Abstract
Objective(s)
Carotid Endarterectomy (CEA) is a commonly performed vascular operation. Yet, post-operative length of stay (LOS) varies greatly even within institutions. In the present study, the morbidity and mortality, as well as financial impact of increased LOS were reviewed in order to establish modifiable factors associated with prolonged hospital stay.
Methods
The Society for Vascular Surgery Vascular Quality Initiative database was used to identify all patients undergoing primary CEA at a single institution between 6/1/2011 and 11/28/2014. Pre-operative patient characteristics, intra-operative details, post-operative factors, long-term outcomes and cost data were reviewed using an Institutional Review Board (IRB) approved prospectively collected database. Multivariate analysis was used to determine statistical difference between patients with LOS ≤ 1 day and >1 day.
Results
Complete 30-day variable and cost data was available for 219 patients with an average follow-up of 12 months. 79 (36%) patients had a LOS > 1 day. Variables determined to be statistically significant predictors of prolonged LOS included pre-operative creatinine (p=0.02) and severe congestive heart failure (p=0.05) with self-pay status (p=0.02) and pre-operative beta-blocker therapy (p=0.04) being protective. Shunt placement (p=0.04), arterial re-exploration and post-operative cardiac (p=0.001) or neurological (p=0.03) complications also resulted in prolonged hospitalization. Specific modifiable risk factors that contributed to increased LOS included operative start time after noon (p=0.04), drain placement (p=0.05), prolonged operative time (101 minutes vs 125 minutes p=0.01), return to the OR (p=0.01), and post-operative hypertension (p=0.02) or hypotension (p=0.04). Of note, there was no difference in LOS associated with technique (conventional vs eversion), patch use (p=0.49), protamine administration (p=0.60), EEG monitoring (p=0.45), measurement of stump pressure (p=0.63), doppler (p=0.36) or duplex (p=0.92). Both hospital charges (p=0.0001) and costs (p=0.0001) were found to be significantly higher in patients with prolonged LOS, with no difference in physician charges (p=.10). Increased LOS after CEA was associated with an increase in 12-month mortality (p=0.05).
Conclusions
Increased LOS was associated with increased hospital charges, costs, as well as significant morbidity and midterm mortality following CEA. Further, this study highlights several modifiable risk factors leading to increased LOS. Identified factors associated with increase LOS can serve as targets for improving care in vascular surgery.
Graphical abstract
Introduction
Healthcare costs continue to grow with some projections anticipating expenditures to reach 20% of U.S. gross domestic product by 2020.[1] Recently there has been a focus on providing high quality healthcare in the most cost effective way. Prolonged hospital length of stay (LOS) after routine procedures has been cited as a quality metric; in order to reduce cost and hospital acquired morbidity. With the shift toward value based purchasing driven by third party payers, metrics such as LOS are more important than ever.
Perioperative care for many operations is becoming pathway-driven and is highly standardized; however, these protocols vary significantly between providers and hospitals.[2] Specifically for CEA, a hospital LOS >1 day has been defined by the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) as a prolonged LOS. Following the Virginia's Vascular Study Group Regional meeting in 2014, our institution sought to better understand our practices and explore the cost implications of prolonged hospitalization after CEA.
There have been a number of papers demonstrating various risk factors for increased LOS after CEA, many of which are non-modifiable patient factors.[3-5] However, we sought to identify opportunities for better pre-operative and discharge planning focusing on modifiable risk factors to shorten LOS and thus improve outcomes. It is hypothesized a number of pre-operative, intra-operative and post-operative factors could be identified and thus modified to reduce the incidence of prolonged LOS in our CEA population.
Patients and Methods
Patients
This study was approved by the University of Virginia Institutional Review Board (IRB) (IRB #17900). All patients undergoing primary elective CEA performed for carotid artery stenosis secondary to cerebrovascular disease at the University of Virginia Health System between 1/1/2011 and 5/28/2014 were reviewed with waiver of consent through the IRB. Patient's undergoing urgent/emergent procedures or procedures by neurosurgeons or neurovascular surgeons were excluded. Data was obtained using the prospectively collected Society for Vascular Surgery's (SVS) Vascular Quality Initiative (VQI) database. Preoperative comorbidities, operative variables, and postoperative complications, as well as 30-day and 1 year mortality were analyzed to determine the difference between patients with post-operative LOS ≤ 1 day and those with LOS > 1 day. Complete 30-day follow up was available on all patients included.
