Abstract
Renal cell cancer (RCC) is a prevalent and lethal disease. At time of diagnosis, most patients present with localized disease. For these patients, the standard of care includes nephrectomy with close monitoring thereafter. While many patients will be cured, 5-year recurrence rates range from 30% to 60%. Furthermore, nearly one-third of patients present with metastatic disease at time of diagnosis. Metastatic disease is rarely curable and typically lethal. Cytotoxic chemotherapy and radiation alone are incapable of controlling the disease. Extensive effort was expended in the development of cytokine therapies but response rates remain low. Newer agents targeting angiogenesis and mTOR signaling emerged in the 2000s and revolutionized patient care. While these agents improve progression free survival, the development of resistance is nearly universal. A new era of immunotherapy is now emerging, led by the checkpoint inhibitors. However, therapeutic resistance remains a complex issue that is likely to persist. In this review, we systematically evaluate preclinical research and clinical trials that address resistance to the primary RCC therapies, including anti-angiogenesis agents, mTOR inhibitors, and immunotherapies. As clear cell RCC is the most common adult kidney cancer and has been the focus of most studies, it will be the focus of this review.
Keywords: Renal cell carcinoma, Resistance, Angiogenesis, Immunotherapy, mTOR
2. Resistance to Anti-Angiogenesis Therapies in Renal Cell Carcinoma
2.1 Background
Hanahan and Weinberg outlined over 15 years ago the principles necessary for the uncontrolled proliferation of cells causing tumor formation1. Included in their original six hallmarks of cancer was angiogenesis, or the formation of new blood vessels. While initially small tumor populations may live by simple diffusion of nutrients, data show that tumor formation and growth eventually requires neovascularization2. Targeting angiogenesis was hypothesized to be especially important in clear cell renal cell carcinoma (RCC), a highly vascular tumor, in part due to its molecular hallmark of VHL inactivation. VHL, or the Von-Hippel Lindau gene, encodes the substrate recognition component of an E3 ubiquitin ligase complex3. When considering both gene mutation and promoter hypermethylation, VHL function is lost in as many as 90% of clear cell RCC tumors, leading to the accumulation of the transcription factor hypoxia inducible factor (HIF)4. HIF triggers an intense hypoxic and pro-angiogenic response3. Targeting this pro-angiogenic response heralded a new era in the therapy of these cancers, dominated by the use of potent single agent anti-angiogenics. While clearly efficacious for many patients in inducing response and establishing disease control for a period averaging several months, as many as 10% of patients demonstrate intrinsic resistance with lack of response to first-line anti-angiogenics5. These patients have a poor prognosis even with subsequent lines of therapy6. For patients that demonstrate an initial response to sunitinib and other similar anti-angiogenic therapies, the response is often not durable. Potential mechanisms of acquired resistance include activation of alternative or compensatory angiogenic pathways and increased tumor invasiveness (Fig. 1)7. In this review, we will focus on acquired or adaptive resistance mechanisms and new therapies designed to address acquired resistance (Table 1).
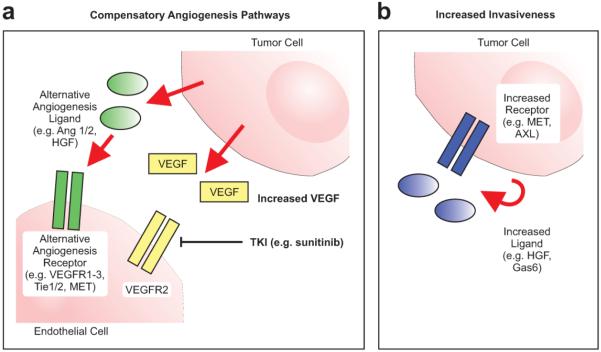
Figure 1. Mechanisms of anti-angiogenesis resistance.
Tumors are capable of continued survival, growth, and proliferation in the setting of persistent VEGFR2 inhibition by several mechanisms including a) activating alternative, compensatory pathways that can continue to support tumor neovascularization and b) reprogramming tumor cells so that they become more invasive, invade deeper into normal tissue, and thus survive using the normal, physiologic vasculature. Ang = angiopoietin, VEGF = vascular epithelial growth factor, VEGFR = vascular epithelial growth factor receptor, TKI = tyrosine kinase inhibitor, HGF = hepatocyte growth factor.
Table 1.
