Abstract
Pancreatic cancer remains one of the major causes of cancer-related mortality. The majority of pancreatic cancer patients are diagnosed at the advanced stage with unresectable and drug resistant tumors. The new treatments with the combination of chemotherapy, molecular targeted therapy, and immunotherapy have shown modest effects on therapeutic efficacy and survival of the patients. Therefore, there is an urgent need to develop effective therapeutic approaches targeting highly heterogeneous pancreatic cancer cells and tumor microenvironments. Recent advances in biomarker targeted cancer therapy and image-guided drug delivery and monitoring treatment response using multifunctional nanoparticles, also referred to as theranostic nanoparticles, offer a new opportunity of effective detection and treatment of pancreatic cancer. Increasing evidence from preclinical studies has shown the potential of applications of theranostic nanoparticles for designing precision oncology approaches for pancreatic cancer therapy. In this review, we provide an update on the current understanding and strategies for the development of targeted therapy for pancreatic cancer using nanoparticle drug carriers. We address issues concerning drug delivery barriers in stroma rich pancreatic cancer and the potential approaches to improve drug delivery efficiency, therapeutic responses and tumor imaging. Research results presented in this review suggest the development of an integrated therapy protocol through image-guided and targeted drug delivery and therapeutic effect monitoring as a promising precision oncology strategy for pancreatic cancer treatment.
Keywords: pancreatic cancer, molecular imaging, targeted therapy, image-guided cancer therapy, theranostic nanoparticles
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in the United States [1, 2]. In 2016, it is estimated that 53,070 new cases of pancreatic cancer will be diagnosed in the US, and 41,789 pancreatic cancer patients will die as a result of this disease [1]. Pancreatic ductal adenocarcinoma (PDAC) is the most common cancer type (over 95%) [2]. Because of its aggressive biological nature and the ineffectiveness of current treatments, PDAC has a mortality rate almost equal to its incidence with a five-year survival rate of 5%. One important reason for the poor survival is that the majority (over 85%) of the patients are diagnosed with advanced diseases and have a poor prognosis [1]. Only few patients (approximately 15%) are diagnosed when they are at the earliest-stage of PDAC and are candidates for the potentially curative surgery [3].
A challenge for screening and early detection of PDAC is that patients lack specific clinical symptoms at the early stage and the risk factors are not well known except for smoking and family history [4]. A major problem is that human pancreatic cancer is highly heterogeneous with a tumor mass from a single patient containing 63 genetic alterations and 12 core signal pathway abnormalities. There are also heterogeneity in tumor biomarker expression among different cancer patients [5]. Currently, blood-test based on biomarkers and imaging methods are clinical standard due to the ease of operation, and relatively noninvasive nature [4, 6]. Tumor biomarkers that allow for the reliable diagnosis of pancreatic cancer have yet to be identified. However, investigations are ongoing to evaluate the effect of several biomarkers for pancreatic cancer detection. So far, the levels of serum carbohydrate antigen (CA 19-9), CA-125, ICAM-1, CEA, mutant Kras DNA, miRNAs, and glypican-1 in exosomes [7, 8] have been evaluated as serum biomarkers for the detection of pancreatic cancer. However, many of those biomarkers are not specific for pancreatic cancer since they are also expressed in other tumor types and some of them on normal and premalignant tissues. It is likely that the combination of serum biomarker detection with the biomarker targeted molecular imaging to localize and characterize pancreatic cancer lesions will improve sensitivity and specificity of the early detection of pancreatic cancer.
At present, various targeted imaging probes have been developed for non-invasive tumor imaging using different imaging modalities. For example, nanoparticle imaging probes targeting plectin-1, mesothelin, uPAR, EGFR, and IGF-1R are under investigations for the detection of pancreatic cancer [9–12]. With the development of nanomedicine, it is expected that nanomaterials modified with PDAC targeting ligands can facilitate the early diagnosis of PDAC so that personalized therapeutic strategy can be designed and applied timely to the patients. Additionally, biomarker targeted imaging nanoparticles are not only promising imaging contrasts for tumor detection, but also for the evaluation of the biomarker expression in primary and metastatic pancreatic cancers for stratifying the patients with biomarker positive tumors for targeted therapy.
Currently, therapeutic options applicable to PDAC are still limited to surgery, chemotherapy and radiotherapy. Most PDAC patients have advanced diseases and are treated by chemotherapy. However, most chemotherapeutics are not very effective with minimal impact on survival in most cases. For example, advanced PDAC patients receiving gemcitabine (2′-2′-difluorodeoxycytidine) or the combination of fluorouracil, oxaliplatin, irinotecan (CPT-11) and folinic acid (FOLFIRINOX) treatment, still have median survival less than 12 months [13]. One reason for this unfavorable treatment outcome is the presence of “desmoplasia” in PDAC, which is known as tumor stroma with characteristics of abnormal and poorly functioning vasculatures, altered extracellular matrix, infiltrating macrophages and proliferation of active fibroblasts. Intense tumor stroma consisting of 50–80% of the PDAC tissues creates a drug delivery barrier for many therapeutic agents, including small molecules, higher molecular weight antibody-based drugs, and nanoparticle formulations. In addition, PDAC cells are highly resistant to chemotherapy drugs [14], because of abnormal levels of gene expression, genetic mutations, activation or inhibition of cellular signal pathways, tumor hypoxia, and the stroma-rich tumor microenvironment [15]. Therefore, new and more potent therapeutic agents and novel treatment protocols will improve the clinical outcomes of pancreatic cancer. Since pancreatic tumor microenvironment plays critical roles in tumor biology, drug delivery and response to therapy, it is necessary to consider the stroma effect for designing and evaluation of new therapeutic agents [16].
In this article, we will provide an overview of the current research regarding potential biomarkers or molecular targets in pancreatic cancer for the development of targeted therapy and imaging for pancreatic cancer PDAC with a particular focus on nanoparticle or theranostic nanoparticle drug carriers.
Pancreatic cancer biomarkers and molecular targets
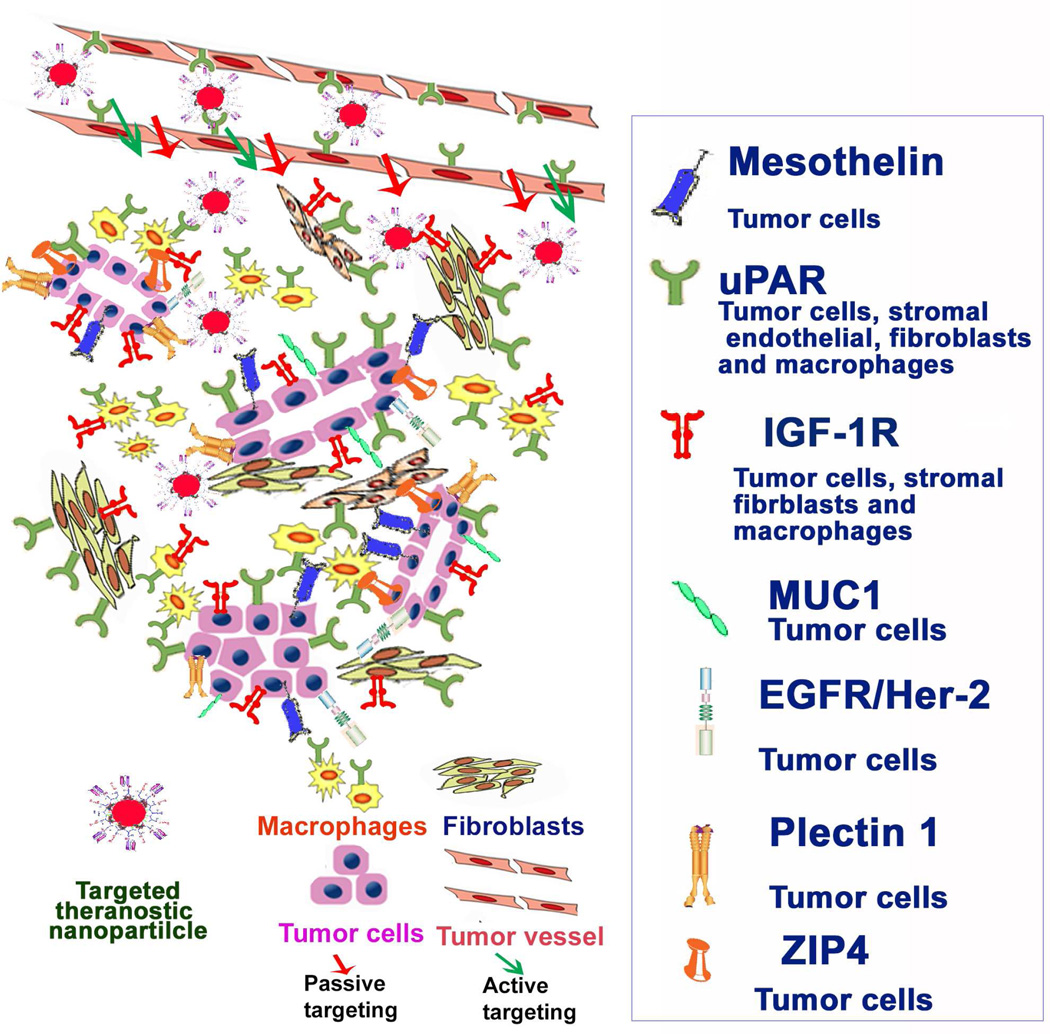
Over the years, intensive efforts have been devoted to identify biomarkers and molecular targets for the development of targeted molecular imaging and therapeutic approaches [17]. In comparison with other solid tumors, PDAC has unique pathological characteristics to consider when developing imaging and drug delivery agents. Several molecules that are currently under evaluation for PDAC targeted treatment based on their roles in tumorigenesis and progressions will be reviewed here (Figure 1). Although these biomarkers may not be specific for PDAC, the detection of the presence of high levels of the biomarkers in suspected pancreatic lesions should not only provide supportive information for diagnosis of pancreatic cancer, but also identify the cell surface molecular targets for the development of biomarker targeted nanoparticle drug carriers, or theranostic nanoparticles. The following are examples of cell surface biomarkers that are highly expressed in pancreatic cancer for the development of tumor targeted imaging and therapeutic agents:
Figure 1. Molecular biomarkers for the development of targeted nanoparticle drug carriers and theranostic nanoparticles for pancreatic cancer therapy.