Definitions
Hospital LOS was determined by calculating the number of days between surgery date and discharge for each patient. Congestive heart failure (CHF) was stratified as mild (slight limitation of physical activity, comfortable at rest, but ordinary physical activity results in fatigue, palpitation, or dyspnea), moderate (marked limitation of physical activity, comfortable at rest, but less than ordinary activity causes fatigue, palpitation, or dyspnea) and severe (unable to carry out any physical activity without discomfort, symptoms of cardiac insufficiency at rest). Similarly, coronary artery disease (CAD) was defined as stable angina (stable pattern or symptoms with or without anti-anginal medication) or unstable angina (new onset, increasing frequency, lasting > 20 min and/or rest angina). Ambulatory status is defined as being able to ambulate without assistance. Prior neurologic event includes history of cortical or occular Transient Ischemic Attach (TIA) or stroke, or vertebrobasilar TIA or stroke. Major amputation included below the knee and above the knee amputations. Dysrhythmia is any new rhythm disturbance requiring treatment with medications or cardioversion, and myocardial infarction (MI) is classified as ECG changes or clinical evidence of MI in conjunction with any abnormality of cardiac biomarker consistent with infarction. Post-operative Hypertension or Hypotension is defined as any variation in blood pressure requiring continuous infusion or more than one dose of vasoactive medication more than one hour after surgery. Arterial re-exploration applies to re-exploration for a defect detected after closure during same operation.
Statistical Analysis
Primary outcomes included preoperative comorbidities, operative variables, and postoperative complications. Secondary outcomes included 1 and 12 month morbidity or mortality, in addition to hospital charges and cost as well as physician charges. Differences between the standard LOS (≤ 1 day) and prolonged LOS (>1 day) study groups were compared using the χ2 test for categorical variables and with Mann-U Whitney for continuous variables. A p-value of <0.05 was used for statistical significance.
Results
Patient Characteristics and Preoperative Risk Factors for Increased LOS
Of the 219 patients undergoing primary CEA over the past 3 years; 79 (36%) had a LOS > 1 day. All patients included in the study had complete 30 day follow-up with an average of 13 month follow-up in the LOS ≤ 1 day and 14 months for those LOS > 1 day (p=.18). The average LOS for all patients was 1.7 +/- 1.5 days. Table I displays pre-operative characteristics for patients undergoing CEA. Self-pay status (p=.02), severe CHF (p=.05), transfer from outside hospital (p=.02), and pre-operative beta-blocker therapy (p=.04) were the only variables to reach statistical significance for predicting prolonged LOS. Table II displays the peri-operative variables recorded and analyzed. Pre-operative arteriogram (p=.05) was the only pre-operative imaging modality found to statistically impact LOS in our population, however, this was used in less than 5% of our population. All procedures were performed by five surgeons with varying experience at an academic medical center with no statistically significant difference in LOS (p=.89) between surgeons.
Table I.
Preoperative patient characteristics of CEA groups by length of stay.
| LOS ≤ 1 | LOS > 1 | ||||
|---|---|---|---|---|---|
| Pre-operative Patient Characteristics | # | % | # | % | p |
| Sex (Male) | 102 | 73 | 49 | 62 | .10 |
| Race (White) | 134 | 96 | 74 | 94 | .51 |
| Surgeon (Highest Volume) | 39 | 28 | 23 | 29 | .89 |
| Medicare | 49 | 35 | 34 | 43 | .24 |
| Medicaid | 4 | 3 | 0 | 0 | .13 |
| Private Insurance | 24 | 17 | 17 | 22 | .43 |