Clinical Strategies for Acquired Resistance in Renal Cell Carcinoma
|
Mechanism of
Resistance |
Therapeut
ic Target |
Design / Results | Reference |
|---|---|---|---|
|
Compensatory
Angiogenesis Pathways |
VEGFR1-3 | Axitinib with superior PFS relative to sorafenib in sunitinib-resistant disease |
Rini et al., 2011 |
| VEGFR/F GFR + mTOR |
Lenvatinib + everolimus improved PFS relative to everolimus alone |
Motzer et al., 2015 |
|
| ALK-1 + VEGFR |
Dalantercept + axitinib in heavily pretreated RCC patients |
NCT01727 336 |
|
| VEGF | VEGFR TKI refractory patients had stable disease on aflibercept |
Pili et al., 2015 |
|
|
| |||
|
Increased Tumor
Invasion |
MET/AXL | Cabozantinib improves PFS and ORR compared to everolimus |
Choueiri et al., 2015 |
|
| |||
|
Persistent AKT
Activation |
AKT | AKT allosteric inhibitor MK2206 not superior to everolimus |
Jonasch et al., 2013 |
|
| |||
|
Compensatory
mTORC2 Signaling |
mTORC1 & mTORC2 |
Apitolisib more toxic, no improvement in PFS compared to everolimus |
Powles et al., 2016 |
|
| |||
|
Immune
Suppression |
PD-1 | Nivolumab improved survival for VEGFR TKI refractory clear cell RCC patients compared to everolimus |
Motzer et al., 2015 |
| PD-1 + VEGF |
Pembrolizumab + aflipercept in VEGFR TKI refractory RCC patients |
NCT02298 959 |
|
| PD-1 + CTLA-4 |
Pembrolizumab + interferon alpha- 2b or ipilimumab in refractory clear cell RCC |
NCT02089 685 |
|
VEGFR = Vascular Epithelial Growth Factor Receptor. FGFR = Fibroblast Growth Factor Receptor. ALK-1 = Activin Receptor Like Kinase.
VEGF = Vascular Epithelial Growth Factor. PFS = Progression Free Survival. RCC = Renal Cell Carcinoma. TKI = Tyrosine Kinase Inhibitor.
MET = Hepatocyte Growth Factor Receptor. AXL = AXL Receptor Tyrosine Kinase.
ORR = Overal Response Rate. AKT = AKT Serine/Threonine Kinase.
mTORC = Mammalian Target of Rapamycin. PD-1 = Programmed Cell Death 1. PDL-1 = Programmed Death Ligand 1. CTLA-4 = Cytotoxic T-Lymphocyte Associated Protein 4.
2.2 Adaptive Resistance via Compensatory Angiogenesis Pathways
The adaptive resistance that emerges in RCC during anti-angiogenesis therapy is distinct among targeted therapies. Most receptor targeted therapies develop emergent resistance through an acquired point mutation, such as occurs frequently in targeting EGFR8. These resistance mutations alter the protein structure, decrease drug binding, or allow for continued receptor signaling via other means. Conversely, resistance to VEGFR TKIs often develop alongside continued target inhibition. A straightforward explanation implicates the fact that many of the leading VEGFR TKIs, such as sunitinib, most potently target VEGFR2. However, other VEGFR proteins exist, specifically VEGFR1 and VEGFR3, which may share redundant functions that are likely important in tumor angiogenesis9. Consistent with this theory, simultaneous inhibition of multiple VEGFR proteins more potently inhibits angiogenesis than inhibition of a single subtype9.
Thus, VEGFR1 and VEGFR3 have emerged as potential functional drivers of resistance and thus desirable pharmacologic targets. To this end, axitinib, a potent inhibitor of VEGFR1-3, was compared to sorafenib, a multi-targeted TKI whose predominant VEGFR target is VEGFR2, in the AXIS study, a phase III second line clinical trial in clear cell RCC patients10. In this study, the axitinib arm had a higher PFS (6.7 versus 4.7 months, one-sided p<0.0001) and established the utility of second-generation angiogenesis inhibitors with broader activity to overcome sunitinib resistance10. Thus, data support the importance of broadly targeting VEGFR1-3 as a strategy to treat acquired anti-angiogenesis resistance.
Tumors, however, also utilize non-VEGFR angiogenesis pathways as resistance mechanisms. For example, increased signaling through fibroblast growth factor receptor (FGFR) in the context of VEGFR2 inhibition has been associated with decreased PFS (p=0.0452) in RCC patients receiving sorafenib11. A recent phase II trial of patients progressing after VEGF inhibition compared lenvatinib, a combined VEGFR1-3 and FGFR inhibitor, either alone or in combination with everolimus, to everolimus alone12. The combination was associated with a longer PFS when compared to everolimus (median PFS 14.6 months versus 5.5 months, p=0.0005) but not compared to lenvatinib alone (7.4 months, p=0.12). These results suggest activity for FGFR-targeted drugs in this clinical context and led to FDA-approval of the combination for anti-angiogenic resistant patients.
Other pathways likely promote tumor angiogenesis in the context of persistent VEGFR2 inhibition. Angiopoietin-1/2 are glycoproteins that are implicated in both physiologic and tumor angiogenesis13. Trebananib is a fusion protein which disrupts the interaction of angiopoietin-1/2 with its receptors, Tie1/2. This agent may have activity in RCC14, though it has not been well tested in anti-angiogenesis resistant disease. Another angiogenesis pathway of interest is ALK-1, a pro-angiogenic receptor in the TGF-beta family15. An ALK-1 ligand trap, dalantercept, is being tested in combination with axitinib in heavily pretreated RCC patients (NCT01727336). VEGF, the canonical ligand for VEGFR, continues to be investigated as a mediator of anti-angiogenesis resistance with multiple trials exploring the VEGF trap aflibercept (NCT02298959)16. Sunitinib resistance has been shown to be mediated by specific epigenetic changes, with either increases in sunitinib dosing or targeted inhibition of specific histone-modifying enzymes both proving capable of eliciting tumor response17. Thus, researchers continue to aggressively target alternative pro-angiogenic pathways as mediators of anti-angiogenesis resistance.