Mesothelin
Mesothelin is a membrane type protein that is normally expressed in mesothelial cells of pleura, peritoneum and pericardium. A high level of mesothelin is detected in various types of cancers, including mesothelioma, non-small cell lung, ovarian and pancreatic cancers [18]. Studies so far have reported that mesothelin is related to cell survival, migration, invasion and tumor progression [19, 20]. It was also uncovered that silencing the expression of mesothelin suppressed tumor growth [21], implying that mesothelin can be a potential marker for cancers, including PDAC [22]. Indeed, mesothelin was detected in PDAC tissues but not in normal pancreas and chronic pancreatitis [23]. It was reported that significantly elevated levels of circulating mesothelin protein were detected in 73 of the 74 patients with pancreatic adenocarcinoma, and in all five patients with benign pancreatic disease, but not in the healthy controls [24] As the most of the other PDAC biomarkers, mesothelin is not a PDAC specific biomarker but can be combined with other biomarkers for confirming diagnosis of PDAC [25]. Recent studies have shed light on the possible role of mesothelin as an antigen for immunotherapy. A positive correlation between prognosis and the presence of a humoral response to mesothelin has been shown in pancreatic cancer patients [26]. It has been reported that modification of imaging agents and drug-delivery systems with anti-mesothelin antibody would improve diagnostic and therapeutic efficiency in mesothelin overexpressing PDAC [27]. Mesothelin antibody conjugated liposomes loaded with iron oxides and doxorubicin (DOX) has shown the ability of simultaneous detection and therapy of PDAC in a pancreatic cancer xenograft animal model [28], suggesting that the role of targeting mesothelin in imaging and therapy of pancreatic cancer. In addition, several other mesothelin targeted complexes are under clinical evaluations as therapeutics, such as SS1(dsFv)-PE38 (a recombinant single chain anti-mesothelin and immunotoxin fusion protein), anti-mesothelin antibody drug conjugates (BAY-94 9343), mesothelin tumor vaccine (CRS-207), and mesothelin-specific chimeric antigen receptors (CAR)[29]. Currently, mesothelin is considered as a cell surface target of pancreatic cancer cells. Therefore, mesothelin targeted therapeutic agents and nanoparticle drug carriers have to overcome the tumor stromal barriers to be delivered into tumor cells.
Urokinase Plasminogen Activator/Urokinase Plasminogen Activator Receptor
Urokinase plasminogen activator (uPA) is a serine proteinase that activates plasminogen into functional plasmin to promote protease activity. Its function involves tumor growth, invasion metastasis, and angiogenesis by activation of matrix metalloproteinases and degradation of extracellular matrix A high level of uPA, therefore, has been associated with a poor prognostic biomarker for cancer patients [30]. Cellular receptor of uPA (uPAR) is anchored on plasma membrane via a glycosylphosphatidylinositol (GPI) and controls uPA mediated plasminogen activation [31]. It is overexpressed in tumor cells, especially invasive tumor cells, and several stromal cell types in tumor microenvironment, including angiogenic tumor endothelial cells, active fibroblasts, and active macrophages [32]. In addition, the soluble form of uPAR (suPAR) in urine was also reported as a useful marker for the identification of a subset of patients with poorer outcome [30]. A marked pathological characteristic of pancreatic cancer is the presence of an extensive tumor stroma that consists of 50-85% of a tumor mass. It has been shown that about 80 to 98% of human pancreatic cancer tissues have a high level of uPAR expression and 58% of them have uPAR gene amplification [33]. The level of uPAR mRNA in pancreatic tumor tissue is 9.6 folds higher than that of the adjacent normal tissues. Among 15 biomarkers examined, uPAR was placed as the first biomarker that could distinguish cancer from normal tissues. Results of another study also showed that uPAR is the most accurate biomarker among 29 pancreatic cancer biomarkers to discriminate between PDAC and chronic pancreatitis [34].
Since high levels of uPAR expression are found in pancreatic cancer and tumor stromal fibroblasts and macrophages, targeting uPAR for the development of tumor imaging and therapy agents has the potential to improve intratumoral delivery and distribution by binding to multiple cell types, increasing retention of small nanoparticles in the tumor tissue, and improving intratumoral cell drug delivery efficiency by receptor-mediated internalization. Therefore, uPAR is an excellent molecular target for the development of targeted imaging and therapy agents for stroma rich pancreatic cancer. Several groups have utilized uPAR or uPA-targeted nanoparticle for tumor imaging and treatment in preclinical studies using pancreatic animal tumor models. Results of our studies also showed that systemic delivery of uPAR targeted magnetic iron oxide nanoparticles (IONPs) led to the selective accumulation of the nanoparticles in orthotopic pancreatic tumors and detection of tumor lesions as small as I mm3 in diameter in a human pancreatic cancer xenograft model by non-invasive NIR optical and MR imaging [12].
