Abstract
In this review, some of the recent developments in probes and assay techniques specific for superoxide (O2•−) and hydrogen peroxide (H2O2) are discussed. Over the last decade, significant progress has been made in O2•− and H2O2 detection due to syntheses of new redox probes, better understanding of their chemistry, and development of specific and sensitive assays. For superoxide detection, hydroethidine (HE) is the most suitable probe, as the product, 2-hydroxyethidium, is specific for O2•−. In addition, HE-derived dimeric products are specific for one-electron oxidants. As red-fluorescent ethidium is always formed from HE intracellularly, chromatographic techniques are required for detecting 2-hydroxyethidium. HE analogs, Mito-SOX and hydropropidine, exhibit the same reaction chemistry with O2•− and one-electron oxidants. Thus, mitochondrial superoxide can be unequivocally detected using HPLC-based methods and not by fluorescence microscopy. Aromatic boronate-based probes react quantitatively with H2O2, forming a phenolic product. However, peroxynitrite and hypochlorite react more rapidly with boronates, forming the same product. Using ROS-specific probes and HPLC assays, it is possible to screen chemical libraries to discover specific inhibitors of NADPH oxidases. We hope that rigorous detection of O2•− and H2O2 in different cellular compartments will improve our understanding of their role in redox signaling.
Keywords: NADPH oxidase, fluorescent probes, oxy radicals, mitochondrial complex I, signal transducer
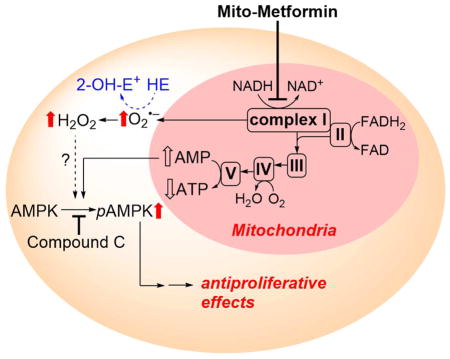
Graphical Abstract
Introduction
Over the past few decades, we have been fortunate to work with organic chemists who synthesized spin traps, fluorescent and chemiluminescent probes, and compounds specifically targeted to mitochondria, as well as many cell biologists, cell signaling researchers, and molecular biologists. Our frequent interactions between synthetic chemists, physical chemists, and biologists have allowed us to identify relevant problems and develop pertinent probes and analytical techniques that can provide solutions to long-standing problems in free radical biology. Important novel probes for detecting reactive oxygen and nitrogen species are being developed in several laboratories here and around the world. Because of these advances, we are now in the position to simultaneously monitor different reactive oxygen and nitrogen species in biological systems, identify inhibitors of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Nox) by screening libraries of chemicals, and even detect reactive oxygen species (ROS) in a cell signaling milieu. In this review, the recent developments of probes and techniques to detect the specific products derived from the interaction between selected ROS and fluorescent and chemiluminescent probes in vitro and in vivo, and possibly under redox signaling conditions, are discussed.
Understanding the reaction chemistry of probes
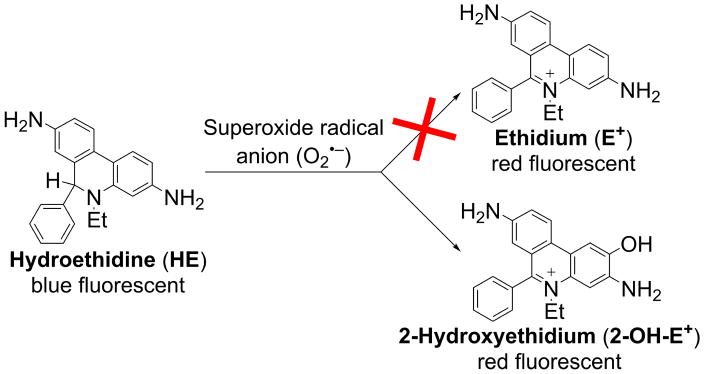
Roger Tsien, who won the 2008 Noble Prize in chemistry for his discovery on genetically engineered green fluorescent proteins, famously wrote, “Being able to do any chemistry was like being the one-eyed man in the kingdom of the blind” [1]. We realized that the free radical biology field would greatly benefit from a rigorous understanding of the chemistry of fluorescent probes, their reaction kinetics with ROS/reactive nitrogen species (RNS), the determination of stoichiometry and specific products, and development of rigorous assays and detection methods. Thus began our quest to understand the reaction mechanism of superoxide and the redox-sensitive fluorescent probe hydroethidine (HE). HE, a two-electron reduction product of ethidium (E+), was thought to react specifically with the superoxide radical anion (O2•−) and get oxidized back to E+ [2]. However, we found that, in a pure superoxide-generating enzymatic system, HE was not oxidized to E+ (Figure 1); rather, a hydroxylated product that has fluorescence characteristics similar to that of E+ was formed [3,4]. The structure of the hydroxylated product was determined to be 2-hydroxyethidium (2-OH-E+, Figure 1) [4].
Figure 1.
Superoxide radical anion reacts with hydroethidine to produce 2-hydroxyethidium but not ethidium.
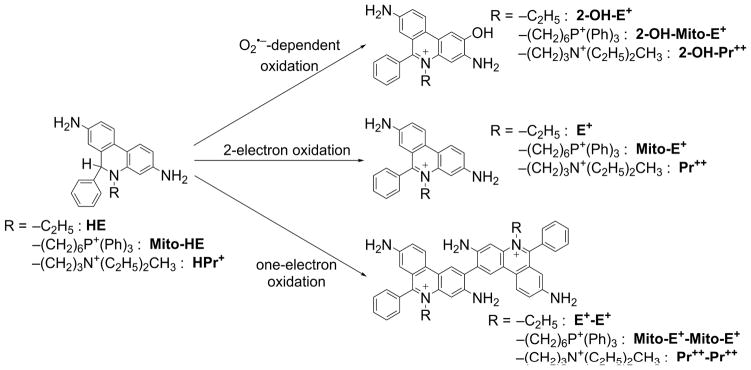
However, in superoxide-generating systems that contain trace levels of redox-active metal ions (iron) or peroxidases, E+ was formed as a nonspecific product (Figure 2). In addition to E+, several dimeric products (nonfluorescent), such as E+-E+, that are characteristic of a radical-radical dimerization mechanism were formed (Figure 2) [5]. Because the reaction of HE radical cation with superoxide is rapid (estimated rate constant ~109 M−1s−1), one-electron oxidation of HE may significantly improve its efficiency in competing for O2•− with other targets of superoxide, including intracellular superoxide dismutase (SOD). Thus, the presence of one-electron oxidants may increase the yield of 2-OH-E+, as the HE-derived radical is a common intermediate for superoxide (or its protonated form, hydroperoxyl radical [HO2•]) and other one-electron oxidants [6,7]. As the one-electron oxidants will also lead to formation of the dimeric products, complete profiling of HE oxidation products is necessary for proper interpretation of the changes in intracellular levels of 2-OH-E+ [8]. Recently, an additional chlorination product of hydroethidine (2-chloroethidium) has been identified as a product of the reaction between HE and hypochlorous acid, and proposed as a specific marker of myeloperoxidase activity in the in vitro and in vivo settings [9,10].