2.3 Adaptive Resistance via Increased Tumor Invasiveness
Increased invasiveness is an adaptation of cancer cells to decreased angiogenesis that allows them to invade into normal tissue and rely on the normal vasculature for metabolic demands7. The receptor tyrosine kinase (RTK) MET may be important in this pathway and thus a promoter of anti-angiogenesis resistance. A patient-derived xenograft model of sunitinib-resistant clear cell RCC was shown to have increased MET expression and phosphorylation relative to sunitinib-sensitive models18. Cabozantinib, a TKI whose targets include VEGFR and MET, was compared to everolimus in clear cell RCC patients with progression on prior VEGFR TKI in the METEOR trial19. Significant improvement in PFS (7.4 versus 3.8 months, p<0.001) and ORR (21% versus 5%, p<0.001) was observed in the cabozantinib arm. Thus, MET is emerging as an important target in the treatment of anti-angiogenesis resistance.
Similarly, the proto-oncogene AXL is a transmembrane RTK to ligand Gas6 that participates in mitogenic signaling, cell adhesion, chemotaxis, and angiogenesis and is highly expressed in RCC20,21. Similar to MET, increased AXL expression was associated with poor clinical outcomes and was over-expressed in pre-clinical models of sunitinib resistance22. Inhibition of AXL or MET in these models impaired the invasive, prometastatic behavior of the sunitinib-resistant cells and restored sunitinib sensitivity and novel inhibitors of AXL signaling are in preclinical development23. Given these observations, important questions remain in order to understand the role of AXL and MET in anti-angiogenesis resistant RCC. Do these RTKs drive anti-angiogenesis resistance via increased invasiveness and promotion of an epithelial-to-mesenchymal transition, a process known to be important in RCC anti-angiogenesis resistance24,25? Alternatively, do AXL and MET drive resistance through enhanced angiogenesis, alternative mechanisms, or some combination? Clinically, what is responsible for the activity seen for cabozantinib in anti-angiogenesis resistant disease? Not only does cabozantinib potently inhibit VEGFR and MET, but it also has activity against AXL. A better understanding of such issues is important as investigators continue to seek improved therapies and biomarkers for anti-angiogenesis resistant RCC.
2.4 mTOR Inhibitors in the Treatment of Anti-Angiogenesis Resistance
Significant evidence exists supporting the role for mTOR signaling in RCC tumorigenesis. AKT/mTOR activation has been seen in preclinical models of RCC anti-angiogenesis resistance26. In these studies, pharmacologic inhibition of the AKT/mTOR pathway was able to overcome sunitinib resistance. Indeed, the mTOR inhibitor everolimus was shown to be superior to placebo in RCC patients who previously progressed on anti-angiogenesis therapies27. Furthermore, the combination of lenvatinib and everolimus offered superior PFS relative to lenvatinib alone, suggesting that the everolimus is contributing significantly to the efficacy of this newly-approved combination12. Whether new generation drugs that target multiple components of the PI3K/mTOR pathway will demonstrate increased activity in anti-angiogenesis resistant disease remains to be answered.
2.5 Conclusion of Anti-Angiogenesis Resistance
The VEGFR TKIs have dominated RCC therapeutics since their introduction. Given their overall tolerability and proven efficacy, they are likely to remain an important component of the medical oncologist’s metastatic RCC armamentarium. Thus, understanding how tumors thrive in the context of VEGFR inhibition and how to therapeutically target compensatory pathways remains an important area of RCC research. New druggable resistance mechanisms are emerging that require further exploration, such as sunitinib-induced epigenetic changes that, when reversed, may increase sunitinib sensitivity28. As shown, the potential mechanisms of resistance are numerous and likely to be distinct for different patients29. Even for the individual patient, resistance mechanisms appear to be capable of changing over time as evidenced by the marginal success of rechallenge with anti-angiogenesis agents30. Thus, to maximize the impact in patient care, we need accompanying biomarkers that demonstrate activation of the targeted pathways in individual patients.
3. Renal Cell Carcinoma Resistance to mTOR Inhibitors
3.1 The PI3K/AKT/mTOR Pathway Dysregulation in Renal Cell Carcinoma
The phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway is a critical driver of cellular protein synthesis, cell cycle and metabolism31. Prominent among the many downstream targets of PI3K is the serine/threonine kinase AKT. While activated by PI3K, optimal AKT activation is achieved by additional phosphorylation by mTOR complex 2 (mTORC2)31 (Fig. 2). Activated AKT phosphorylates a large number of substrates, including tuberous sclerosis complex (TSC) 1/2, which releases inhibition of mTOR complex 1 (mTORC1). mTORC1 is involved in critical cellular bioenergetic pathways such as protein synthesis and glucose metabolism. The PI3K/AKT/mTOR pathway is commonly dysregulated in human cancer including clear cell RCC32. Given these observations, efforts to pharmacologically modulate the PI3K/AKT/mTOR pathway in cancer and particularly RCC have been robust over the years.