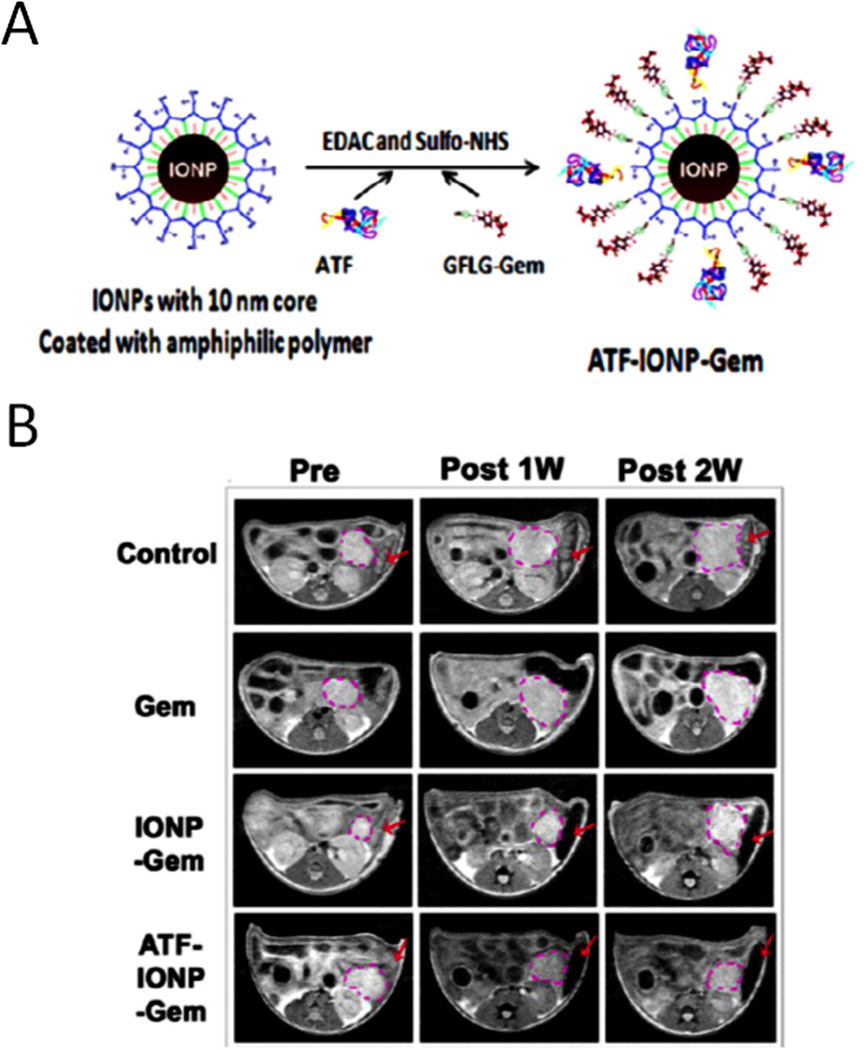
Recent studies have demonstrated the ability of overcoming drug resistant mechanisms by receptor-mediated internalization of nanoparticle-drug complexes. It has been shown that endocytosis of uPA-uPAR complex led to nuclear translocation of the complex, suggesting the presence of an endosomal escape mechanism for targeted nanoparticles and its therapeutic payload [12, 35]. In addition to be a pair of attractive PDAC target and targeting ligand, uPA/uPAR has been reported related to aggressive and drug-resistant phenotype [36]. Downregulation of uPA expression in pancreatic cancer cells sensitizes to drug treatment [30, 37]. To overcome the physical barrier of the stroma in drug delivery, theranostic nanoparticles targeting uPAR have been developed by our group and their anti-tumor effects have been examined in a human pancreatic cancer xenograft model in nude mice. Amino-terminal fragment (ATF) peptides of uPA were conjugated onto IONPs carrying a conditional release chemotherapy drug, gemcitabine (ATF-IONP-Gem) [38]. Systemic delivery of uPAR-targeted ATF-IONP-Gem resulted in significant growth inhibition of pancreatic tumors (Figure 2).
Figure 2. uPAR targeted theranostic nanoparticles for the treatment of pancreatic cancer.
A. Preparation of uPAR targeted iron oxide nanoparticle (IONP) loaded with gemcitabine. B. Axial T2-weighted MR images of the tumor-bearing mice before, one week, and two weeks after receiving theranostic nanoparticles. The location and size of the cancer lesions (pink dotted circles) were seen by MR images. Red arrows point out the MRI contrast change (darkening) in the spleen. Reprinted with permission from [38]. Copyright (2016) American Chemical Society.
Growth factor receptors
Insulin Growth Factor 1 Receptor (IGF-1R)
IGF-1R is a cell receptor with tyrosine kinase activity. It is overexpressed in many tumors including PDAC [39]. IGF-1R signal pathway is well known to be associated with cancer cell growth, invasion and metastasis. Detection of a high level of IGF-1R in a lesion in the pancreas by molecular imaging should assist in detection of pancreatic cancer as well as selection of IGF-1R targeted therapeutic agents. Recently, IGF-1R targeted imaging probes have been shown to be able to detect PDAC using different imaging modalities such as fluorescent imaging [40], MRI [9] and PET imaging [41]. It has been shown that cancers overexpressing IGF-1R are more resistant to drug- and radiation-induced apoptosis. Following chemotherapy, drug resistant cancer cells further increase the level of IGF-1R expression [42]. Therefore, targeting IGF-1R may allow for overcoming drug resistance when treating pancreatic tumors [43]. Additionally, targeting IGF-1R that is highly expressed in tumor cells as well as tumor stromal fibroblasts and macrophages is a suitable approach for stroma-rich pancreatic cancer [9, 44]. Currently, a humanized monoclonal antibody specific to IGF-1R, named Dalotuzumab or MK-0646, has been developed for advanced pancreatic cancer treatment. MK-0646 specifically binds with IGF-1R and blocks its interaction with the IGF-I/ II ligands, which enhances gemcitabine-induced apoptosis and inhibits the MEK/Erk and the PI3-kinase/Akt signaling [45]. A randomized phase II study of MK-0646 is being conducted in combination with gemcitabine or gemcitabine plus erlotinib for advanced pancreatic cancer (NCT00769483) [45].
Epidermal growth factor receptor (EGFR and HER-2/neu)
EGFR is a membrane of epidermal growth receptor family that regulates cell growth and proliferation in response to the binding of growth factor [46]. Upon ligand binding, EGFR forms homo- or heterodimeric complexes (usually with HER2) and in turn activates intracellular signaling cascades, resulting in cell proliferation, production of proangiogenic factors, increase in cell invasion, and resistance to the apoptotic cell death [47]. EGFR is highly expressed in 45–95% of human pancreatic cancer tissues [48]. Therefore, targeting EGFR is an appealing approach for selective cancer therapy. Single-chain anti-EGFR antibody conjugated nanoparticles have been shown to target to orthotopic pancreatic tumors in a human pancreatic cancer xenograft model, implying the potential of EGFR-targeted delivery of therapeutic agents for PDAC using nanoparticle drug carriers [49]. Anti-EGFR monoclonal antibody was covalently conjugated onto Poly(lactic-co-glycolic acid) (PLGA) nanoparticles encapsulated with gemcitabine for selective PDAC chemotherapy with improved treatment response [50], demonstrating that this polymeric drug delivery system offers an excellent targeted drug delivery potency with translational potential by targeting of EGFR. It has been shown that another EGFR family cellular surface protein, Her-2/neu, is overexpressed in 30 to 52% of human pancreatic cancer tissues [51, 52]. Although anti-Her2 antibody treatment alone has not shown significant anti-tumor growth effect, the combination of antibodies for EGFR and Her-2/neu has demonstrated synergistic effect in human pancreatic cancer xenograft models in nude mice [53]. Anti-Her-2 antibody (Herceptin) or Her-2 affibody have been used as targeting ligands to develop Her-2 targeted imaging and therapy nanoparticles [54, 55].
For the development of nanoparticle based imaging and therapeutic agents for pancreatic cancer, EGFR and Her-2 targeted nanoparticles are delivered into tumor tissues by the enhanced permeability and retention effect (EPR) effect mediated by leaky tumor vessels. The majority of EGFR or Her2 targeted nanoparticle drug carriers may be trapped in the tumor stroma area before reaching EGFR or HER-2 overexpressing tumor cells.
Plectin-1
Plectin-1 is a multidomain protein with versatile binding properties[56]. Plectin-1 has been shown to interact with intermediate filament proteins of various types and to physically link intermediate filament proteins with microfilaments and microtubules [57, 58]. Plectin-1 expression is low in normal pancreatic ductal cells but is overexpressed in PDAC, indicating that plectin plays important roles in PDAC progression and would be a good target for PDAC [56]. A peptide named Plectin-1 Targeting Peptide (PTP) with the sequence of KTLLPTP was identified and attached to a magnetofluorescent nanoparticle for imaging of PDAC in vivo by intravital confocal microscopy [56]. In a later report, PTP peptide was labeled with 111In for single photon emission computed tomography (SPECT) detection of PDAC in an orthotopic and liver metastasis murine model [59]. More recently, an engineered adeno-associated virus (AAV) vector fusing with PTP (AAV-PTP) was developed for specifically targeting PDAC cells in vitro and in vivo [60]. Results from this study demonstrated a 37-fold preference of AAV-PTP for PDAC tumor over normal organs, confirming that plectin-1 is a promising target for PDAC. Current studies focus on the development of plectin-1 targeted imaging and therapeutic agents for the detection PDAC and ultimately therapy of PDAC in human patients.