| Veterans Affairs | 2 | 1 | 0 | 0 | .29 |
| Self-Pay | 9 | 6 | 0 | 0 | .02 |
| Never Smoker | 25 | 17 | 13 | 17 | .79 |
| Quit Smoke | 59 | 42 | 41 | 52 | .16 |
| Current Smoker | 56 | 40 | 24 | 32 | .22 |
| Hypertension | 75 | 82 | 38 | 83 | .46 |
| No Diabetes Mellitus | 79 | 56 | 41 | 52 | .56 |
| Diet Controlled Diabetes Mellitus | 7 | 5 | 4 | 5 | .98 |
| Medication Controlled Diabetes Mellitus | 30 | 21 | 20 | 25 | .51 |
| Insulin Controlled Diabetes Mellitus | 21 | 15 | 14 | 18 | .60 |
| Stable Coronary Artery Disease | 27 | 19 | 13 | 17 | .60 |
| Unstable Coronary Artery Disease | 4 | 3 | 3 | 4 | .70 |
| Coronary Artery Bypass or Coronary Stent >5 years ago | 26 | 19 | 12 | 15 | .53 |
| Coronary Artery Bypass or Coronary Stent <5 years ago | 21 | 15 | 7 | 9 | .19 |
| Mild Congestive Heart Failure | 12 | 9 | 11 | 14 | .22 |
| Moderate Congestive Heart Failure | 5 | 4 | 1 | 1 | .12 |
| Severe Congestive Heart Failure | 0 | 0 | 2 | 5 | .05 |
| Asymptomatic Chronic Obstructive Pulmonary Disease | 6 | 4 | 2 | 3 | .51 |
| Medication Chronic Obstructive Pulmonary Disease | 23 | 17 | 10 | 13 | .45 |
| O2 dependent Chronic Obstructive Pulmonary Disease | 1 | 1 | 0 | 0 | .45 |
| Currently on Dialysis | 1 | 1 | 0 | 0 | .45 |
| Ambulatory | 138 | 99 | 76 | 96 | .26 |
| Transfer from Outside Hospital | 2 | 1 | 6 | 8 | .02 |
| Transfer from Nursing Home | 0 | 0 | 1 | 1 | .18 |
| Pre-operative Beta Blocker | 100 | 71 | 66 | 84 | .04 |
| Pre-operative Aspirin | 128 | 91 | 70 | 89 | .50 |
| Pre-operative Plavix | 32 | 23 | 22 | 28 | .41 |
| Pre-operative Statin | 112 | 80 | 62 | 79 | .89 |
| Pre-operative Angiotensin Converting Enzyme Inhibitor | 48 | 34 | 19 | 24 | .11 |
| Pre-operative Anticoagulation | 7 | 5 | 6 | 8 | .44 |
| Prior Neurological Event | 64 | 46 | 41 | 52 | .38 |
| Prior Major Amputation | 2 | 2 | 1 | 2 | .33 |
| History of Carotid Endarterectomy or Stent | 18 | 13 | 8 | 10 | .47 |
The table above demonstrates the prevalence of preoperative variables and the p value calculated by Chi Square analysis for each variable. Statistically significant variable (p<0.05) are in bold.
Table II. Pre-operative work-up and Intra-operative variables by length of stay.
| LOS ≤ 1 | LOS > 1 | ||||
|---|---|---|---|---|---|
| Variables | # | % | # | % | p |
| Pre-op Duplex | 127 | 91 | 72 | 91 | .92 |
| Pre-op Magnetic Resonance Angiography | 21 | 15 | 18 | 23 | .14 |
| Pre-op CT Angiography | 115 | 82 | 57 | 73 | .22 |
| Pre-op Arteriogram | 4 | 3 | 7 | 9 | .05 |
| Anatomic High Risk | 19 | 14 | 6 | 8 | .18 |
| Type Skin Prep (Most Common) | 75 | 54 | 42 | 53 | .73 |
| Side (Right) | 72 | 51 | 39 | 49 | .77 |
| Type (Eversion) | 33 | 24 | 16 | 20 | .57 |
| OR Start Time (Before Noon) | 104 | 74 | 49 | 62 | .04 |
| EEG Monitoring | 1 | 1 | 0 | 0 | .45 |
| Stump Pressure Measured | 23 | 16 | 15 | 19 | .63 |
| Intra-op Indication for Shunt | 9 | 6 | 11 | 14 | .04 |
| Shunt | 21 | 15 | 18 | 23 | .15 |
| Patch Manufacturer | 92 | 66 | 51 | 65 | .57 |
| Patch Angioplasty | 134 | 96 | 74 | 94 | .49 |
| Dextran Administration | 59 | 42 | 35 | 44 | .76 |
| Heparin Administration | 140 | 100 | 78 | 99 | .18 |
| Protamine Administration | 89 | 64 | 53 | 67 | .60 |
| Drain Placement | 92 | 66 | 62 | 78 | .05 |
| Completion Doppler | 20 | 14 | 15 | 19 | .36 |
| Completion Duplex | 31 | 22 | 17 | 22 | .92 |
| Re-explore Artery | 0 | 0 | 5 | 6 | .01 |
| Start Antibiotics within 1 hour of operation | 140 | 100 | 78 | 99 | .10 |
| Stop Antibiotics within 24 hours after operation | 127 | 91 | 66 | 84 | .12 |
| 1st or 2nd Gen Cephalosporin | 134 | 96 | 72 | 91 | .17 |
The table above demonstrates the prevalence of intraoperative variables and the p value calculated by Chi Square analysis for each variable. Statistically significant variable (p<0.05) are in bold.