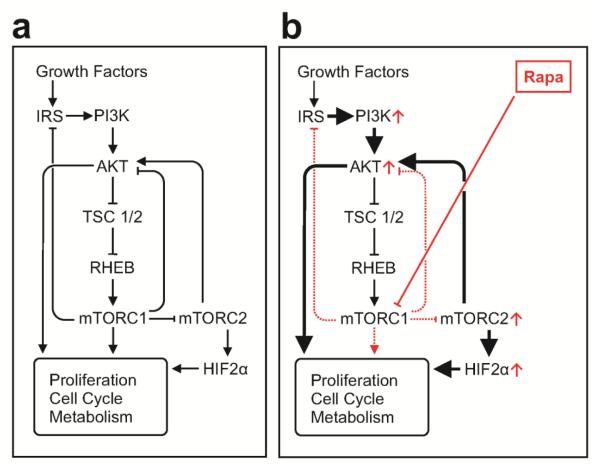
Figure 2. Regulation of the PI3K/AKT/mTOR Pathway.
The PI3K/AKT/mTOR pathway is critical to multiple cellular functions and is thus tightly regulated under a) physiologic conditions. However, this pathway becomes dysregulated through a variety of mechanisms in tumors. b) While sometimes an effective anti-tumor maneuver, inhibiting mTORC1 with rapamycin analogs typically leads to resistance through a variety of mechanisms including loss of inhibition of mTORC2, feedback activation of PI3K and AKT, and up-regulation of HIF-2 alpha. RAPA = rapamycin analogs.
3.2 Targeting Resistance to mTOR Inhibitors in Renal Cell Carcinoma
Everolimus and temsirolimus, the approved rapamycin analogs for RCC, are classically considered to be mTORC1-specific33. Not only do the rapamycin analogs not inhibit mTORC2, they may relieve mTORC1-mediated suppression of mTORC234. mTORC2 activation has been shown to increase expression of the clear cell RCC tumor driver HIF-2α and thus may promote tumor growth and rapamycin analog resistance35.
In addition, compensatory activation of PI3K and AKT has been observed in the context of mTORC1 inhibition36. Thus, PI3K, AKT, and mTORC2 have emerged as candidates for mediating resistance to the rapamycin analogs (Fig. 2).
Interestingly, patterns of rapamycin analog resistance may be influenced by the genomics of the individual tumor. For example, in a retrospective analysis of RCC patients exhibiting durable disease control with rapamycin analogs, somatic mutations in TSC and/or mTOR were identified in 3 of 5 patients37. Thus, further work is needed to explore strategies to target the late resistance that emerges in some patients with somatic mutations in this pathway.
Based on the discussed mechanisms, strategies to prevent and treat mTORC1 inhibitor resistance have focused on different members of the PI3K/AKT/mTOR pathway. The AKT inhibitor perifosine was evaluated in advanced RCC, but no advantage was observed relative to established second line treatments38. In a phase II study in RCC patients who had failed prior therapies, the AKT allosteric inhibitor MK2206 was not superior to everolimus, however notable responses to MK2206 were seen in a subset of patients39. The role of AKT inhibitors in the care of RCC patients therefore remains to be defined.
Combined inhibition of mTORC1 and mTORC2 in addition to mTORC1 would potentially prevent reactive mTORC2 signaling, limit reactive AKT and HIF-2α induction, and may limit the development of resistance to rapamycin analogs (Fig. 2). A phase II trial comparing the dual mTORC and PI3K inhibitor apitolisib to everolimus in VEGFR-TKI resistant RCC patients demonstrated superior PFS for everolimus and increased grade 3/4 toxicity for apitolisib40. While informative, this phase II trial had important design flaws that hinder our ability to draw definitive conclusions. More work is necessary to understand whether the approach of targeting multiple members of the PI3K/AKT/mTOR pathway is safe and effective for overcoming resistance.
4. Immunotherapy for Renal Cell Carcinoma: Resistance and Novel Therapeutic Approaches
4.1 Regulation of the Anti-Tumor Immune Response in Renal Cell Carcinoma
Evidence continues to accumulate that a patient’s immune system can play an important antitumor role leading to the theory that cancers must evade the host immune system in order to flourish1. RCC in particular may be capable of eliciting an immune response, thus requiring the tumor to develop evasion mechanisms to proliferate. Spontaneous remissions of RCC have been observed, suggesting endogenous immune mediated anti-tumor response41. Furthermore, it has been observed that higher proliferative capacity of intratumoral CD8+ T cells correlates with improved RCC patient survival42. Therapies given to patients to either broadly stimulate the host immune response (e.g. high dose interleukin-2) or target specific immune evasion pathways (e.g. immune checkpoint inhibitors; CPI) have produced responses and thus further strengthen the evidence that RCC is capable of eliciting an immune response that can be re-trained for patient benefit43. Continuing our focus on drug resistance, we will consider the use of immunotherapy in anti-angiogenesis resistant disease and RCC that has progressed on prior immunotherapy regimens.