Mucin-1
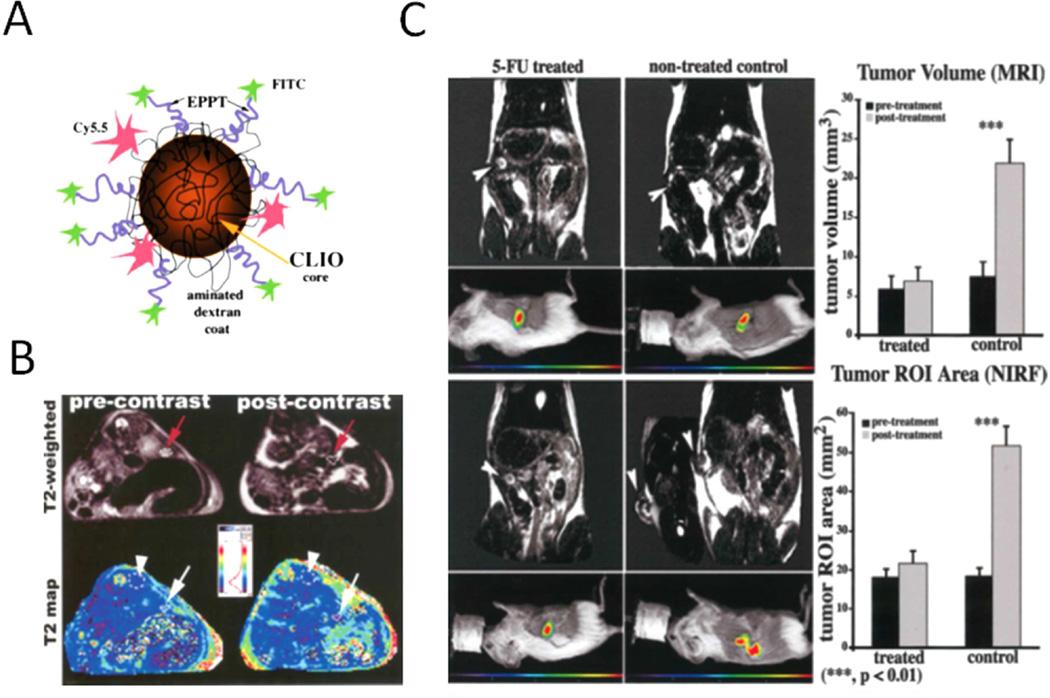
Mucin is a family of high-molecular-weight glycoproteins with a heavily O-glycosylated tandem repeat region (TRR). On the basis of their structural characteristics, mucins can be divided into membrane type (MUC1, MUC3A/B, MUC4, MUC11–13) and secreted type (MUC2, MUC5AC, MUC5B, MUC6, MUC19 and MUC7) [61]. Specifically, mucin 1 is abnormally expressed in almost all human epithelial cell adenocarcinomas, such as human breast, ovarian, colorectal and pancreatic carcinomas. Besides, the fact that mucin-1 is heavily glycosylated in the health tissue but underglycosylated in neoplastic tissues makes mucin a cellular target for imaging and possible therapeutics. At present, investigations have also been focused on the potential to use MUC-1 as a target for immunotherapy. Several MUC-1 monoclonal antibodies have been reported for cancer imaging and therapy [62]. By taking advantages of nanotechnology, mucin-1 targeted multifunctional magnetic IONOs were produced for monitoring treatment response of 5-FU to PDAC [63]. A decreased MRI signal was observed in 5-FU treated PDAC mouse model (Figure 3). Another study reported that about 75–80% of mucin-1 protein was downregulated when HPAF-II pancreatic cells were treated by curcumin or curcumin encapsulated nanoformulations, a naturally polyphenol with significant cancer prevention activity [64], implying the potency of tracking the PDAC chemotherapy response by targeting mucin-1.
Figure 3. Imaging of pancreatic cancer by targeting mucin-1.
A. Design of pancreatic cancer diagnosis probe, mucin-1 targeted superparamagnetic iron oxide nanoparticles (CLIO-EPPT). B. Representative T2-weighted images (top) and corresponding T2 maps (bottom) of animals bearing PDAC before (left) and 24 h after (right) i.v. injection of CLIO-EPPT. A significant (46.5% 6 3.2%, p < 0.01) reduction in average T2 relaxation rates was observed in the tumor at 24 h after injection. C. Left, T2-weighted MR images and corresponding color-coded NIRF images of mice bearing PDAC before (top) and after (bottom) treatment with 5-FU. Right, Quantitative evaluation of differential tumor growth by MRI and NIRF optical imaging. Tumor growth was inhibited by 5-FU as observed by using CLIO-EPPT with MRI. Reprinted with permission from [62] and [63]. Copyright (2016) Wiley-Liss, Inc.
Zinc Transporter 4 (ZIP4)
Zinc is an essential trace element and catalytic/structural component required by many metalloenzymes, such as carbonic anhydrase and matrix metalloproteinases (MMPs) that overexpressed in tumor. One of the zinc transporters, Zrt-, Irt-like proteins 4 (ZIP4), is overexpressed in pancreatic cancer cells but not in normal ductal epithelium cells in human pancreatic cancer tissues [65, 66]. A recent study from examination of fine needle aspiration (FNA) and surgical specimens revealed that ZIP4 was significantly overexpressed in pancreatic cancer cells. Interestingly, ZIP4 level in FNA samples was significantly associated with tumor differentiation and patient survival, indicating that targeting of ZIP4 has the potential to direct detection and targeted therapy of PDAC [67]. A study utilized RNA interference technique to specifically silence ZIP4 expression successfully inhibited pancreatic cancer growth and significantly increased the survival rate of pancreatic cancer xenografts[68].
CURRENT ADVANCES IN CANCER NANOTECHNOLOGY FOR DETECTION AND THERAPY of PANCREATIC CANCER
Conventional chemotherapy drugs for PDAC have low efficiency in intratumoral delivery and distribution due to the presence of drug delivery barriers in pancreatic cancer tissues. Relative high levels of combination of several potent drugs, such as FOLFIRINOX, are used for the treatment of pancreatic cancer and result in severe systemic toxicity but only modestly improved the therapeutic response. In recent years, nanoparticle drug carriers have attracted great attention for improving drug formulation, enhancing tumor accumulation, and reducing side effects of anticancer drugs. Various types of nanoparticles with large surface area, biocompatibility and high drug loading capacity have been engineered for drug delivery into pancreatic cancer. Some nanoparticle drug carriers are also imaging contrasts agents, also termed as theranostic nanoparticles for their capacity in both therapy delivery and non-invasive imaging, which allow monitoring nanoparticle drug delivery. This function is important for selecting the most efficiency drug delivery systems for pancreatic cancer patients. Heterogeneous distributions of tumor vasculatures, dense stroma components, and ductal cancer cells in pancreatic cancer tissues will require non-invasive imaging to evaluate targeted drug delivery in tumors in a timely manner.
To address clinical challenges in PDAC treatment, new nanotherapeutics have been developed using various targeting ligands that bind to cell receptors upregulated in pancreatic cancer cells and tumor microenvironment. In this aspect, anti-tumor agents, such as, small molecular drugs, small interfering RNAs (siRNAs), antisense nucleotides, toxins, antibodies are all potential agents for the development of nanotheranostics for PDAC [13, 69, 70], presenting a new perspective on PDAC treatment using molecular therapy approaches. Currently, targeted theranostic nanoparticles are mostly in preclinical studies. Clinical trials using various nanoparticle drug carriers are focused on safety and therapeutic efficacy (Table 1). Clinical protocols for image-guided drug delivery and monitoring therapeutic responses using theranostic nanoparticles have yet to be developed and approved by the FDA. In the following sections, we discuss about current status and examples of the development of theranostic nanoparticles using different nanomaterials.
Table 1.