Figure 2.
Products formed from hydroethidine (HE), MitoSOX (Mito-HE), and hydropropidine (HPr+) as a function of oxidant identity/oxidation mechanism.
Rigorous identification of ROS and RNS using the fluorescent probes
The proper use of molecular probes for detection and characterization of reactive oxidizing and nitrating species requires a detailed knowledge of the chemistry, reaction kinetics, stoichiometry, and product(s) formed from the reaction between ROS/RNS and fluorescent or chemiluminescent probes. Clearly, accurate interpretation of the fluorescence data requires sufficient knowledge of the ROS/RNS reaction chemistry with the fluorescent dyes. Sometimes, determination of minor, but specific, products can provide major new information regarding the identity of the ROS/RNS species [11–13].
Intracellular levels of the fluorescent probes
With very few exceptions, fluorogenic probes for ROS are not present in sufficient concentrations in cells to efficiently compete with other targets of superoxide or hydrogen peroxide. This may be regarded as an advantage, as in such cases the probe is not expected to significantly disturb the system, providing a way for biorthogonal ROS detection. It has, however, two important consequences for ROS measurements: (i) only a fraction of O2•− or H2O2 is detected and, therefore, the assay is semi-quantitative at best, and (ii) changes in intracellular concentration of the probe will affect its effectiveness in competition with other targets, resulting in different yields of the products detected. Therefore, it is critically important to monitor intracellular probe levels for an accurate interpretation of the changes in the amounts of the oxidation product(s).
Is the red fluorescence formed from HE truly indicative of the reactive oxygen and nitrogen species?
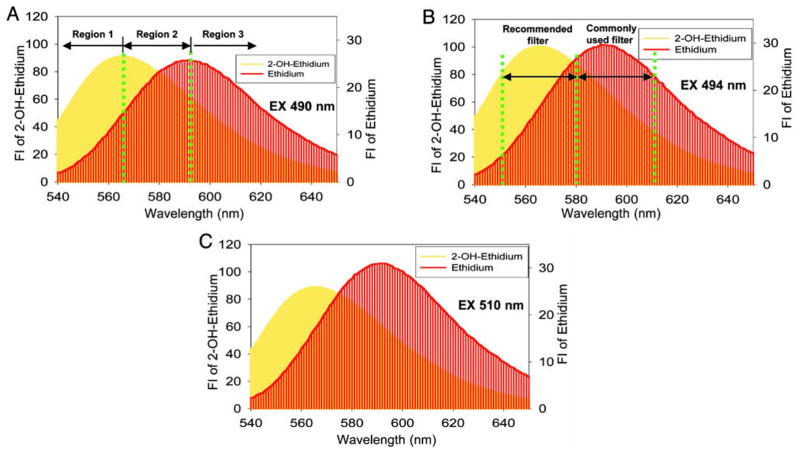
Both 2-OH-E+ and E+, derived from HE oxidation, have very similar fluorescence characteristics (Figure 3) [2,4,14]. Furthermore, the relative intracellular concentrations of 2-OH-E+ and E+ generally are not known. Consequently, the use of fluorescence microscopy to monitor red fluorescence does not measure just one species, as demonstrated [4], and measurement of “red fluorescence” derived from HE-treated cells is not an indicator of intracellular superoxide. What was measured in almost all of these studies is either the nonspecific or one-electron oxidation of HE, or a mixture of nonspecific and the superoxide-specific (2-OH-E+) products of HE oxidation. As such, it is incorrect to conclude that the red fluorescence obtained from HE is due only to 2-hydroxyethidium, the product of reaction of O2•− with HE. Although use of shorter wavelength excitation light (~400 nm) for more selective detection of 2-OH-E+ has been proposed [15,16], without knowing the relative contribution from E+ and 2-OH-E+ to the signal intensity, the fluorescence signal cannot be equated to cellular superoxide level/production. Regardless of the excitation wavelength, monitoring the red fluorescence does not provide data on the intracellular probe level and, therefore, misses the important factor, which undoubtedly controls the signal intensity.
Figure 3.
Fluorescence spectral overlap between 2-hydroxyethidium (2-OH-E+) and ethidium (E+) as a function of the wavelength of the excitation light. None of the excitation light tested allowed selective detection of 2-OH-E+. (Adapted from [4].)
Is Mito-SOX-derived red fluorescence indicative of mitochondrial superoxide?
Mitochondria-targeted hydroethidine (Mito-SOX or mito-hydroethidine [Mito-HE]) is a redox probe in which the HE molecule is conjugated to a triphenylphosphonium group (TPP+) via an alkyl side chain [5,15]. Mito-SOX is targeted to mitochondria because of the presence of the TPP+ moiety. As the “business end” of this probe is HE moiety, the reaction chemistry of HE and Mito-SOX is identical in all aspects. Figure 4 shows the one-electron oxidation and superoxide-dependent hydroxylation products of HE and Mito-SOX. For example, both HE and Mito-SOX form the corresponding 2-hydroxylated products, 2-OH-E+ from HE and 2-hydroxy-Mito-ethidium (2-OH-Mito-E+) from Mito-SOX, as the only products in a pure superoxide-generating system (e.g., xanthine/xanthine oxidase) [5,7,14]. In the presence of trace metal ions and/or peroxidase, one-electron oxidation products are formed from both HE and Mito-SOX (Figure 4) [5,7]. Because mitochondria are enriched with iron-containing hemeproteins and hemes, Mito-SOX is more susceptible to undergoing a one-electron oxidation than HE. As a result, Mito-SOX is able to form red-fluorescent Mito-E+ (the ethidium analog of Mito-SOX); without knowing the relative concentrations of Mito-E+ and 2-OH-Mito-E+, it is impossible to assign the red fluorescence derived from Mito-SOX to mitochondrial superoxide. Pharmacological or genetic (e.g., overexpression of manganese SOD) manipulations directed at decreasing mitochondrial superoxide levels may not be sufficient for accurate interpretation of Mito-SOX-derived fluorescence, as they may also affect cellular and/or mitochondrial probe uptake and its mitochondrial level. Thus, in our opinion, most studies wherein Mito-SOX red fluorescence was equated to mitochondrial superoxide require reevaluation and reinvestigation using rigorous methodologies employing high performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS) techniques.
Figure 4.
Oxidant-dependent distribution of the oxidation products of HE and Mito-SOX (Mito-HE) probes. HE and its products of oxidation are shown in blue; Mito-HE and its oxidation products are shown in red.
DCF and DHR-derived fluorescence–Are these useful?