The roles of distinct immune cell types in mediating anti-tumor immunity are underexplored. Effector T (Teff) cells, such as CD8+ cytotoxic lymphocytes, can provide specific immunity against infections and tumors44. Proliferation and differentiation of Teff cells requires cytokines such as interleukin-2 (IL-2) and can be modulated via “checkpoint” pathways such as the programmed cell death-1 (PD-1) signaling pathway45. While IL-2 directly supports T cell differentiation and effector functions, PD-1 signaling inhibits T cell proliferation, cytokine production and suppresses key metabolic pathways46,47. Tumor cells often express PD-1 ligand (PDL-1) that activates PD-1 and thus can contribute to tumor associated T cell dysfunction48. Contemporary attempts to modulate and improve T cell mediated anti-tumor immunity in metastatic RCC have therefore focused on relieving the functional exhaustion of anti-tumor T cells through the use of CPIs.
4.2 Immunotherapy for the Treatment of Anti-Angiogenesis Resistant Renal Cell Carcinoma
The net impact of anti-angiogenesis therapies on the anti-RCC immune response remains unclear. Liu et al. identified increased T cell infiltration in primary RCC tumors treated with anti-angiogenesis therapies49. This T cell infiltration consisted of both cytotoxic CD8(+) Teff cells as well as CD4(+)FOXP3(+) regulatory T cells, a population capable of suppressing Teff cells. In addition, increased PDL-1 was also observed in anti-angiogenesis treated tumors as well as sunitinib-treated RCC cell lines and xenografts. Thus, while anti-angiogenesis therapies seem to have a positive effect on the anti-tumor immune response by stimulating tumor infiltration by Teff cells, this effect may be blunted by concomitant recruitment of immunosuppressive immune cells and up-regulation of tumor cell PDL-1 that inhibits Teff cells49.
Strategies targeting the immunosuppressive PD-1/PDL-1 signaling in anti-angiogenesis resistant disease in order to relieve Teff cell suppression are emerging. An initial study by Brahmer et al. included patients with various tumor types, including RCC. The anti-PD-1 antibody nivolumab was well tolerated with evidence of anti-tumor activity50. In another study, nivolumab produced an ORR of 27% in RCC patients (the majority of whom had progressed on prior anti-angiogenesis therapies)51. Several of these responses were durable in nature, lasting over 12 months. Another phase II trial with metastatic RCC patients previously treated with anti-angiogenesis therapies identified a 20% ORR with nivolumab52. A large, phase III trial (Checkmate 025) compared nivolumab with everolimus in 821 RCC patients with anti-angiogenesis resistant disease53. Nivolumab treatment resulted in greater ORR (25% versus 5%, p<0.001) and longer OS (25.0 months versus 19.6 months, p=0.002) compared to everolimus. Nivolumab has now been approved to treat patients with metastatic RCC whose disease progressed on prior anti-angiogenic therapy.
Rather than reserving CPIs like nivolumab for patients who are resistant to anti-angiogenesis therapies, several investigators are seeking to combine CPIs and anti-angiogenesis drugs in an attempt to increase response rates and prevent or delay resistance. Several lines of data provide rationale for this innovative combination. The previously referenced study by Liu et al. noted an increase in Teff cells in tumors treated with anti-angiogenesis agents. Thus, if one could couple this increased Teff cell infiltration with strategies to block the immunosuppressive PD-1/PDL-1 signaling, the result could theoretically be synergistic anti-tumor response49.
VEGFR TKIs may act through multiple pathways to increase the anti-tumor effects of immune therapies (Fig.3). As shown by Voron et al., tumor infiltrating Teff cells also express VEGFR1 and VEGFR2. VEGF-A produced in the tumor microenvironment signals through VEGFR1/2 to enhance expression of PD-1 and other inhibitory receptors involved in Teff cell suppression. Moreover, T cell PD-1 expression was decreased after anti-angiogenic therapy54. Because VEGFR TKIs may also increase the immune suppressive PD-L1 on tumor cells49 their ability to simultaneously decrease PD-1 on Teff cells appears paradoxical. However, the direct effects of VEGFR TKI on T cells may strengthen the rationale of simultaneous rather than serial treatment of RCC tumors with VEGFR TKI and immune therapies. Several clinical trials, detailed below, are testing this hypothesis that VEGFR TKIs may augment the immune-stimulating properties of CPIs.
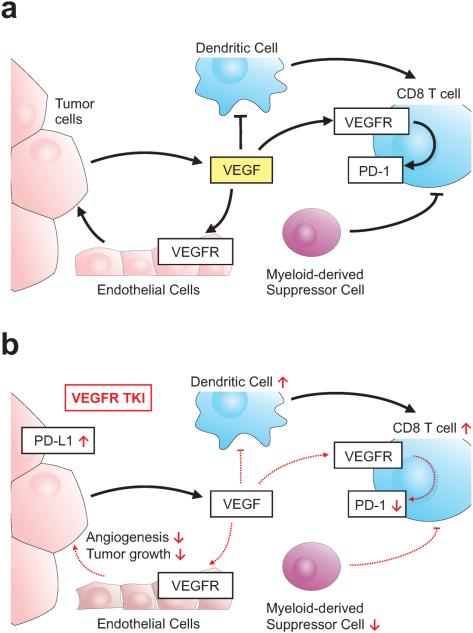
Figure 3. Impact of VEGF on Anti-Tumor T Cell Function.