Selected nanomedicines for pancreatic cancer
| Drug product | Nanocarrier | Active ingredient | Status | Conclusion |
|---|---|---|---|---|
| Abraxane | Albumin | paclitaxel | FDA approved |
Increased overall survival |
| MM-398 | liposome | irinotecan | FDA approved |
Increased overall survival |
| EndoTAG-I | cationic liposome |
paclitaxel | phase II | May increase overall survival |
| Genexol-PM | micelle | paclitaxel | Marketed in Europe, Korea |
Inhibits primary tumor growth and metastases |
| NK105 | micelle | paclitaxel | Phase I | Decrease in size of metastatic lesions |
| NC-6004 | micelle | cisplatin | Phase III | May increase overall survival |
| Lipoplatin | liposome | cisplatin | Phase II/III | Symptom relief |
| Rexin-G | Retroviral expression vectors |
doxorubicin | Phase I/II clinical tria |
Increased overall survival |
| NK911 | Polymeric micelles |
doxorubicin | Phase 2 | No update data is available for phase II |
| SGT 53-01 | Transferrin targeted liposome |
p53 gene | Phase II | May increase overall survival |
| Atu027 | Liposome | anti-PKN3 siRNA | Phase IIa | Safe and well tolerated |
Chemotherapy and small molecular drugs
Nanoparticles have been investigated as key drug carriers for the development of targeted therapeutic agents for efficient delivery of conventional chemotherapeutic drugs [71]. Abraxane® or nab-Paclitaxel (130 nm size), is human albumin nanoparticles encapsulated with paclitaxel. The FDA has recently approved Abraxane in combination with gemcitabine for the treatment of metastatic pancreatic cancer as the results of clinical trials that showed improved overall survival by 1.8 months compared to gemcitabine alone. The delivery mechanisms of Abraxane are thought to be mediated by passive targeting tumors by the EPR effect through the leaky tumor vessels and active targeting to the serum protein acidic and rich in cysteine [72]. The observed efficacy in pancreatic cancer therapy is also considered to be mediated by disrupting the tumor stroma from the initial nanoparticle-drug delivery that further increases drug delivery into the tumor [73–76]. Preclinical studies showed that modification of the human albumin-paclitaxel nanoparticles by conjugating tumor necrosis factor-related apoptosis-inducing ligand [77] or RGD integrin targeting ligand [76] enhanced the therapeutic effect. It was also reported that albumin-bound formulation of paclitaxel nanoparticles in the combination with other chemotherapy drugs, such as gemcitabine, significantly increased overall survival of pancreatic cancer patients with metastatic diseases [78]. Another promising clinical trial for pancreatic cancer therapy involved co-delivering chemotherapy agents with Peglyated hyaluronidase (PEGrHuPH20) [73]. Delivery of PEGrHuPH20 into pancreatic cancer tissues leads to the degradation of extracellular matrix, hyaluronic acid, in the tumor stroma and reduction of the interstitial pressure, allowing improved drug delivery into the tumor bed and enhanced therapeutic responses. However, PEGrHuPH20 only acts on hyaluronic acid and has no effect on other stromal components, especially stromal cell barrier and dense fibrotic collagen.
Gemcitabine is a nucleoside analog commonly used as standard chemotherapy for pancreatic cancer therapy. Although it enhances the effects in treating patients with advanced PDAC and improving survival of the patients’ efficiently [79], systemic toxicity is associated with relatively high doses (1000 mg/m2) applied. Moreover, gemcitabine (Gem) can be rapidly inactivated by deoxycytidine deaminase after in vivo injection (half-life is about 17 min), which reduces the effectiveness of the drug. Various nanocomplexes, such as liposome/Gem [80], Silica Nanoparticle/Gem[81], biopolymer/Gem[82] and iron oxide nanoparticle/Gem [38], were designed to increase gemcitabine in vivo half-life, enhance therapeutic effects and reduce systemic toxicity in PDAC patients. Another concern for gemcitabine is drug resistance induced by deficiency or inhibition of nucleoside transporters in tumor cells that facilitate gemcitabine cellular internalization and intracellular phosphorylation for activation. Receptor targeted nanoparticle drug carriers that facilitate internalization of nanoparticle-drug to tumor cells should increase drug concentration in tumor cells and bypass the g-glycoprotein mediated multidrug resistance.
Paclitaxel was used in several studies as a chemotherapeutic agent due to its effect on the treatment of gemcitabine-resistant PDAC. For example under magnetic resonance imaging (MRI) guidance, systemic delivery of paclitaxel encapsulated in poly(ethyleneoxide)copoly(D,L-lactide) in combination with ultrasound enhanced intratumoral paclitaxel accumulation by increasing tumor perfusion and blood vessel and cell membrane permeability, leading to improved tumor growth inhibition [83].
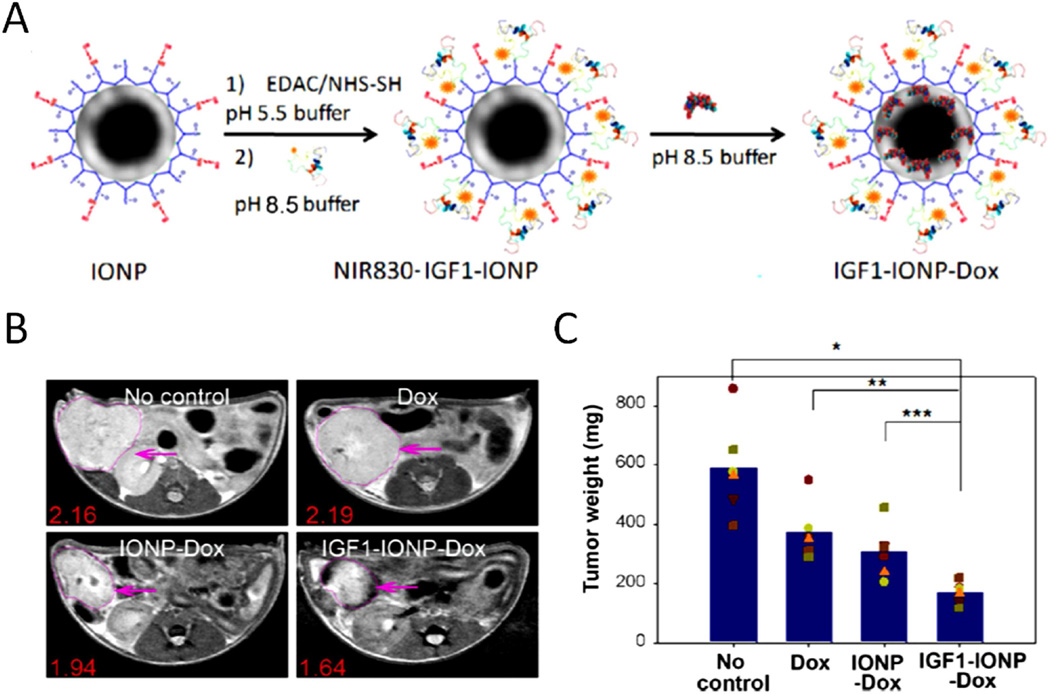
One feature in drug resistant PDAC tumor is the dense tumor stroma that blocks drug or drug complex reaching cancer cells after extravasation from tumor blood vessels. Nanoparticle drug carriers targeting tumor stromal fibroblasts and macrophages offer the opportunity for enhanced intratumoral retention and distribution of nanoparticle-drug and drug delivery into tumor cells. In addition to uPAR-targeted theranostic nanoparticles, IGF-1R that expresses at a high level in pancreatic cancer cells and tumor stromal fibroblasts and macrophages is another cellular receptor for the development of targeted nanoparticles that have the potential of improving drug delivery in stroma rich pancreatic cancer tissues. For example, IGF-1R targeted IONPs carrying anthracycline doxorubicin (Dox) have been developed and have shown to deliver nanoparticle-drug into IGF1R-expressing tumor cells and tumor associated stromal cells [84]. The effect of the theranostic IONPs was demonstrated in an orthotopic human pancreatic cancer patient tissue derived xenograft (PDX) model that recapitulated heterogeneous tumor cells and enriched tumor stroma in human pancreatic cancer. Results of this study demonstrated that systemic delivery of IGF1R targeted IGF1-INOP-Dox theranostic nanoparticles efficiently delivered to pancreatic tumors and were detectable by optical and MR imaging. Repeated delivery of IGF1-IONP-Dox led to breaking tumor stromal drug delivery barriers and significant tumor growth inhibition in this pancreatic PDX model in nude mice (Figure 4). Therefore, the IGF-1R-targeted theranostic IONP is a promising drug delivery system for the treatment of drug resistant pancreatic cancer.