Both dichlorodihydrofluorescein (DCFH2) and dihydrorhodamine (DHR) have been used to monitor intracellular oxidative stress, hydrogen peroxide, and other oxidants derived therefrom [16,17]. We and other researchers have reported that DCFH2 does not directly react with hydrogen peroxide (H2O2) and that intracellular iron, heme, or peroxidase is required or peroxynitrite-derived radicals are involved [16,18]. We suggested that DCFH2-derived fluorescence may be used to track oxidant-induced intracellular iron signaling as formation of DCF was inhibited by a transferrin receptor antibody blocking the iron uptake [18]. However, a major drawback in using DCFH2 and DHR to measure ROS/RNS is that the one-electron oxidized intermediate, DCFH radical (DCFH•/DCF•−) or DHR radical (RhH•), forms superoxide in the presence of oxygen [19–21]. This means that these dyes themselves generate O2•−, leading to the formation of H2O2 and to fluorescence signal amplification, which may be unrelated to the original event responsible for initial probe oxidation [22,23]. This is a case of a reporter molecule making “news.” In such a case, any pharmacological or genetic manipulation aimed at decreasing the intracellular H2O2 levels may attenuate the DCFH2-derived fluorescence signal, even if the initiation of DCFH2 oxidation was H2O2-independent (e.g., release of cytochrome c from mitochondria during apoptosis). Clearly, one does not glean any useful information concerning the nature of the intracellular oxidant formed by monitoring DCFH2 or DHR-derived fluorescence. In contrast to DCFH2 and DHR, the Amplex Red radical intermediate does not redox cycle to generate O2•−. Most redox probes undergo one-electron oxidation to a probe radical; however, in some cases, the redox-probe-derived radical stimulates superoxide and H2O2 formation. In other cases, redox-probe-derived radicals do not react with oxygen but react with superoxide to form the specific product, as reported for hydroethidine oxidation.
Small molecular weight probes for detecting hydrogen peroxide
For decades, it has been known that boronates react stoichiometrically with H2O2 [24] to form the corresponding hydroxyl product. However, the reaction rate constant is very low (~ 1 M−1s−1); thus, in a cellular milieu, unless the local concentration of the boronate is very high, it is unlikely that the boronate probe is the predominant target for H2O2 and thus can be used as a bioorthogonal probe [25]. Under extracellular conditions using high concentrations of the coumarin boronate (CBA) probe, H2O2 formation was monitored with and without the catalase enzyme by observing the fluorescence of the product, 7-hydroxycoumarin [12,26,27]. Most boronates, including the CBA probe, react very rapidly with peroxynitrite and hypochlorous acid, forming the corresponding phenols as a major product [27–29]. In contrast to the H2O2/boronate reaction, the peroxynitrite/boronate reaction is not sensitive to catalase [26–30].
The Amplex Red probe is used to detect and quantitate H2O2 because of its high sensitivity. In this assay, the Amplex Red probe is oxidized to resorufin by added horseradish peroxidase (HRP) and H2O2 generated endogenously. Resorufin has a high extinction coefficient in the visible absorption region (~570 nm), so it can be conveniently and continuously monitored [26,30]. However, one has to be cognizant of artifacts introduced by light, reductants, and the photosensitized oxidation mechanism [31]. As continuous monitoring of Amplex Red oxidation may lead to significant photosensitization of Amplex Red by resorufin [32], the data from real-time monitoring should be validated by end-point measurements of the samples incubated in the dark. It has been also reported that Amplex Red can be converted to resorufin by peroxynitrite in an HRP-catalyzed reaction [33] and in an HRP-independent, carboxylesterase-catalyzed reaction [34]. The Amplex Red-based assay is predominantly limited to cell-free and extracellularly generated H2O2 as the reaction requires a catalyst and the HRP is cell impermeable. It is, however, likely that another intracellular heme peroxidase could catalyze the oxidative conversion of the Amplex Red to resorufin.
Simultaneous monitoring of O2•− and H2O2: UHPLC analysis
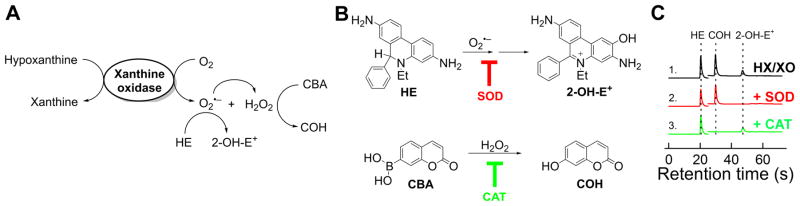
Using ultra high pressure liquid chromatography (UHPLC), the products formed from O2•−/HE and CBA/H2O2 reactions can be detected within 60 s (Figure 5) [12,26]. Using the appropriate standards, 2-OH-E+ and 7-hydroxycoumarin (COH), the actual concentrations of these products can be rigorously measured. Figure 5C shows a series of HPLC chromatograms obtained from incubations containing a mixture of HE and CBA probes with hypoxanthine/xanthine oxidase alone (trace 1), and with either SOD (trace 2), or catalase (CAT, trace 3) [26]. Whereas 2-OH-E+ was inhibited by superoxide dismutase, COH (a product formed from CBA and H2O2 reaction) was not inhibited. In contrast, COH was inhibited by catalase but not by SOD.
Figure 5. Simultaneous monitoring of O2•− and H2O2 using UHPLC in HTS compatible manner using hypoxanthine/xanthine oxidase system as a source of O2•− and H2O2 with HE and CBA as the probes thereof.
(A) Scheme of formation of O2•− and H2O2 and oxidation of the probes; (B) Chemical structures of the probes used and the products formed; (C) UHPLC traces recorded. (Adapted from [26].)
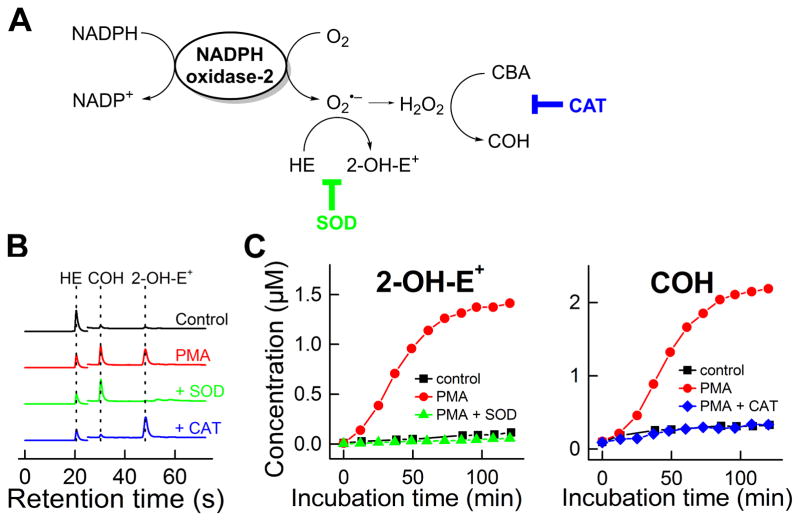
We applied this approach to simultaneously monitor O2•− and H2O2 formation upon Nox2 activation in differentiated human promyelocytic leukemia (HL-60) cells (Figure 6) [12,26]. Figure 6B shows the UHPLC traces obtained from incubations containing HE and CBA and differentiated HL-60 cells alone, plus phorbol ester (PMA), plus PMA and SOD, and plus PMA and catalase. The time-course in O2•− and H2O2 formation from activated and resting HL-60 cells is shown in Figure 6C. Thus, using this approach, one can rigorously monitor over time and quantitate the probes’ oxidation products from the reaction with O2•− and H2O2 generated from activated Nox2.