Vascular epithelial growth factor (VEGF) produced in the tumor microenvironment has multiple influences on the anti-tumor immune response including a) inhibition of dendritic cell function and enhancing PD-1 expression on T lymphocytes. b) Evidence suggests that VEGFR tyrosine kinase inhibitors (TKIs) may promote some aspects of the anti-tumor immune response by reducing PD-1 expression on lymphocytes, reversing VEGF-induced dendritic cell inhibition, and inhibiting myeloid-derived suppressor cell function. These pro-immune properties of VEGFR TKIs though are balanced by evidence that these drugs can increase PDL-1 expression on tumor cells. Clinical trials are underway to evaluate the combination of VEGFR TKIs and PD-1/PDL-1 check point inhibitors.
VEGF signaling in cell populations that are able to modulate Teff activation, such as dendritic cells or myeloid-derived suppressor cells (MDSCs) may also influence the outcome of therapies combining VEGFR inhibition and immunomodulation (Fig.3). Interestingly, VEGF-mediated dendritic cell dysfunction may be important in metastatic cancer patients55. In addition, VEGFR TKI therapy has the potential to modulate antitumor immunity by reversing MDSC-mediated immunosuppression56. MDSCs are elevated in the blood of metastatic RCC patients and sunitinib treatment nearly normalized their levels. The decline of MDSCs was associated with improved T cell function and reduced numbers of immunosuppressive T regulatory cells. In vitro treatment of human MDSCs with sunitinib blocked their suppressive function56. While the molecular mechanisms of VEGF on tumor-associated immune cells remain underexplored, these results suggest that the combination of anti-angiogenic agents with CPIs may be complementary in RCC.
Several ongoing clinical trials are evaluating combination regimens that include CPIs and anti-angiogenesis therapies. A combination treatment of nivolumab with either pazopanib or sunitinib is currently being evaluated in a phase I trial for untreated metastatic RCC patients (NCT01472081). Preliminary data show a manageable toxicity profile and encouraging anti-tumor activity with both combinations57. Specifically, the response rate by the first assessment (6 weeks) was 41% in the sunitinib arm and 56% in the pazopanib arm. Other combinations of CPIs and anti-angiogenesis therapies are also being investigated (NCT01984242, NCT02420821). While many trials target untreated patients, others are testing this combination of anti-angiogenesis therapies and CPIs in anti-angiogenesis resistant disease (NCT02298959). Results from this innovative treatment strategy are eagerly anticipated.
4.3 Treatment of Renal Cell Carcinoma after Progression on Prior Immunotherapy
With the increased utilization of immunotherapies, an important question arises about “cross-resistance” of immunotherapies. Hypothetically, RCC tumors that progressed on prior immunotherapy may have developed immune escape mechanisms that predispose to failure of a subsequent immunotherapy. On the other hand, combination immunotherapy could lead to greater efficacy. As promising as CPIs and other new cancer therapies appear, resistant disease is likely to persist as a major cause of mortality in RCC. Thus, understanding the biology and treatment options for these patients is a high priority.
Returning to the cytokine era, it was observed that interferon-α patients were less likely to respond to HD-IL243. However, while some degree of cytokine cross-resistance may exist, the data suggest that cross-resistance among cytokines and CPIs is less likely. For example, in a phase II trial of nivolumab in metastatic RCC, approximately 25% of patients had previously progressed on HD-IL252. Conversely, a case report documents a metastatic RCC patient who failed CPI but had a near-complete response to HD-IL258. Thus, while ideally prospective studies would address the issue of cross-resistance between CPIs and cytokines, the available evidence suggests CPIs have activity in cytokine-resistant disease and contemporary CPI trials are proceeding without prior cytokine therapies as an exclusion criterion (NCT01472081, NCT02089685).
New antibodies targeting PD-1, PDL-1, and CTLA-4 are being rapidly developed. In addition, blockade of other T cell immunoregulatory receptors, such as TIM3 or LAG3, are being investigated in pre-clinical and clinical studies59. Therefore, a pressing question is emerging: can resistance to one CPI be overcome by administration of a different CPI either in monotherapy or combination? This issue is difficult to address, as prior T cell modulating antibody therapy is a common exclusion criterion in RCC immunotherapy trials. However, as additional CPIs become approved, more patients are likely be exposed to sequential CPIs, which will lend to our collective experience in this arena. For now, most of the data are found in the melanoma literature. Several melanoma trials have documented responses in patients who had previously progressed on ipilimumab by switching to a different CPI or CPI combination60,61. Thus, the melanoma experience suggests that patients that progressed on CPI therapy may benefit from the use of a subsequent CPI, which is a paradigm worthy of testing in RCC.
4.4 Conclusion
The RCC CPI era is in full motion with the approval of nivolumab for RCC patients who had progressed on prior anti-angiogenesis therapies53. Currently, there is an explosion of basic, translational, and clinical research focusing on the optimal utilization of this new class of T cell modulators. As progress continues, CPIs are likely to move past the treatment of anti-angiogenesis resistance and into the arena of first line therapies. However, as the use of these new therapies expands, one can expect that resistance to CPIs will also become a more pressing clinical issue. New strategies are emerging to address this emerging clinical need, including new agents and combinations. The goal of these investigative strategies is to optimize CPI therapy and target resistance mechanisms.