Figure 4. IGF-1 receptor targeted IONP for imaging guided tumor therapy.
A. Preparation of the DOX capsulated NIR830-IGF1-IONPs. B. T2-weighted MRI confirms the accumulation of IGF1-IONP-Dox in the tumor site and tumor growth inhibition in different groups. Pink arrows indicate the locations of pancreatic PDX-tumor lesions. Red numbers are the mean of relative MRI signal intensities of MRI image slices from the entire tumor. A 10.2% MRI signal decrease was detected in nontargeted IONP-Dox-treated tumor, while a 24.1% MRI signal decrease was seen in IGF1-IONP-Dox-treated tumor. C. Tumor growth inhibition. The mean tumor weight (navy bar) and individual tumor weight distributions as color symbols after the treatment are shown. Adapted with permission from [84]. Copyright 2016 American Chemical Society.
In addition to the single drug treatment, many groups are investigating the potency of combination therapies of PDAC to improve treatment response and capitalize on the drug's initial promises. For example, FOLFIRINOX (Leucovorin Calcium, Fluorouracil, Irinotecan Hydrochloride and Oxaliplatin), OFF (Oxaliplatin, Fluorouracil and Leucovorin Calcium), Gem-Cisplatin and Gem-Oxaliplatin are four FDA approved drug combinations for PDAC. The other combinations, such as chemotherapy-radiation therapy [85], photoherapy-chemotherapy [69] and gene therapy-chemotherapy [81], have also been investigated. Because each drug kill cancer cells through different mechanisms, these combination therapies usually show improved therapeutic effects compared to the single drug treatment. Unfortunately, systemic toxicity often becomes worse in combination therapy. Thus, it is important to develop nanoparticles simultaneously carrying multiple drugs to enhance therapeutic response and overcome drug resistance while reducing side effects in PDAC therapy.
siRNA
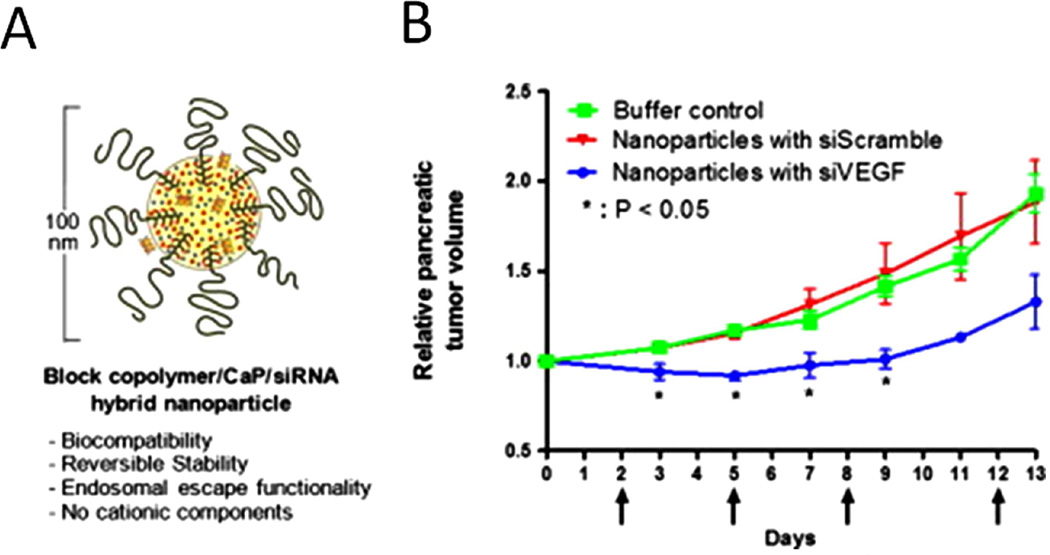
RNA interference, or gene silencing, is referring to utilization of small RNA (siRNA) molecules to specifically turn off the expression of a target gene. It has great therapeutic potential for diseases caused by abnormal gene expression and genetic mutation. The main challenges in applying this approach are the poor stability of RNA in vivo and its inability to pass the cell membrane. In this regard, various nanomaterials provide possible carriers for delivery siRNAs [86]. Polymer/calcium phosphate/siRNA hybrid nanoparticles carrying siRNA for vascular endothelial growth factor (VEGF) siRNA was delivered into subcutaneous BxPC3 tumor following systemic delivery and significant VEGF gene silencing was achieved [87] (Figure 5). Recently, another micelle with poly(ethylene glycol)-block-charge-conversional polymer /calcium phosphate from the same group was used to transferred VEGF siRNA into spontaneous pancreatic tumors and found obvious tumor ablation [70]. Due to the unique shape, single wall carbon nanotube was also reported for delivery of siRNA into pancreatic cells for potential therapeutic application [88]. Interestingly, it has been shown that knocking-down expression of critical genes not only suppresses the tumor growth, but also enhances efficacy of chemotherapy. For example, silencing AURKA genes can induce apoptosis and increase cytotoxicity of taxanes in pancreatic cancer cells [89]. In another study, ubiquitin ligase ITCH was specifically silenced by anti-ITCH siRNA and poly(propylenimine) dendrimers complex. It was reported that down-regulation of ITCH would sensitize cells to chemotherapeutic agents and that RNAi based therapy would act as a booster for conventional chemotherapeutic agents. Indeed, the growth of PDAC co-administered with gemcitabine and ITCH-shRNA [90]. However, the in vivo safety and biocompatibility of these siRNA carriers have to be clarified. Further, studies are also necessary before drawing any conclusions regarding the efficacy of the siRNA targeted therapy for PDAC treatment.
Figure 5. Polymer hybrid nanoparticles encapsulated with VEGF siRNA for pancreatic cancer therapy.
A. Design of hybrid polymer nanoparticle for siRNA delivery. B. Therapeutic effect of pancreatic cancer by the hybrid nanoparticles with siVEGF. Adapted with permission from [87]. Copyright (2016) Elsevier.
Photodynamic therapy
In comparison with conventional chemotherapy, laser-triggered photodynamic therapy (PDT) is an alternative tumor ablation regimen, especially for those chemo/radio resistant cancers. It utilizes light at a specific wavelength to excite nontoxic photosensitizers to produce toxic reactive oxygen species (ROS) to destroy tissues and vasculartures for both benign and malignant tumors [91, 92]. PDT can be a noninvasive therapy approach with minimal systemic effects, due to its killing of cancer cells by local light activation [93]. The first report on the application of PDT in clinic was reported in 2002 and 16 patients were received PDT after intravenously injection of photosensitizer (PS), meso-tetrahydroxyphenyl chlorin (mTHPC) [94]. Substantial tumor necrosis was observed and the median survival time after PDT is 9.5 months (range 3–40). Although further studies about using PDT in PDAC are required to confirm the therapeutic efficacy, this study encouraged the ablation of PDAC by PDT. More recently, the second-generation PS, named verteporfin, was evaluated by the same group in 15 patients with advanced PDAC. Tumor necrosis was induced with lesser side effects compared to their previous study [95]. In addition to PDT single treatment, photofrin and gemcitabine combined therapy is now under phase I clinic trial (NCT01770132). Overall, PDT presents an attractive alternative to the traditional therapeutic approaches. Accumulating studies on applications of PDT have been widely reported in recent years. Yu et al. encapsulated PS into amphiphilic sodium alginate-derivative nanoparticles and tested their PDT effects in Panc-1 human pancreatic cancer cells [96]. Under ultraviolet irradiation, the high level of reactive oxygen species generated by the treatment resulted in strong phototoxicity and apoptosis. However, reduction of side effects of PDT, optimization of the light penetration, the pre-existing hypoxic tumor microenvironment and the potential toxicity of PS are still challenging for applications of PDT in clinic, especially for PDAC treatment. Therefore, combination of PDT with other PDAC treatment approaches, development of new PS and drug delivery using novel nanomaterials may address those problems.