Peri- and Postoperative Outcomes and Increased LOS after EVAR
Operating room start time after noon (p=.04), drain placement (p=.05) and re-exploration of the artery (p=.01) resulted in significantly increased LOS (Table II). Patients with intra-operative indication for shunt placement (p=.04) were found to have prolonged LOS, however, overall shunt use was not associated with LOS (p=.15). Dextran, heparin, protamine and eversion versus conventional endarterectomy with or without patch were not a predictor of prolonged LOS in this study. Finally, the routine use of completion Duplex scanning was not found to significantly impact LOS.
Post-operative complications and variables contributing to prolonged LOS can be found in Table III. Return to the operating room (p=.01), post-operative hypertension (p=.02) or hypotension (p=.04) all had significantly prolonged hospital stays. Neurologic and Cardiac injury were a major predictors of increased LOS with patients having new neurologic event (p=.01), cranial nerve injury (p=.04), post-operative myocardial infarction (MI) (p<.001), or new dysrhythmias (p<.001) all having statistically significant prolonged LOS. Of note, there were no wound complications in our study population and we found no difference in discharge medications between the groups. Finally, patients with prolonged LOS had higher 12 month (p<.05) mortality compared to patients discharged on post-operative day one.
Table III.
Post-operative management of patients based on length of stay.
| LOS ≤ 1 | LOS > 1 | ||||
|---|---|---|---|---|---|
| Post-Operative Complications | # | % | # | % | p |
| Reperfusion Symptoms | 0 | 0 | 1 | 1 | .18 |
| New Neurologic Event | 0 | 0 | 5 | 6 | .01 |
| Cranial Nerve Injury | 1 | 1 | 4 | 5 | .04 |
| Return to Operating Room | 0 | 0 | 5 | 6 | .01 |
| Post-op Hypertension (Requiring Vasoactive Med) | 19 | 14 | 21 | 27 | .02 |
| Post-Op Hypotension (Requiring Vasoactive Med) | 6 | 4 | 9 | 11 | .04 |
| Myocardial Infarction | 0 | 0 | 6 | 8 | <.001 |
| Dysrhythmia | 0 | 0 | 6 | 8 | <.001 |
| Wound Complication | 0 | 0 | 0 | 0 | 1 |
| Discharged on Aspirin | 130 | 93 | 71 | 90 | .44 |
| Discharged on Plavix | 34 | 24 | 26 | 33 | .17 |
| Discharged on Statin | 116 | 83 | 63 | 80 | .57 |
| Discharged on Beta Blocker | 83 | 59 | 56 | 71 | .09 |
| Discharged on ACE-Inhibitor | 48 | 34 | 21 | 27 | .24 |
| Discharged on Anticoagulant | 6 | 4 | 3 | 4 | .86 |
| 30 day mortality | 2 | 1 | 2 | 2 | .28 |
| 12 month Mortality | 3 | 2 | 4 | 5 | .05 |
The table above demonstrates the prevalence of postoperative variables and the p value calculated by Chi Square analysis for each variable. Statistically significant variable (p<0.05) are in bold.
Table IV demonstrates the continuous variables assessed with Mann-U Whitney test for statistical significance. Pre-operative creatinine (p=.02) and total procedure time (p=.01) were predictive of extended LOS. Pre-operative peak systolic velocity (PSV), end diastolic velocity (EDV), and internal versus external carotid velocity ratios were not correlated with LOS. Age also was not a predictor of LOS with and mean of 67 +/- 8.8 years in the early discharge and 71 +/- 8.9 years for prolonged LOS.
Table IV. Continuous Variables.