5. Summary
Tumors, in particular renal cell carcinomas, are complex systems with great molecular and genetic heterogeneity62. Furthermore, by their very nature, cancers are genetically unstable. Collectively, these factors likely contribute to the remarkable ability of cancers to adapt, evade, and resist efforts to cure patients. Resistance is likely to continue to be an important issue in the medical oncology clinic for the foreseeable future. As we have demonstrated, numerous resistance mechanisms exist, even among patients treated with the same medications. This malleability of the tumor is enhanced by the polyclonal molecular, genomic, and epigenetic nature of RCC, which theoretically facilitates tumor evolution when faced with a selective pressure (i.e. therapy). Future efforts should focus on identifying the relevant resistance pathways in the individual patient through the use of novel biomarkers so as to personalize treatment plans. Such work will improve our ability to understand, therapeutically target, and even prevent resistance mechanisms that emerge in these ever-adapting tumors.
Highlights.
Therapeutic resistance is a common problem in the treatment of kidney cancer
Resistance mechanisms are diverse, even among patients treated with the same drugs
Therapies for relapsed patients should be tailored to the resistance mechanism
6. Acknowledgements
SMH is supported by the Urology Care Foundation Research Scholar Award. WKR is supported by the NCI, K24 CA172355. KEB is supported by a Cancer Research Institute postdoctoral fellowship and the Brock Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. doi:10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. doi:10.1056/nejm197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson ML, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14:4726–4734. doi: 10.1158/1078-0432.CCR-07-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England journal of medicine. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Busch J, et al. Intrinsic resistance to tyrosine kinase inhibitors is associated with poor clinical outcome in metastatic renal cell carcinoma. BMC cancer. 2011;11:295. doi: 10.1186/1471-2407-11-295. doi:10.1186/1471-2407-11-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. doi:10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balak MN, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. doi:10.1158/1078-0432.ccr-06-1570. [DOI] [PubMed] [Google Scholar]
- 9.Tammela T, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. doi:10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. doi:10.1016/s0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 11.Ho TH, et al. The impact of FGFR1 and FRS2alpha expression on sorafenib treatment in metastatic renal cell carcinoma. BMC cancer. 2015;15:304. doi: 10.1186/s12885-015-1302-1. doi:10.1186/s12885-015-1302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. The Lancet. Oncology. 2015;16:1473–1482. doi: 10.1016/S1470-2045(15)00290-9. doi:10.1016/s1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, et al. The role of angiopoietins as potential therapeutic targets in renal cell carcinoma. Translational oncology. 2014;7:188–195. doi: 10.1016/j.tranon.2014.02.003. doi:10.1016/j.tranon.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkins MB, et al. Trebananib (AMG 386) in Combination With Sunitinib in Patients With Metastatic Renal Cell Cancer: An Open-Label, Multicenter, Phase II Study. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.60.6012. doi:10.1200/jco.2014.60.6012. [DOI] [PubMed] [Google Scholar]
- 15.Hawinkels LJ, Garcia de Vinuesa A, Ten Dijke P. Activin receptor-like kinase 1 as a target for anti-angiogenesis therapy. Expert opinion on investigational drugs. 2013;22:1371–1383. doi: 10.1517/13543784.2013.837884. doi:10.1517/13543784.2013.837884. [DOI] [PubMed] [Google Scholar]
- 16.Pili R, et al. Randomized phase II study of two different doses of AVE0005 (VEGF Trap, aflibercept) in patients (pts) with metastatic renal cell carcinoma (RCC): An ECOG-ACRIN study [E4805] J Clin Oncol. 2015;33 doi: 10.1016/j.clgc.2017.04.023. abstr 4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. doi:10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciamporcero E, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Molecular cancer therapeutics. 2015;14:101–110. doi: 10.1158/1535-7163.MCT-14-0094. doi:10.1158/1535-7163.mct-14-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choueiri TK, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. doi:10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–3455. doi: 10.1038/onc.2009.212. doi:10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 21.Chung BI, Malkowicz SB, Nguyen TB, Libertino JA, McGarvey TW. Expression of the proto-oncogene Axl in renal cell carcinoma. DNA and cell biology. 2003;22:533–540. doi: 10.1089/10445490360708946. doi:10.1089/10445490360708946. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2015 doi: 10.1038/onc.2015.343. doi:10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kariolis MS, et al. An engineered Axl 'decoy receptor' effectively silences the Gas6-Axl signaling axis. Nat Chem Biol. 2014;10:977–983. doi: 10.1038/nchembio.1636. doi:10.1038/nchembio.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews. Molecular cell biology. 2014;15:178–196. doi: 10.1038/nrm3758. doi:10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammers HJ, et al. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Molecular cancer therapeutics. 2010;9:1525–1535. doi: 10.1158/1535-7163.MCT-09-1106. doi:10.1158/1535-7163.mct-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makhov PB, et al. Modulation of Akt/mTOR signaling overcomes sunitinib resistance in renal and prostate cancer cells. Molecular cancer therapeutics. 2012;11:1510–1517. doi: 10.1158/1535-7163.MCT-11-0907. doi:10.1158/1535-7163.mct-11-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motzer RJ, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. doi:10.1016/s0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 28.Adelaiye R, et al. Sunitinib dose escalation overcomes transient resistance in clear cell renal cell carcinoma and is associated with epigenetic modifications. Molecular cancer therapeutics. 2015;14:513–522. doi: 10.1158/1535-7163.MCT-14-0208. doi:10.1158/1535-7163.mct-14-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porta C, et al. Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology. 2013;84:115–122. doi: 10.1159/000342099. doi:10.1159/000342099. [DOI] [PubMed] [Google Scholar]