Conclusion and perspectives
The combination of high doses of several chemotherapeutic drugs has been used for the treatment of pancreatic cancer patients. Despite aggressive chemotherapy, there is only modest improvement in therapeutic response and survival of the patients, however, with drawback of severe systemic toxicity. Targeted nanoparticle drug carriers offer promising approaches for selective delivery of highly potent chemotherapy drugs into pancreatic cancer tissues while markedly reducing systemic toxicity. Through appropriate surface modifications, nanoparticle drug carriers or theranostic nanoparticles offer a possibility of reducing or overcoming the therapeutic limitations of conventional chemotherapy by targeted delivery to cancer cells without obvious toxicity to healthy tissue. The ability of high capacity of loading one or multiple therapeutic agents of the nanoparticle drug carriers make them suitable drug delivery system for targeted therapy of drug resistant pancreatic cancer using high doses of potent chemotherapeutic drugs. The large surface of the nanoparticles allows conjugating agents that act upon tumor stroma to break the drug delivery barrier to improve drug delivery into pancreatic cancer cells. Due to the presence of a physical barrier limiting the amount of therapeutic agents delivered into pancreatic tumor tissues and tumor cells, chemotherapy agents have to be highly toxic to effectively kill tumor cells. Since nanoparticle drug carriers are not able to pass the tight junctions of normal endothelial cells to enter most normal tissues and organs, the toxicity can be reduced. For macrophages in the spleen and Kupffer cells in the liver, those tissue macrophages are mostly post mitotic cells and are relatively resistant to chemotherapy drugs acting on proliferating cell populations. Furthermore, highly potent but also highly cytotoxic drugs could be encapsulated in the targeted nanoparticles to be delivered into pancreatic cancer tissues.
In summary, research progresses of the development of PDAC biomarkers and treatment strategies discussed in this review paved the way for further development of targeted cancer therapeutic agents, and multifunctional theranostic nanoparticle drug carriers for effective treatment of pancreatic cancer. Theranostic nanoparticles are promising new therapeutic agents for the development and translation of integrated image-guided and targeted therapeutic agents for personalized treatment of pancreatic cancer patients. The ability of non-invasive imaging to monitor nanoparticle-drug delivery is essential for maximizing the effect of biomarker-targeted therapy. Additionally, the development of novel stroma breaking or stroma penetrating nanoparticles, should allow therapeutic agents to overcome stroma barriers to reach to pancreatic cancer cells, which is highly likely to improve the therapeutic effect of current treatment agents. At the same time, nanoparticles as promising carriers provide new opportunities for some highly potent drugs that previously failed in clinical development due to low solubility, inappropriate pharmacokinetics, lack of bioavailability, or severe systemic toxicity. Through multidisciplinary cooperations among biologists, chemists, engineers, and clinicians, we believe that more powerful nanotherapeutic agents will be developed to address critical clinical issues on pancreatic cancer detection and therapy and to significantly improve the outcome of current therapy of pancreatic cancer.
Supplementary Material
Highlight.
Early diagnosis and effective treatment of pancreatic cancer still present challenges.
Cell surface biomarkers have been identified for the development of targeted imaging and therapeutic agents for detection and treatment of pancreatic cancer.
Theranostic nanoparticles are promising drug delivery platforms for the development of targeted and image-guided therapy for stroma rich pancreatic cancer.
Major challenges for translation of targeted nanoparticle drug carriers into clinical applications for detection and treatment of pancreatic cancer have been discussed.
Acknowledgments
This research project was supported by the NIH/NCI grants U01CA151810, 1U01CA198913, R01CA154846 and the Nancy Panoz Endowed Chair Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.Kooby DA, Gillespie TW, Liu Y, Byrd-Sellers J, Landry J, Bian J, Lipscomb J. Impact of Adjuvant Radiotherapy on Survival after Pancreatic Cancer Resection: An Appraisal of Data from the National Cancer Data Base. Ann Surg Oncol. 2013;20:3634–3642. doi: 10.1245/s10434-013-3047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur S, Baine MJ, Jain M, Sasson AR, Batra SK. Early diagnosis of pancreatic cancer: challenges and new developments. Biomark Med. 2012;6:597–612. doi: 10.2217/bmm.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo M. Pancreatic Cancer. New Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Niu G, Fang X, Chen X. Preclinical molecular imaging of tumor angiogenesis. Q J Nucl Med Mol Im. 2010;54:291–308. [PMC free article] [PubMed] [Google Scholar]
- 7.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brand RE, Nolen BM, Zeh HJ, Allen PJ, Eloubeidi MA, Goldberg M, Elton E, Arnoletti JP, Christein JD, Vickers SM, Langmead CJ, Landsittel DP, Whitcomb DC, Grizzle WE, Lokshin AE. Serum Biomarker Panels for the Detection of Pancreatic Cancer. Clin Cancer Res. 2011;17:805–816. doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou H, Qian W, Uckun FM, Wang L, Wang YA, Chen H, Kooby D, Yu Q, Lipowska M, Staley CA, Mao H, Yang L. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano. 2015;9:7976–7991. doi: 10.1021/acsnano.5b01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang LL, Sajja HK, Cao ZH, Qian WP, Bender L, Marcus AI, Lipowska M, Wood WC, Wang YA. uPAR-targeted Optical Imaging Contrasts as Theranostic Agents for Tumor Margin Detection. Theranostics. 2014;4:106–118. doi: 10.7150/thno.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh A, Xu J, Mattheolabakis G, Amiji M. EGFR-targeted gelatin nanoparticles for systemic administration of gemcitabine in an orthotopic pancreatic cancer model. Nanomed-Nanotechnol. 2016;12:589–600. doi: 10.1016/j.nano.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Mao H, Cao ZH, Wang YA, Peng XH, Wang XX, Sajja HK, Wang LY, Duan HW, Ni CC, Staley CA, Wood WC, Gao XH, Nie SM. Molecular Imaging of Pancreatic Cancer in an Animal Model Using Targeted Multifunctional Nanoparticles. Gastroenterology. 2009;136:1514–1525. doi: 10.1053/j.gastro.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saridaki Z, Androulakis N, Vardakis N, Vamvakas L, Kabouraki E, Kalbakis K, Hatzidaki D, Voutsina A, Mavroudis D, Georgoulias V, Souglakos J. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Brit J Cancer. 2012;107:1932–1937. doi: 10.1038/bjc.2012.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melisi D, Budillon A. Pancreatic Cancer: Between Bench and Bedside. Curr Drug Targets. 2012;13:729–730. doi: 10.2174/138945012800564130. [DOI] [PubMed] [Google Scholar]
- 15.Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, Yao Q, Li M. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets. 2011;15:817–828. doi: 10.1517/14728222.2011.566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB, Cheng H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016;16:3268–3277. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 17.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 18.Zervos E, Agle S, Freistaedter AG, Jones GJB, Roper RL. Murine mesothelin: characterization, expression, and inhibition of tumor growth in a murine model of pancreatic cancer. J Exp Clin Canc Res. 2016;35 doi: 10.1186/s13046-016-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng CN, Jia W, Tang Y, Zhao HL, Jiang YS, Sun SC. Mesothelin regulates growth and apoptosis in pancreatic cancer cells through p53-dependent and -independent signal pathway. J Exp Clin Canc Res. 2012;31 doi: 10.1186/1756-9966-31-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin Binding to CA125/MUC16 Promotes Pancreatic Cancer Cell Motility and Invasion via MMP-7 Activation. Sci Rep-Uk. 2013;3 doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CL, Wu TC, Hung CF. Control of human mesothelin-expressing tumors by DNA vaccines. Gene Ther. 2007;14:1189–1198. doi: 10.1038/sj.gt.3302974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 23.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 24.Johnston FM, Tan MCB, Tan BR, Porembka MR, Brunt EM, Linehan DC, Simon PO, Plambeck-Suess S, Eberlein TJ, Hellstrom KE, Hellstrom I, Hawkins WG, Goedegebuure P. Circulating Mesothelin Protein and Cellular Antimesothelin Immunity in Patients with Pancreatic Cancer. Clin Cancer Res. 2009;15:6511–6518. doi: 10.1158/1078-0432.CCR-09-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu L, Liu YL, Chen GY. Diagnostic value of mesothelinin pancreatic cancer: a meta-analysis. Int J Clin Exp Med. 2014;7:4000–4007. [PMC free article] [PubMed] [Google Scholar]