| LOS ≤ 1 day | LOS > 1 day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Continuous Variables | Mean | sd | Med | 25% | 75% | Mean | sd | Med | 25% | 75% | p |
| Body Mass Index (kg/m2) | 28.3 | 5.5 | 27.6 | 24.5 | 31.1 | 28.2 | 5.3 | 27.6 | 24.7 | 31.4 | .93 |
| Creatinine (mg/dl) Pre-op | 1.1 | 0.5 | 0.9 | 0.8 | 1.1 | 1.2 | 0.5 | 1.1 | 0.8 | 1.3 | .02 |
| Hemoglobin (g/dl) Pre-op | 13.7 | 1.6 | 13.9 | 12.9 | 15.0 | 13.3 | 1.8 | 13.5 | 12.1 | 14.6 | .11 |
| Right Peak Systolic Velocity | 302.0 | 185.7 | 328.5 | 110.5 | 453.0 | 281.9 | 170.9 | 253.0 | 124.0 | 419.0 | .74 |
| Right End Diastolic Velocity | 100.6 | 70.3 | 99.0 | 30.0 | 149.0 | 92.1 | 93.8 | 55.5 | 29.5 | 136.0 | .31 |
| Right ICA/CCA Ratio | 4.5 | 3.3 | 4.2 | 1.3 | 6.7 | 3.9 | 3.0 | 3.6 | 1.4 | 5.3 | .37 |
| Left Peak Systolic Velocity | 252.1 | 167.4 | 208.0 | 114.0 | 367.5 | 288.5 | 187.1 | 225.0 | 139.0 | 386.0 | .38 |
| Left End Diastolic Velocity | 89.1 | 76.9 | 57.5 | 34.0 | 113.0 | 97.4 | 80.7 | 79.0 | 37.0 | 128.0 | .57 |
| Left ICA/CCA Ratio | 3.2 | 3.0 | 2.2 | 1.1 | 4.7 | 4.3 | 3.4 | 3.1 | 1.2 | 7.6 | .23 |
| Heart Rate On Arrival to OR | 71.6 | 15.7 | 70.0 | 62.0 | 80.0 | 73.3 | 17.2 | 72.0 | 66.0 | 80.0 | .16 |
| Highest Intra-Op Heart Rate | 81.4 | 15.0 | 80.0 | 72.5 | 90.0 | 80.9 | 17.6 | 80.0 | 74.0 | 90.0 | .80 |
| Total Procedure Time (min) | 133.6 | 32.7 | 128.0 | 113.5 | 150.0 | 149.1 | 42.4 | 144.0 | 115.0 | 159.0 | .01 |
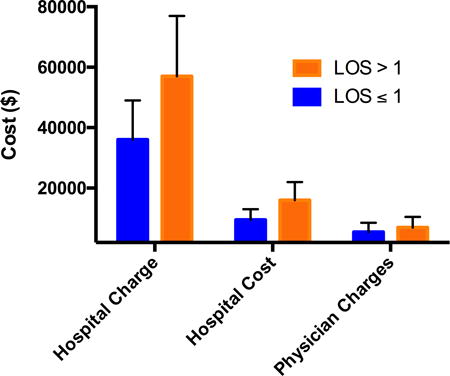
| Hospital Charges | 36000 | 13000 | 33000 | 29000 | 38000 | 57000 | 20000 | 47000 | 37000 | 64000 | <.001 |
| Hospital Cost | 9500 | 3500 | 8500 | 7500 | 10000 | 16000 | 6000 | 13000 | 10000 | 18000 | <.001 |
| Physician Charges | 5500 | 3000 | 6000 | 3500 | 6000 | 7000 | 3500 | 6500 | 5500 | 8000 | .10 |
The table above demonstrates the average, standard deviation, 25% and 75% ranges for continuous variables in addition to the p value calculated by Mann-Whitney U analysis for each variable. Statistically significant variable (p<0.05) are in bold.
Hospital cost and physician charge data was obtained through the Clinical Data Repository (CDR) at our institution. Patients with hospital stay >1 day have a statistically significant increase in hospital charges (p<0.001) with a mean hospital bill of $57,000 +/- 12,000 in the prolonged LOS group as opposed to $36,000 +/- 13,000 for patients discharged on post-operative day one. Hospital cost were also found to be significant (p<.001), with a cost of $16,000 +/-6,000 for prolonged LOS versus $9,500 +/- 3,500 for LOS ≤ 1 day. Yet, there was no difference in physician charges (p=.10) between the two groups $5,500 +/- 3,000 compared to $7,000 +/-3,500 for prolonged LOS.
Discussion
In the present study, a number of risk factors, including multiple modifiable risk factors were found to be predictive of increased LOS. Multiple pre-operative characteristics, known to place patients at risk, were shown to independently predict prolonged LOS including transfer from outside hospital, severe CHF with beta-blocker therapy, and self-pay status. While we are often unable to change these characteristics in our patient population, this information is important to consider when setting a standard metric for LOS. Intra-operative factors predicting LOS > 1 day included operating room start time after noon, intraoperative indication for shunt, drain placement, arterial re-exploration, and total operating room time. Many of these factors are adjustable requiring strict attention to moderate the risk for increased morbidity and mortality associated with increased LOS. Finally, post-operative complications are good targets for modifiable risk reduction including new neurologic event, cranial nerve injury, MI, dysrhythmia, and hypertension or hypotension requiring vasoactive medications.