Figure 6. Simultaneous monitoring of O2•− and H2O2 generated from NADPH oxidase-2 (in differentiated HL60 cells stimulated with PMA).
(A) Scheme of formation of O2•− and H2O2 and oxidation of the probes; (B) UHPLC traces; (C) Time course of formation of 2-OH-E+ and COH upon stimulation of the cells with PMA. (Adapted from [26].)
Does Nox4 generate detectable O2•−?
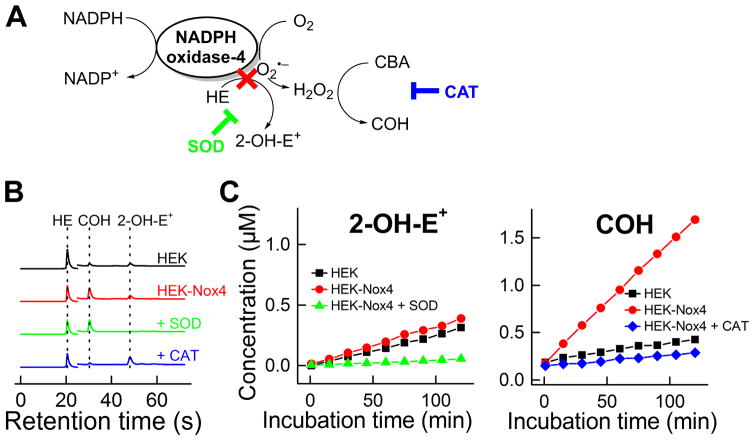
A considerable amount of controversy is present in the literature with regard to O2•− formation and detection from Nox4 [35]. So far, only fluorescence and spectrophotometric detection methods have been used; clearly, more rigorous methods need to be applied. We investigated this problem using the UHPLC/HE/CBA approach (Figure 7). Figure 7B shows the UHPLC traces obtained from incubations containing human embryonic kidney (HEK) cells (control), HEK cells expressing Nox4 (HEK-Nox4), and both HE and CBA. There rate of 2-OH-E+ formation from HEK cells and HEK-Nox4 cells was almost the same, suggesting no detectable O2•− generation from Nox4. There was, however, a marked increase in catalase-sensitive COH formation from H2O2 reaction with CBA in HEK-Nox4 cells. From these results, we can conclude that no detectable O2•− was released from the active site of the enzyme Nox4 and that the major detectable species released from Nox4 is H2O2 [26].
Figure 7. Monitoring of oxidants generated from NADPH oxidase-4 (in HEK-Nox4 cells).
(A) Scheme of formation of H2O2 from Nox4 and oxidation of the probes, (B) UHPLC traces, (C) Time course of formation of 2-OH-E+ and COH in wild-type and Nox4-transfected HEK-293 cells. (Adapted from [26].)
Opportunities in Nox inhibitor discovery
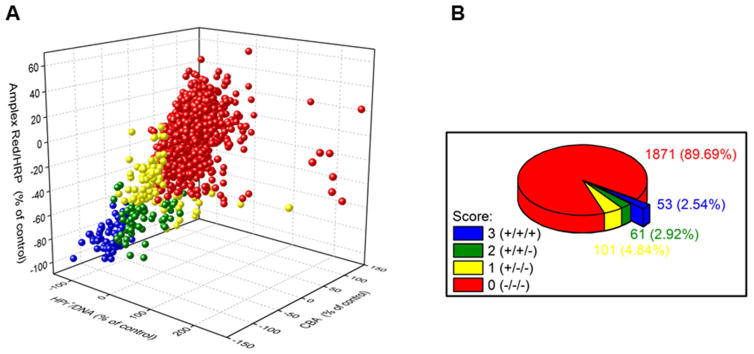
The only known function of all Nox isoforms (Nox1–Nox5, Duox1, Duox2) is generation of reactive oxygen species [36]. Thus, a rigorously developed ROS assay is critical for monitoring of enzyme activity and assessing the effects of putative Nox inhibitors. However, only a limited number of Nox inhibitors are currently available [37,38]. We propose that one of the reasons for the slow progress is the lack of availability of specific and selective ROS assays. Although EPR spin trapping is a method for specific detection of superoxide in extracellular milieu, and we used it successfully for trapping Nox2-derived superoxide [26], this method is not compatible with high throughput screening but can be used in the confirmatory assays. After we became convinced that we are able to reliably and reproducibly obtain similar results with 40+ compounds using our assays based on HE, CBA, HPr+ (hydropropidine), and Amplex Red [26], we screened a small library of >2,000 bioactive and US Food and Drug Administration (FDA)-approved drugs for Nox2 inhibition. We used the differentiated HL-60 cells as the source of Nox2 and identified about 50 drugs as potential inhibitors of Nox2-mediated ROS formation (Figure 8) [12]. This screen also identified compounds such as diphenyleneiodonium (DPI), the most frequently used Nox inhibitor. Clearly, much more work needs to be done with regard to dose response, mechanisms, off-target effects, toxicity, and related factors. But, we are now in a position to use the developed assays for other Nox isoforms, the appropriate cells, and ROS-assay-based high-throughput screening.
Figure 8. Results of screening of the library of >2,000 bioactive compounds at the Broad Institute using three plate-reader-based assays: hydropropidine/DNA (for O2•−), CBA (for H2O2), and Amplex Red/HRP (for H2O2).
(A) Correlation of the results from the three probes; (B) Pie chart showing the percentage of positive hits in single (+/−/−), two (+/+/−), and three (+/+/+) assays. (Adapted from [12].)
