Google Scholar]
- Schuh K., Cartwright E.J., Jankevics E., Bundschu K., Liebermann J., Williams J.C., Armesilla A.L., Emerson M., Oceandy D., Knobeloch K.P. Plasma membrane Ca2+ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 2004;279:28220–28226. doi: 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- Shao M., Ghosh A., Cooke V.G., Naik U.P., Martin-DeLeon P.A. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev. Biol. 2008;313:246–255. doi: 10.1016/j.ydbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U., Conine C.C., Shea J.M., Boskovia A., Derr A.G., Bing X.Y., Belleannee C., Kucukural A., Serra R.W., Sun F. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.A., Michael R., Aravindan R.G., Dash S., Shah S.I., Galileo D.S., Martin-DeLeon P.A. Anatase titanium dioxide nanoparticles in mice: evidence for induced structural and functional sperm defects after short-, but not long-, term exposure. Asian J. Androl. 2015;17:261–268. doi: 10.4103/1008-682X.143247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathes D.C., Abayasekara D.R.E., Aitken R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- Wennemuth G., Babcock D.F., Hille B. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 2003;122:115–128. doi: 10.1085/jgp.200308839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirleitner B., Vanderzwalmen P., Stecher A., Spitzer D., Schuff M., Schwerda D., Bach M., Schechinger B., Zech N.H. Dietary supplementation of antioxidants improves sperm quality of IVF patients in terms of motility, sperm count, and nuclear vacuolization. Int. J. Vitam. Nut. Res. 2012;82:391–398. doi: 10.1024/0300-9831/a000136. [DOI] [PubMed] [Google Scholar]
- World Health Organization . fifth ed. 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]