Pre-operative patient characteristics that increase LOS are typically non-modifiable; however, they serve as important predictors of LOS that can be used for discharge planning.[6, 7] These pre-operative predictors can be used to identify individuals with high likelihood of discharge to a skilled nursing facility.[2, 8, 9] The strongest predictor of prolonged LOS after CEA was transfer from outside hospital; this selects for sick patients with complex disease who are referred to our quaternary care center for management.[10] In our population, severe CHF and pre-operative creatinine were statistically significant, however, the difference in the mean creatinine was 1.1 versus 1.2 raising the question of clinical significance in this variable especially since there was no difference in patients on dialysis.[11] Additionally, self pay status and beta blocker therapy were found to be protective; however, only 4% of the population qualified as self pay, but all of them were discharged on post-operative day 1. In our population, there was no difference in LOS stratified by age as demonstrated by several groups.[12-14] It is important to note there was also no difference in LOS between patients with history of neurologic events, major amputation, respiratory or cardiac comorbidities, including obesity with equal distribution in BMI.[6, 15] The same applies for pre-operative medications including pre-operative use of clopidogrel and aspirin with no difference in post-operative LOS. [16]
There is wide variation in the imaging modality requested prior to CEA.[17-19] In this study we found pre-operative cerebral arteriogram as the only imaging modality correlating with LOS. Yet, only 5% of patients underwent arteriogram and on review of the cases they were patients referred from neurology with complex intracranial and extracranial cerebrovascular disease. Additionally, there was no difference in velocities on duplex between the two groups suggesting even patients with critical stenosis are safe to discharge on post-operative day one. However when scheduling a patient for CEA, our study demonstrates it is critical to start the operation before noon or they are significantly more likely to require hospitalization beyond post-operative day one as demonstrated by other groups.[3] Finally, we highlighted no difference in LOS between patients with various intra-operative cerebral monitoring, including EEG and routine measurement of stump pressure as seen by other groups.[20]
Procedure details are potentially modifiable and represent opportunities to reduce LOS, and must be taken into consideration when attempting to improve outcomes. While inherent differences in practice between surgeons might explain some variability in intraoperative variables, it is important to note that in the present series there were no differences in LOS for the five surgeons performing CEA. Our surgeons have different practices for heparin administration, use of dextran, protamine administration, and performance of completion duplex scan; however, none of these factors served as independent predictors of prolonged LOS. At our institution we have a 20% rate of eversion endarterectomy with no difference in LOS as reported by Diao et al.[21] In patients undergoing conventional CEA with patch angioplasty bovine pericardial patch is the first choice as described by Bisdas et al.[22] In the present study, we found patients with intra-operative indication for shunt were significantly more likely to have prolonged LOS. However, as demonstrated by other groups there was no difference in patients undergoing shunt placement for CEA, only the subset with intra-operative indications.[20, 23] As expected, patients requiring re-exploration and prolonged operative time resulted in a complicated post-operative course with longer hospitalization.[24] These factors likely serve as markers of case complexity and are difficult to modify.[4]
Post-operative complications influence LOS and represent a great opportunity for improvement. Neurologic and cardiac post-operative complications were some of the strongest predictors of prolonged LOS. For this reason, it is critical to optimize medical management of cardiovascular disease pre-operatively and return patients to their medications as soon as possible after their operation.[25] Post-operative hemodynamic instability after CEA is a well-described phenomenon presenting a major barrier to post-operative day one discharge.[6, 7, 26-28] In our CEA population, post-operative hypertension or hypotension affects over 25% and 10% of our patients requiring prolonged LOS respectively. This is a major target for improvement as we have advancements in intraoperative monitoring and anesthetic techniques. Another important finding in this study is the significantly higher 1-year mortality in patients with prolonged LOS after CEA highlighting the long-term impact of peri-procedural complications. These patients have a small physiologic window and do not respond well to hospital-acquired infections, disruption of their complex medication regimens, and invasive procedures.[10, 25, 29] Of note, there was no mortality difference at 30 days suggesting many of the patients who died within 1 year were patients suffering neurologic complications who survived their hospitalization, but have a significantly higher mortality over the next year due to these complications.
The final significant finding in this study is the increase in cost to the healthcare system for patients with prolonged LOS.[5, 30-32] We demonstrated significantly higher hospital costs and charges with no change in physician's charges. As we move toward bundled payments for procedure related hospital admissions, it is critical to reduce the hospital cost because the charges will be fixed regardless of the amount of care a patient requires. As demonstrated by Glaser et al the hospital is losing money if patients require more than one-day hospitalization after elective operations. [4] It is interesting to note that there was a wide variation in charges identified in this study with wide standard deviations. This illustrates the ongoing problem with identifying and reducing medical expense in our country, all patients received the same operation, but cost differed greatly.[31] In the future we will need to streamline efforts and reduce variability in “brands” and types of diagnostic and monitoring equipment used to provide the most cost efficient care to patients. For CEA this could mean routine shunting instead of selective shunting with more expensive EEG monitoring.