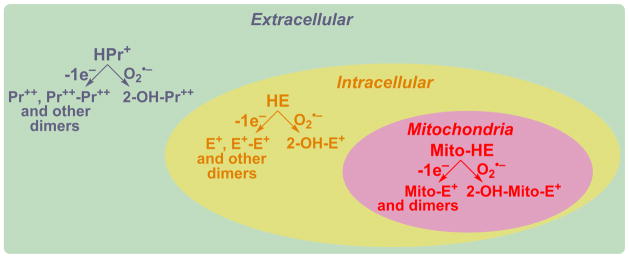
Detection of O2•− in different cellular compartments
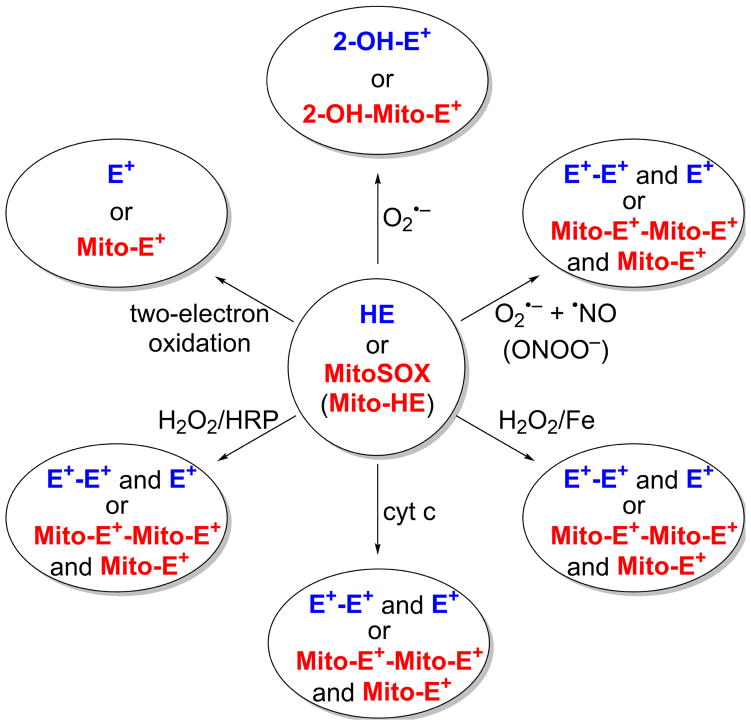
As ROS/RNS are generated by various cellular machineries in different cellular compartments, targeting ROS/RNS-specific probes to sites of oxidant generation is essential. Advances made with regard to rigorous understanding of the chemistry and determinations of products formed from the redox probes are making it possible to achieve this ultimate goal. Figure 9 shows products formed from O2•− and one-electron oxidants reaction with extracellular, intracellular, and mitochondrial HE analogs. As shown, all of these probes share the same reaction chemistry. We envision that, if HE is attached to a peptide targeted to a nucleus, lysosome, or peroxisome, O2•− and other oxidant formation in these organelles can specifically be probed. Investigators have previously attached a lysosome-targeting peptide to a boronate for measuring lysosomal H2O2 [39]. This is an interesting new approach that is likely to be pursued by other investigators. However, as we stressed earlier, regardless of the targeting moiety, the free radical chemistry of the probe remains the same as previously demonstrated by us [2,5,28].
Figure 9.
Detection of site-specific formation of O2•− and other oxidants using HE and its site-specific analogs. (Adapted from [60].)
In vivo imaging of ROS: A pipe-dream or a plausible reality?
New sensitive probes that are relatively nontoxic are being developed primarily in Chang’s laboratory with a view to ultimately translating to the clinic [25,40,41]. These include boronate-based bioluminescent [41,42] and fluorine-18 containing positron emission tomography (PET) probes [40]. Several PET probes including fluorodeoxyglucose (FDG) are now used in the clinic for diagnosing tumor growth [43]. However, regardless of the technology’s enhanced sensitivity, the basic ROS chemistry of the radiolabeled probes is the same as described for unlabeled probes. Thus, advantages and limitations of radiolabeled probes will be the same as those described for unlabeled ROS probes.
With the emerging synthetic capability that now exists for developing radiolabeled boronate-based bioluminescent probes and fluorescent probes yielding products that emit light in the red and infrared regions, and with our increased mechanistic understanding of ROS/fluorescent and chemiluminescent probe chemistry, it is highly likely that specific ROS imaging will be achieved in preclinical rodent models in the very near future.
Mitochondrial ROS and redox signaling
The role of ROS (particularly H2O2) in cellular signaling through redox mechanisms (redox signaling) in pathophysiological processes is gaining traction [44–46]. Recent reports emphasized that mitochondria-generated ROS act as signaling molecules regulating cell proliferation, stimulating mitogenic signaling in vascular cells and pro-tumorigenic signaling in cancer cells [47]. We initially used mitochondria-targeted antioxidants (Mito-CP, Mito-Tempol, Mito-Vit-E, and Mito-Q) and reported that at very low concentrations these compounds inhibited tumor cell proliferation [47–50]. The antiproliferative effects were attributed to mitigation of mitochondrial ROS [39]. In a subsequent publication, we used a mitochondria-targeted acetamido analog (the nitroxide group in Mito-CP was converted to an acetamido group), and this compound that is devoid of the antioxidant group (nitroxide) also effectively inhibited tumor cell proliferation [51]. Clearly, further research is required to fully understand the action mechanisms of mitochondria-targeted cationic compounds [51,52].
Mitochondria-targeted metformin: redox signaling mechanism
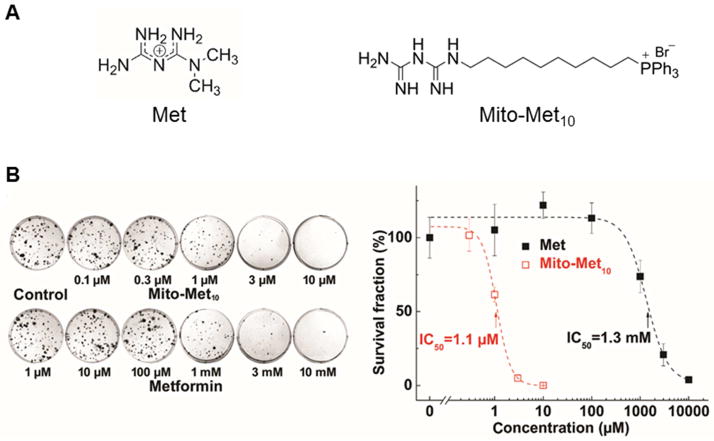
In a recent publication [52], we showed that a mitochondria-targeted metformin (Met) analog called Mito-Met, synthesized by attaching a TPP+ group to Met via a 10-carbon aliphatic side chain (Figure 10A) was nearly 1,000-fold more effective than Met in inhibiting pancreatic ductal adenocarcinoma (PDAC) cell proliferation (Figure 10B).
Figure 10. Effects of Met and Mito-Met10 on proliferation of pancreatic cancer cells.
(A) Chemical structures of metformin (Met) and Mito-metformin (Mito-Met10). (B) Effects of Mito-Met10 and Met (24-h treatment) on colony formation in MiaPaCa-2 cells. (B, right) The calculated survival fraction plotted against concentration of Met and Mito-Met10. (Adapted from [52].)
Mito-Met treatment dramatically inhibited mitochondrial respiration and potently inhibited complex I activity and complex I-dependent oxygen consumption in pancreatic cancer cells. As compared to the untargeted Met, Mito-Met much more potently (>200-fold) inhibited the mitochondrial complex I activity in PDACs. The effect of Mito-Met was more selective in that much higher concentrations were required to inhibit complex I-dependent oxygen consumption in human pancreatic epithelial nestin-expressing (HPNE) cells [52].
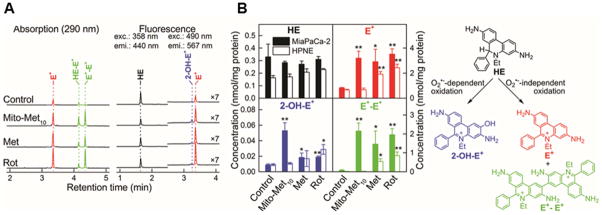
A consequence of mitochondrial complex I inhibition is enhanced generation of superoxide and hydrogen peroxide [53]. However, this aspect has not been satisfactorily demonstrated in cancer cells under conditions where the drug inhibits only tumor cell proliferation without exerting cytotoxicity. Using the cell-permeable probe HE, with HPLC-based detection of specific oxidation products, we detected a marked increase in the formation of 2-hydroxyethidium, a specific marker product of the superoxide reaction with HE, along with increased diethidium product (indicative of enhanced one-electron oxidant formation) in pancreatic cancer cells treated with Mito-Met but not Met (Figure 11) [52]. At the same concentration Mito-Met did not stimulate O2•− formation in control, nontumorigenic HPNE cells, consistent with the lack of inhibition of mitochondrial complex I in these cells. Thus, superoxide produced upon inhibition of mitochondrial complex I can be detected using the cell-permeable probe HE, coupled with HPLC-based analysis of 2-hydroxyethidium.