- 30.Porta C, Paglino C, Grunwald V. Sunitinib re-challenge in advanced renal-cell carcinoma. Br J Cancer. 2014;111:1047–1053. doi: 10.1038/bjc.2014.214. doi:10.1038/bjc.2014.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nature reviews. Drug discovery. 2014;13:140–156. doi: 10.1038/nrd4204. doi:10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cancer Genome Atlas Research, N Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. doi:10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacinto E, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature cell biology. 2004;6:1122–1128. doi: 10.1038/ncb1183. doi:10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 34.Figlin RA, Kaufmann I, Brechbiel J. Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: new strategies for overcoming resistance to VEGFR and mTORC1 inhibitors. International journal of cancer. Journal international du cancer. 2013;133:788–796. doi: 10.1002/ijc.28023. doi:10.1002/ijc.28023. [DOI] [PubMed] [Google Scholar]
- 35.Toschi A, Lee E, Gadir N, Ohh M, Foster DA. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. The Journal of biological chemistry. 2008;283:34495–34499. doi: 10.1074/jbc.C800170200. doi:10.1074/jbc.C800170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly KE, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. doi:10.1158/0008-5472.can-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voss MH, et al. Tumor genetic analyses of patients with metastatic renal cell carcinoma and extended benefit from mTOR inhibitor therapy. Clin Cancer Res. 2014;20:1955–1964. doi: 10.1158/1078-0432.CCR-13-2345. doi:10.1158/1078-0432.ccr-13-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho DC, et al. Two phase 2 trials of the novel Akt inhibitor perifosine in patients with advanced renal cell carcinoma after progression on vascular endothelial growth factor-targeted therapy. Cancer. 2012;118:6055–6062. doi: 10.1002/cncr.27668. doi:10.1002/cncr.27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonasch E, Corn PG, Pagliaro LC, Lara P, Wang X. Randomized phase II CTEP study of MK2206 versus everolimus in VEGF inhibitor refractory renal cell carcinoma patients. ASCO Annual Meeting. 2013 [Google Scholar]
- 40.Powles T, et al. Randomized Open-Label Phase II Trial of Apitolisib (GDC-0980), a Novel Inhibitor of the PI3K/Mammalian Target of Rapamycin Pathway, Versus Everolimus in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2016;34:1660–1668. doi: 10.1200/JCO.2015.64.8808. doi:10.1200/jco.2015.64.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escudier B. Emerging immunotherapies for renal cell carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 8):viii35–40. doi: 10.1093/annonc/mds261. doi:10.1093/annonc/mds261. [DOI] [PubMed] [Google Scholar]
- 42.Nakano O, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Research. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 43.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma : a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14:1014–1022. doi: 10.1038/ni.2703. doi:10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. doi:10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siska PJ, Rathmell JC. T cell metabolic fitness in antitumor immunity. Trends in immunology. 2015;36:257–264. doi: 10.1016/j.it.2015.02.007. doi:10.1016/j.it.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keir ME, Butte MJ, Freeman GJ, Sharpel AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. doi:10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu XD, et al. Resistance to Antiangiogenic Therapy Is Associated with an Immunosuppressive Tumor Microenvironment in Metastatic Renal Cell Carcinoma. Cancer immunology research. 2015 doi: 10.1158/2326-6066.CIR-14-0244. doi:10.1158/2326-6066.CIR-14-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. doi:10.1200/jco.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. doi:10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motzer RJ, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. doi:10.1200/jco.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motzer RJ, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. The New England journal of medicine. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. doi:10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8(+) T cells in tumors. Journal of Experimental Medicine. 2015;212:139–148. doi: 10.1084/jem.20140559. doi:10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabrilovich D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 56.Ko JS, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. doi:10.1158/1078-0432.ccr-08-1332. [DOI] [PubMed] [Google Scholar]
- 57.Amin A, Plimack ER, Infante JR, Ernstoff MS, Rini BI. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) ASCO Annual Meeting. 2014 [Google Scholar]
- 58.Brayer J, Fishman M. Regression of metastatic clear cell kidney cancer with interleukin-2 treatment following nivolumab (anti-PD-1) treatment. Journal of immunotherapy (Hagerstown, Md. : 1997) 2014;37:187–191. doi: 10.1097/CJI.0000000000000024. doi:10.1097/cji.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 59.Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA-4 and PD-1 blockade and new combinations. Seminars in oncology. 2015;42:363–377. doi: 10.1053/j.seminoncol.2015.02.015. doi:10.1053/j.seminoncol.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. doi:10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 61.Wolchok JD, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. New Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. doi:10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerlinger M, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–233. doi: 10.1038/ng.2891. doi:10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]