The limitations of this study include the retrospective nature of the study, the relatively small sample size achieved with a single center review, and lack of long-term disease specific complication data beyond one year. The VSQIP database does an outstanding job capturing peri-operative data and 30-day outcomes; however, long term mortality data was more difficult to obtain through our CDR. Given our moderate sample size it is difficult to perform multivariate analysis to control for confounding variables including operative time as a surrogate of operative difficulty. It would be very helpful to have more information on procedure related complications beyond 30 days including re-hospitalization and neurologic events to determine if LOS impacts these factors.
This study provides an important message for surgeons performing CEA on patients with complex cerebrovascular disease. Given the current culture of value driven healthcare with a focus on quality and cost, it is more important than ever to provide the most efficient and effective care to vascular patients. As we move into future value based purchasing driven by third party payers, we will be required to provide high quality care, by standard metrics, at a low cost. While many of the factors predicting prolonged length of stay in our population are non-modifiable, this study demonstrates the importance of identifying patients at risk for post-operative complications and optimizing medical management of these comorbidities.
Conclusion
Increased LOS was associated with increased hospital charges, costs, as well as significant morbidity and midterm mortality following CEA. Further, this study highlights several modifiable risk factors leading to increased LOS. Identified factors associated with increase LOS can serve as targets for improving care in vascular surgery.
Acknowledgments
We would like to give special thanks to Mary Baldwin for her hard work and assistance in data collection and database maintenance. National Institutes of Health under Award Number T32HL007849 supported this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52. 54, 56-61 passim. [PubMed] [Google Scholar]
- 2.Calligaro KD, et al. Critical pathways can improve results with carotid endarterectomy. Semin Vasc Surg. 2004;17(3):253–6. doi: 10.1016/s0895-7967(04)00049-3. [DOI] [PubMed] [Google Scholar]
- 3.Ho KJ, et al. Contemporary predictors of extended postoperative hospital length of stay after carotid endarterectomy. J Vasc Surg. 2014;59(5):1282–90. doi: 10.1016/j.jvs.2013.11.090. [DOI] [PubMed] [Google Scholar]
- 4.Glaser J, et al. Factors that determine the length of stay after carotid endarterectomy represent opportunities to avoid financial losses. J Vasc Surg. 2014;60(4):966–72 e1. doi: 10.1016/j.jvs.2014.03.292. [DOI] [PubMed] [Google Scholar]
- 5.Young KC, et al. Hospital resource use following carotid endarterectomy in 2006: analysis of the nationwide inpatient sample. J Stroke Cerebrovasc Dis. 2010;19(6):458–64. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez N, et al. Factors related to short length of stay after carotid endarterectomy. Vasc Endovascular Surg. 2002;36(6):425–37. doi: 10.1177/153857440203600603. [DOI] [PubMed] [Google Scholar]
- 7.Ricotta JJ. Regarding: “timing of postcarotid complications: a guide to safe discharge planning”. J Vasc Surg. 2001;34(1):178–9. doi: 10.1067/mva.2001.116105. [DOI] [PubMed] [Google Scholar]
- 8.Ho KJ, et al. Predictors and consequences of unplanned hospital readmission within 30 days of carotid endarterectomy. J Vasc Surg. 2014;60(1):77–84. doi: 10.1016/j.jvs.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney AB, et al. Integrated care pathways for vascular surgery: an analysis of the first 18 months. Postgrad Med J. 2002;78(917):175–7. doi: 10.1136/pmj.78.917.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melin AA, et al. Preoperative frailty Risk Analysis Index to stratify patients undergoing carotid endarterectomy. J Vasc Surg. 2015;61(3):683–9. doi: 10.1016/j.jvs.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Reil T, et al. The safety of carotid endarterectomy in patients with preoperative renal dysfunction. Ann Vasc Surg. 2002;16(2):176–80. doi: 10.1007/s10016-001-0149-x. [DOI] [PubMed] [Google Scholar]