Figure 11. Effect of Met, Mito-Met10 and rotenone (ROT) on the oxidation profile of HE probe in tumorigenic (MiaPaCa-2) and normal (HPNE) pancreas cells.
(A) HPLC traces from MiaPaCa-2 cells; (B) Quantitative data showing the distribution of the HE oxidation products and chemical scheme of probe oxidation. (Adapted from [52].)
Are we detecting the signaling ROS?
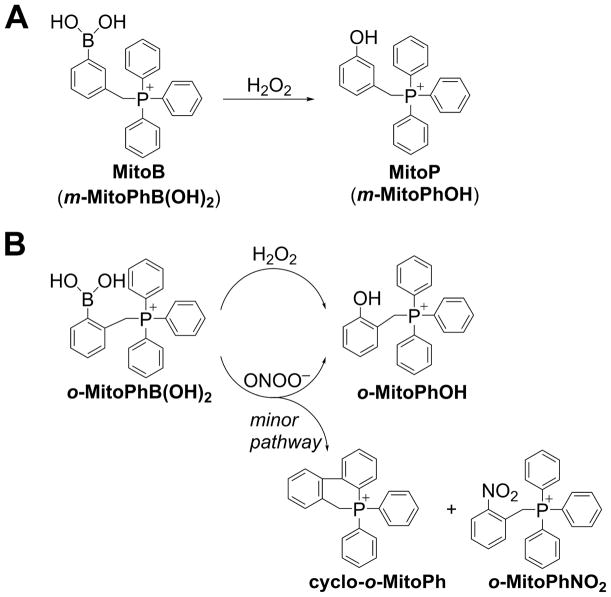
Although we detected Mito-Met-induced O2•−, which is presumably the precursor to the actual signaling species, H2O2, we have failed to detect H2O2 using the Amplex Red probe. As discussed earlier, boronate-based probes, despite their low reactivity with H2O2, may enable detection of intracellular H2O2 when used at high concentrations. Mitochondria-targeted boronates (e.g., Mito-B, Figure 12A) have been successfully used to detect mitochondrial H2O2 by monitoring the phenolic product using mass spectrometry [11,13,54]. The use of ortho-isomer of Mito-B (ortho-MitoPhB(OH)2, Figure 12B) not only will enable monitoring of the extent of probe oxidation, but also differentiation between H2O2 and peroxynitrite as possible oxidants, by monitoring the distribution of the products formed (Figure 12B) [11–13]. However, when using mitochondria-targeted probes, one has to be cautious and make sure that the probe, at the levels used, does not inhibit complex I and artifactually induce additional H2O2. It has been proposed that mitochondria-targeted probes, including Mito-SOX, may affect mitochondrial function [55–57]. Also, the HE probe at a high concentration is toxic; this has been attributed to the cytotoxicity of HE oxidation products, which, being lipophilic cations, can accumulate in mitochondria and affect mitochondrial function [58]. Additional careful and rigorous research is needed using a variety of conditions (different probe concentrations, time of incubation, cell lines, etc.) before Mito-B (or other mitochondria-targeted probes) can be used confidently to detect mitochondrial ROS.
Figure 12. The use of mitochondria-targeted boronate probes for detection of H2O2.
(A) Oxidation of MitoB probe by H2O2; (B) Products formed upon oxidation of ortho-MitoPhB(OH)2 probe by H2O2 vs. ONOO−.
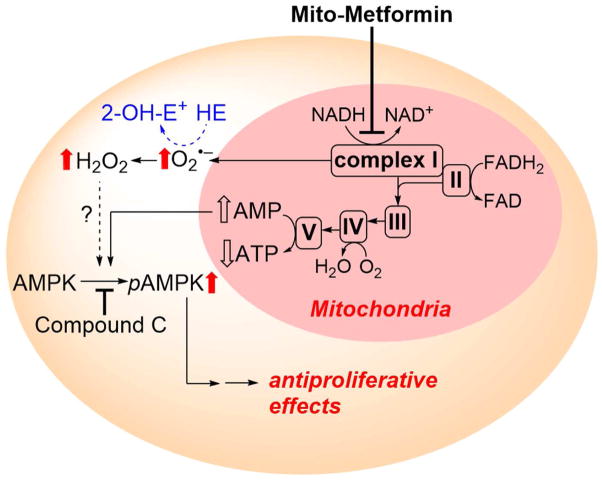
The proposed mechanism by which mitochondria-targeted compounds such as Mito-Met inhibit tumor cell proliferation is shown in Figure 13. Mitochondrial complex I is proposed as a primary target for Mito-Met, although we do not yet have any proof of association or binding between Mito-Met and complex I. As we observed a marked decrease in the complex I activity, we attribute the increased O2•− production to complex I inhibition. Based on the results obtained with compound c, a potent and reversible 5′-adenosine monophosphate-activated protein kinase (AMPK) inhibitor that counteracted the antiproliferative effects of Mito-Met, we proposed a role for AMPK signaling [52].
Figure 13.
Proposed pathways of redox signaling induced by treatment of pancreatic cancer cells with Mito-Met10, leading to inhibition of cell proliferation. HE probe was used to detect intracellular superoxide formation. (Adapted from [52].)
At present, the exact mechanisms linking H2O2 and antiproliferative effects of Mito-Met are still unknown. Emerging results suggest that H2O2 regulates cell signaling by oxidizing specific thiol proteins [59]. The investigators attributed the enhanced signaling by H2O2 to a redox relay—a peroxiredoxin-mediated redox signaling mechanism that transfers the oxidizing equivalent of H2O2 to a target protein [59]. In this mechanism, peroxiredoxins react rapidly with H2O2 and form an oxidized intermediate involving a sulfenic acid. This oxidized peroxiredoxin intermediate transfers the oxidizing equivalents to target proteins, including the transcription factor signal transducer and activator of transcription 3 (STAT3), forming a mixed disulfide intermediate that is reduced by thioredoxin. There are several peroxiredoxins, cytosolic and mitochondrial, and they are all susceptible to oxidation in the presence of H2O2. This type of “redox relay” between H2O2 and the target protein (i.e., STAT3) is novel; if these signaling processes are linked to specific ROS whose source and structure are properly identified and ultimately linked to cell proliferation, we undoubtedly will improve our understanding of redox signaling by ROS.
Concluding remarks
Cellular superoxide can be detected using an HE probe coupled with the detection of the specific product, 2-hydroxyethidium, using HPLC and LC-MS techniques. In addition, HE-derived products (ethidium and ethidium-ethidium dimer) indicative of the presence of two-electron and one-electron oxidants can be detected in cell lysates and mitochondria. These marker products were generated and detected upon treatment of pancreatic cancer cells with mitochondria-targeted metformin analog, under conditions in which little or no cytotoxicity was induced by Mito-Met. We speculate that the O2•− and H2O2 derived from it are involved in redox signaling upon inhibition of mitochondrial complex I.