- 12.Pol RA, et al. Safety and efficacy of carotid endarterectomy in octogenarians. Ann Vasc Surg. 2013;27(6):736–42. doi: 10.1016/j.avsg.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Killeen KL, et al. Carotid reconstruction in nonagenarians: is surgery a viable option? Ann Vasc Surg. 2008;22(2):190–4. doi: 10.1016/j.avsg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Salameh JR, Myers JL, Mukherjee D. Carotid endarterectomy in elderly patients: low complication rate with overnight stay. Arch Surg. 2002;137(11):1284–7. doi: 10.1001/archsurg.137.11.1284. discussion 1288. [DOI] [PubMed] [Google Scholar]
- 15.Khandanpour N, et al. The effects of increasing obesity on outcomes of vascular surgery. Ann Vasc Surg. 2009;23(3):310–6. doi: 10.1016/j.avsg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Saadeh C, Sfeir J. Discontinuation of preoperative clopidogrel is unnecessary in peripheral arterial surgery. J Vasc Surg. 2013;58(6):1586–92. doi: 10.1016/j.jvs.2013.05.092. [DOI] [PubMed] [Google Scholar]
- 17.Arous EJ, et al. National variation in preoperative imaging, carotid duplex ultrasound criteria, and threshold for surgery for asymptomatic carotid artery stenosis. J Vasc Surg. 2015 doi: 10.1016/j.jvs.2015.04.438. [DOI] [PubMed] [Google Scholar]
- 18.Brinjikji W, et al. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. 2015:1–16. doi: 10.3171/2015.1.JNS142452. [DOI] [PubMed] [Google Scholar]
- 19.Makris GC, et al. Advances in MRI for the evaluation of carotid atherosclerosis. Br J Radiol. 2015;88(1052):20140282. doi: 10.1259/bjr.20140282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen TQ, Lind L, Harris EJ., Jr Selective shunting during carotid endarterectomy. Vascular. 2005;13(1):23–7. doi: 10.1258/rsmvasc.13.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Diao YP, et al. Efficacy analysis of two surgical procedures of carotid endarterectomy in the treatment of carotid artery stenosis. Zhonghua Yi Xue Za Zhi. 2013;93(27):2135–8. [PubMed] [Google Scholar]
- 22.Bisdas T, et al. Early neurologic outcome after bovine pericardium versus venous patch angioplasty in 599 patients undergoing carotid endarterectomy. Vascular. 2010;18(3):147–53. doi: 10.2310/6670.2010.00022. [DOI] [PubMed] [Google Scholar]
- 23.Hans SS, Catanescu I. Selective shunting for carotid endarterectomy in patients with recent stroke. J Vasc Surg. 2015;61(4):915–9. doi: 10.1016/j.jvs.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AH, et al. Reoperation for neurological complications following carotid endarterectomy. Br J Surg. 2003;90(7):832–7. doi: 10.1002/bjs.4121. [DOI] [PubMed] [Google Scholar]
- 25.Mackey WC, et al. Perioperative myocardial ischemic injury in high-risk vascular surgery patients: incidence and clinical significance in a prospective clinical trial. J Vasc Surg. 2006;43(3):533–8. doi: 10.1016/j.jvs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Posner SR, et al. Uncomplicated carotid endarterectomy: factors contributing to blood pressure instability precluding safe early discharge. Vascular. 2004;12(5):278–84. doi: 10.1258/rsmvasc.12.5.278. [DOI] [PubMed] [Google Scholar]
- 27.Sternbach Y, et al. Hemodynamic benefits of regional anesthesia for carotid endarterectomy. J Vasc Surg. 2002;35(2):333–9. doi: 10.1067/mva.2002.121579. [DOI] [PubMed] [Google Scholar]
- 28.Tan TW, et al. The need for treatment of hemodynamic instability following carotid endarterectomy is associated with increased perioperative and 1-year morbidity and mortality. J Vasc Surg. 2014;59(1):16–24 e1. 2. doi: 10.1016/j.jvs.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cikrit DF, et al. Short-stay carotid endarterectomy in a tertiary-care Veterans Administration hospital. Am J Surg. 2004;188(5):544–8. doi: 10.1016/j.amjsurg.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Liapis C, et al. Reducing the postoperative cost of carotid endarterectomy. Results of a randomized study. J Cardiovasc Surg (Torino) 2003;44(1):143–4. [PubMed] [Google Scholar]
- 31.Dorafshar AH, et al. Cost analysis of carotid endarterectomy: is age a factor? Ann Vasc Surg. 2004;18(6):729–35. doi: 10.1007/s10016-004-0107-5. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan P. Economics of carotid revascularization. Catheter Cardiovasc Interv. 2011;77(4):473–4. doi: 10.1002/ccd.23001. [DOI] [PubMed] [Google Scholar]