The superoxide radical anion generated from Nox enzymes can be detected using the cell-impermeable HE analog, hydropropidine, with the detection of the characteristic hydroxylated product (2-hydroxypropidium). Superoxide generated from Nox enzymes can also be detected intracellularly and extracellularly using the HE probe.
Superoxide detection with HE and Mito-SOX in cytosolic and mitochondrial compartments using fluorescence microscopy is not recommended due to many artifacts, including the multiplicity of the products formed.
Boronate-based probes are useful for detecting cellular peroxynitrite and hydrogen peroxide. Although the rate of reaction between boronates and H2O2 is low, one can use higher concentrations of the probe and determine the H2O2 (catalase-sensitive) generated from Nox or use probes that are targeted to and concentrated in subcellular compartments (e.g., mitochondria).
Many commercially available ROS kits contain probes for which the chemistry and mechanism of reactions with ROS are unknown. In some cases, even the chemical structure of the probes is unknown. Our advice to researchers interested in ROS measurements is to avoid use of these probes and assays as they lack rigor and transparency.
Hydroethidine can detect O2•− formed upon inhibition of mitochondrial complex I
Mitochondria-targeted boronates can be used to detect mitochondria-derived H2O2
HPLC-based assays enable rigorous analysis of cellular production of O2•− and H2O2
Rigorous O2•−/H2O2 detection will help elucidate the mechanisms of redox signaling
Acknowledgments
Funding: This work was supported by grants from the NIH National Cancer Institute (R01 CA152810 to B. Kalyanaraman).
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsien R. Roger Tsien: bringing color to cell biology. A passion for chemistry, color, and conversations with cells is what drives Roger Tsien Interview by Ruth Williams. J Cell Biol. 2007;179:6–8. doi: 10.1083/jcb.1791pi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zielonka J, Kalyanaraman B. Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: another inconvenient truth. Free Radic Biol Med. 2010;48:983–1001. doi: 10.1016/j.freeradbiomed.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 20031;34:359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–32. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zielonka J, Srinivasan S, Hardy M, Ouari O, Lopez M, Vasquez-Vivar J, Avadhani NG, Kalyanaraman B. Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: identification of homo- and heterodimers. Free Radic Biol Med. 2008;44:835– 46. doi: 10.1016/j.freeradbiomed.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zielonka J, Sarna T, Roberts JE, Wishart JF, Kalyanaraman B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch Biochem Biophys. 2006;456:39–47. doi: 10.1016/j.abb.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalski R, Michalowski B, Sikora A, Zielonka J, Kalyanaraman B. On the use of fluorescence lifetime imaging and dihydroethidium to detect superoxide in intact animals and ex vivo tissues: A reassessment. Free Radic Biol Med. 2014;67:278–284. doi: 10.1016/j.freeradbiomed.2013.10.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dranka BP, Zielonka J, Kanthasamy AG, Kalyanaraman B. Alterations in bioenergetic function induced by Parkinson’s disease mimetic compounds: lack of correlation with superoxide generation. J Neurochem. 2012;122:941–51. doi: 10.1111/j.1471-4159.2012.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talib J, Maghzal GJ, Cheng D, Stocker R. Detailed protocol to assess in vivo and ex vivo myeloperoxidase activity in mouse models of vascular inflammation and disease using hydroethidine. Free Radic Biol Med. 2016;97:124–135. doi: 10.1016/j.freeradbiomed.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Maghzal GJ, Cergol KM, Shengule SR, Suarna C, Newington D, Kettle AJ, Payne RJ, Stocker R. Assessment of myeloperoxidase activity by the conversion of hydroethidine to 2-chloroethidium. J Biol Chem. 2014;289:5580–95. doi: 10.1074/jbc.M113.539486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zielonka J, Sikora A, Adamus J, Kalyanaraman B. Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods Mol Biol. 2015;1264:171–181. doi: 10.1007/978-1-4939-2257-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielonka J, Zielonka M, VerPlank L, Cheng G, Hardy M, Ouari O, Ayhan MM, Podsiadly R, Sikora A, Lambeth JD, Kalyanaraman B. Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J Biol Chem. 2016;291:7029–44. doi: 10.1074/jbc.M115.702787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikora A, Zielonka J, Adamus J, Debski D, Dybala-Defratyka A, Michalowski B, Joseph J, Hartley RC, Murphy MP, Kalyanaraman B. Reaction between peroxynitrite and triphenylphosphonium-substituted arylboronic acid isomers: identification of diagnostic marker products and biological implications. Chem Res Toxicol. 2013;26:856–67. doi: 10.1021/tx300499c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zielonka J, Vasquez-Vivar J, Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 15.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–43. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994;16:149–56. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 17.Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescien assay. Anal Biochem. 1983;134:111–6. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- 18.Tampo Y, Kotamraju S, Citambar CR, Kalivendi SV, Joseph J, Kalyanaraman B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res. 2003;92:56–63. doi: 10.1161/01.res.0000048195.15637.ac. [DOI] [PubMed] [Google Scholar]
- 19.Rota C, Chignell CF, Mason RP. Evidence for free radical formation during the oxidation of 2′-7′-dichlorofluorescin to the fluorescent dye 2′-7′-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic Biol Med. 1999;27:873–81. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 20.Wrona M, Wardman P. Properties of the radical intermediate obtained on oxidation of 2′,7′-dichlorodihydrofluorescein, a probe for oxidative stress. Free Radic Biol Med. 2006;41:657–67. doi: 10.1016/j.freeradbiomed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Folkes LK, Patel KB, Wardman P, Wrona M. Kinetics of reaction of nitrogen dioxide with dihydrorhodamine and the reaction of the dihydrorhodamine radical with oxygen: implications for quantifying peroxynitrite formation in cells. Arch Biochem Biophys. 2009;484:122–6. doi: 10.1016/j.abb.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: A self-fulfilling prophesy? Free Radic Biol Med. 2006;40:968–975. doi: 10.1016/j.freeradbiomed.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Kuivila HG. Electrophilic displacement reactions. III. Kinetics of the reaction between hydrogen peroxide and benzeneburonic acid. J Am Chem Soc. 1954;76:870–4. [Google Scholar]
- 25.Lippert AR, Van de Bittner GC, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res. 2011;44:793–804. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zielonka J, Cheng G, Zielonka M, Ganesh T, Sun A, Joseph J, Michalski R, O’Brien WJ, Lambeth JD, Kalyanaraman B. High-throughput assays for superoxide and hydrogen peroxide: Design of a screening workflow to identify inhibitors of NADPH oxidases. J Biol Chem. 2014;289:16176–16189. doi: 10.1074/jbc.M114.548693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zielonka J, Sikora A, Joseph J, Kalyanaraman B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: direct reaction with boronate-based fluorescent probe. J Biol Chem. 2010;285:14210–6. doi: 10.1074/jbc.M110.110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zielonka J, Sikora A, Hardy M, Joseph J, Dranka BP, Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem Res Toxicol. 2012;25:1793–9. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sikora A, Zielonka J, Lopez M, Joseph J, Kalyanaraman B. Direct oxidation of boronates by peroxynitrite: Mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic Biol Med. 2009;47:1401–1407. doi: 10.1016/j.freeradbiomed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielonka J, Zielonka M, Sikora A, Adamus J, Joseph J, Hardy M, Ouari O, Dranka BP, Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J Biol Chem. 2012;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, Summers FA, Mason RP. Photooxidation of Amplex Red to resorufin: implications of exposing the Amplex Red assay to light. Free Radic Biol Med. 2012;53:1080–7. doi: 10.1016/j.freeradbiomed.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summers FA, Zhao B, Ganini D, Mason RP. Photooxidation of Amplex Red to resorufin: implications of exposing the Amplex Red assay to light. Methods Enzymol. 2013;526:1–17. doi: 10.1016/B978-0-12-405883-5.00001-6. [DOI] [PubMed] [Google Scholar]
- 33.Dębski D, Smulik R, Zielonka J, Michałowski B, Jakubowska M, Dębowska K, Adamus J, Marcinek A, Kalyanaraman B, Sikora A. Mechanism of oxidative conversion of Amplex® Red to resorufin: pulse radiolysis and enzymatic studies. Free Radic Biol Med. 2016;95:323–332. doi: 10.1016/j.freeradbiomed.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miwa S, Treumann A, Bell A, Vistoli G, Nelson G, Hay S, von Zglinicki T. Carboxylesterase converts Amplex red to resorufin: Implications for mitochondrial H2O2 release assays. Free Radic Biol Med. 2016;90:173–83. doi: 10.1016/j.freeradbiomed.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nisimoto Y, Diebold BA, Constentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–20. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–9. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 37.Altenhofer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH. Evolution of NADPH Oxidase Inhibitors: Selectivity and Mechanisms for Target Engagement. Antioxid Redox Signal. 2015;23:406–27. doi: 10.1089/ars.2013.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teixeira G, Szyndralewiez C, Molango S, Carnesecchi S, Heitz F, Wiesel P, Wood JM. Therapeutic potential of NOX1/4 inhibitors. Br J Pharmacol. 2006 doi: 10.1111/bph.13532. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D, Kim G, Nam SJ, Yin J, Yoon J. Visualization of endogenous and exogenous hydrogen peroxide using a lysosome-targetable fluorescent probe. Sci Rep. 2015;5:8488. doi: 10.1038/srep08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carroll V, Michel BW, Blecha J, VanBrocklin H, Keshari K, Wilson D, Chang CJ. A boronate-caged [18F]FLT probe for hydrogen peroxide detection using positron emission tomography. J Am Chem Soc. 2014;136:14742–5. doi: 10.1021/ja509198w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van de Bittner GC, Dubikovskaya EA, Bertozzi CR, Chang CJ. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc Natl Acad Sci U S A. 2010;107:21316–21. doi: 10.1073/pnas.1012864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieracki NA, Gantner BN, Mao M, Horner JH, Ye RD, Malik AB, Newcomb ME, Bonini MG. Bioluminescent detection of peroxynitrite with a boronic acid-caged luciferin. Free Radic Biol Med. 2013;61:40–50. doi: 10.1016/j.freeradbiomed.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spick C, Herrmann K, Czernin J. 18F-FDG PET/CT and PET/MRI Perform Equally Well in Cancer: Evidence from Studies on More Than 2,300 Patients. J Nucl Med. 2016;57:420–30. doi: 10.2967/jnumed.115.158808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winterbourn CC, Hampton WB. Redox biology: signaling via a peroxiredoxin sensor. Nat Chem Biol. 2015;11:5–6. doi: 10.1038/nchembio.1722. [DOI] [PubMed] [Google Scholar]
- 45.Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. J Mol Cell Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diebold L, Chandel NS. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.04.198. in press, http://dx.doi.org/10.1016/j.freeradbiomed.2016.04.198. [DOI] [PubMed]
- 48.Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng G, Zielonka J, McAllister D, Tsai S, Dwinell MB, Kalyanaraman B. Profiling and targeting of cellular bioenergetics: inhibition of pancreatic cancer cell proliferation. Br J Cancer. 2014;111:85–93. doi: 10.1038/bjc.2014.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng G, Zielonka J, McAllister DM, Mackinnon AC, Joseph J, Dwinell MB, Kalyanaraman B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng G, Zielonka J, McAllister D, Hardy M, Ouari O, Joseph J, Dwinell MB, Kalyanaraman B. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015;365:96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng G, Zielonka J, Ouari O, Lopez M, McAllister D, Boyle K, Barrios CS, Weber JJ, Johnson BD, Hardy M, Dwinell MB, Kalyanaraman B. Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Res. 2016;76:3904–15. doi: 10.1158/0008-5472.CAN-15-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochemé HM, Logan A, Prime TA, Abakumova I, Quin C, McQuaker SJ, Patel JV, Fearnley IM, James AM, Porteous CM, Smith RA, Hartley RC, Partridge L, Murphy MP. Using the mitochondria-targeted ratiometric mass spectrometry probe MitoB to measure H2O2 in living Drosophila. Nat Protol. 2012;7:946–58. doi: 10.1038/nprot.2012.035. [DOI] [PubMed] [Google Scholar]
- 55.Cheng G, Zielonka J, Kalyanaraman B. Modulatory effects of MitoSOX on cellular bioenergetics: a cautionary note. Free Radic Biol Med. 2011;51:S37–S38. [Google Scholar]
- 56.Roelofs BA, Ge SX, Studlack PE, Polster BM. Low micromolar concentrations of the superoxide probe MitoSOX uncouple neural mitochondria and inhibit complex IV. Free Radic Biol Med. 2015;86:250–258. doi: 10.1016/j.freeradbiomed.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polster BM, Nicholls DG, Ge SX, Roelofs BA. Use of Potentiometric Fluorophores in the Measurement of Mitochondrial Reactive Oxygen Species. Methods Enzymol. 2014;547:225–250. doi: 10.1016/B978-0-12-801415-8.00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyublinskaya OG, Zenin VV, Shatrova AN, Aksenov ND, Zemelko VI, Domnina AP, Litanyuk AP, Burova EB, Gubarev SS, Negulyaev YA, Nikolsky NN. Intracellular oxidation of hydroethidine: Compartmentalization and cytotoxicity of oxidation products. Free Radic Biol Med. 2014;75:60–68. doi: 10.1016/j.freeradbiomed.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Sobotta MC, Liou W, Stöcker S, Talwar D, Oehler M, Ruppert T, Scharf AN, Dick TP. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling TP1. Nat Chem Biol. 2015;11:64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 60.Kalyanaraman B, Dranka BP, Hardy M, Michalski R, Zielonka J. HPLC-based monitoring of products formed from hydroethidine-based fluorogenic probes - The ultimate approach for intra-and extracellular superoxide detection. Biochim Biophys Acta. 2014;1840:739–744. doi: 10.1016/j.bbagen.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]