- 26.Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, Onners B, Uram JN, Laheru DA, Lutz ER, Solt S, Murphy AL, Skoble J, Lemmens E, Grous J, Dubensky T, Brockstedt DG, Jaffee EM. Safety and Survival With GVAX Pancreas Prime and Listeria Monocytogenes-Expressing Mesothelin (CRS-207) Boost Vaccines for Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:1325-+. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yong KT, Roy I, Swihart MT, Prasad PN. Multifunctional nanoparticles as biocompatible targeted probes for human cancer diagnosis and therapy. J Mater Chem. 2009;19:4655–4672. doi: 10.1039/b817667c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L, Ke XF, He ZY, Yang DQ, Gong H, Zhang YY, Jing XL, Yao JZ, Chen JM. A MSLN-targeted multifunctional nanoimmunoliposome for MRI and targeting therapy in pancreatic cancer. Int J Nanomed. 2012;7:5053–5065. doi: 10.2147/IJN.S34801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly RJ, Sharon E, Pastan I, Hassan R. Mesothelin-targeted agents in clinical trials and in preclinical development. Mol Cancer Ther. 2012;11:517–525. doi: 10.1158/1535-7163.MCT-11-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorio C, Mafficini A, Furlan F, Barbi S, Bonora A, Brocco G, Blasi F, Talamini G, Bassi C, Scarpa A. Elevated urinary levels of urokinase-type plasminogen activator receptor (uPAR) in pancreatic ductal adenocarcinoma identify a clinically high-risk group. Bmc Cancer. 2011;11:448–457. doi: 10.1186/1471-2407-11-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidenius N, Blasi F. The urokinase plasminogen activator system in cancer: Recent advances and implication for prognosis and therapy. Cancer Metast Rev. 2003;22:205–222. doi: 10.1023/a:1023099415940. [DOI] [PubMed] [Google Scholar]
- 32.Buechler P, Reber HA, Tomlinson JS, Hankinson O, Kallifatidis G, Friess H, Herr I, Hines OJ. Transcriptional Regulation of Urokinase-type Plasminogen Activator Receptor by Hypoxia-Inducible Factor 1 Is Crucial for Invasion of Pancreatic and Liver Cancer. Neoplasia. 2009;11:U196–U119. doi: 10.1593/neo.08734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hildenbrand R, Niedergethmann M, Marx A, Belharazem D, Allgayer H, Schleger C, Strobel P. Amplification of the Urokinase-Type Plasminogen Activator Receptor (uPAR) Gene in Ductal Pancreatic Carcinomas Identifies a Clinically High-Risk Group. Am J Pathol. 2009;174:2246–2253. doi: 10.2353/ajpath.2009.080785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zheng B, Robbins DH, Lewin DN, Mikhitarian K, Graham A, Rumpp L, Glenn T, Gillanders WE, Cole DJ, Lu XH, Hoffman BJ, Mitas M. Accurate discrimination of pancreatic ductal adenocarcinoma and chronic pancreatitis using multimarker expression data and samples obtained by minimally invasive tine needle aspiration. International Journal of Cancer. 2007;120:1511–1517. doi: 10.1002/ijc.22487. [DOI] [PubMed] [Google Scholar]
- 35.Stepanova V, Lebedeva T, Kuo A, Yarovoi S, Tkachuk S, Zaitsev S, Bdeir K, Durnler I, Marks MS, Parfyonova Y, Tkachuk VA, Higazi AAR, Cines DB. Nuclear translocation of urokinase-type plasminogen activator. Blood. 2008;112:100–110. doi: 10.1182/blood-2007-07-104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonias SL, Hu JJ. Urokinase receptor and resistance to targeted anticancer agents. Front Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asuthkar S, Stepanova V, Lebedeva T, Holterman AL, Estes N, Cines DB, Rao JS, Gondi CS. Multifunctional roles of urokinase plasminogen activator (uPA) in cancer stemness and chemoresistance of pancreatic cancer. Mol Biol Cell. 2013;24:2620–2632. doi: 10.1091/mbc.E12-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee GY, Qian WP, Wang LY, Wang YA, Staley CA, Satpathy M, Nie SM, Mao H, Yang LL. Theranostic Nanoparticles with Controlled Release of Gemcitabine for Targeted Therapy and MRI of Pancreatic Cancer. Acs Nano. 2013;7:2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arcaro A. Targeting the insulin-like growth factor-1 receptor in human cancer. Front Pharmacol. 2013;4:30. doi: 10.3389/fphar.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JY, Lee JY, Zhang Y, Hoffman RM, Bouvet M. Targeting the insulin growth factor-1 receptor with fluorescent antibodies enables high resolution imaging of human pancreatic cancer in orthotopic mouse models. Oncotarget. 2016;7:18262–18268. doi: 10.18632/oncotarget.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.England CG, Kamkaew A, Im HJ, Valdovinos HF, Sun H, Hernandez R, Cho SY, Dunphy EJ, Lee DS, Barnhart TE, Cai W. ImmunoPET Imaging of Insulin-Like Growth Factor 1 Receptor in a Subcutaneous Mouse Model of Pancreatic Cancer. Mol Pharm. 2016:30. doi: 10.1021/acs.molpharmaceut.6b00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peled N, Wynes MW, Ikeda N, Ohira T, Yoshida K, Qian J, et al. Insulin-like growth factor-1 receptor (IGF-1R) as a biomarker for resistance to the tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. Cell. Oncol. 2013;36:277–288. doi: 10.1007/s13402-013-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhn DJ, Berkova Z, Jones RJ, Woessner R, Bjorklund CC, Ma WC, Davis RE, Lin P, Wang H, Madden TL, Wei CM, Baladandayuthapani V, Wang M, Thomas SK, Shah JJ, Weber DM, Orlowski RZ. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood. 2012;120:3260–3270. doi: 10.1182/blood-2011-10-386789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, et al. Epidermal growth factor receptor and insulin like growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3484–3493. doi: 10.1002/cncr.26661. [DOI] [PubMed] [Google Scholar]
- 45.Javle MM, Varadhachary GR, Fogelman DR, Shroff RT, Overman MJ, Ukegbu L, et al. Randomized phase II study of gemcitabine (G) plus anti-IGF-1R antibody MK-0646, G plus erlotinib (E) plus MK-0646 and G plus E for advanced pancreatic cancer. J. Clin. Oncol. 2011;29 doi: 10.1186/s13045-018-0616-2. (suppl; abstr 4026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritter CA, Arteaga CL. The epidermal growth factor receptor-tyrosine kinase: A promising therapeutic target in solid tumors. Semin Oncol. 2003;30:3–11. doi: 10.1053/sonc.2003.50027. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Heinrich EL, Lu JM, Lee W, Choi AH, Luu C, Chung V, Fakih M, Kim J. Epidermal Growth Factor Receptor Signaling to the Mitogen Activated Protein Kinase Pathway Bypasses Ras in Pancreatic Cancer Cells. Pancreas. 2016;45:286–292. doi: 10.1097/MPA.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Mao H, Wang YA, Cao Z, Peng X, Wang X, Duan H, Ni C, Yuan Q, Adams G, Smith MQ, Wood WC, Gao X, Nie S. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small. 2009;5:235–243. doi: 10.1002/smll.200800714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal S, Yadav S, Gupta S. EGFR Targeted PLGA Nanoparticles Using Gemcitabine for Treatment of Pancreatic Cancer. J Biomed Nanotechnol. 2011;7:137–138. doi: 10.1166/jbn.2011.1238. [DOI] [PubMed] [Google Scholar]
- 51.Safran H, Steinhoff M, Mangray S, Rathore R, King TC, Chai L, Berzein K, Moore T, Iannitti D, Reiss P, Pasquariello T, Akerman P, Quirk D, Mass R, Goldstein L, Tantravahi U. Overexpression of the HER-2/neu oncogene in pancreatic adenocarcinoma. Am J Clin Oncol-Canc. 2001;24:496–499. doi: 10.1097/00000421-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka Y, Friess H, Kobrin MS, Buchler M, Kunz J, Beger HG, Korc M. Overexpression of Her2/Neu Oncogene in Human Pancreatic-Carcinoma. Hum Pathol. 1993;24:1127–1134. doi: 10.1016/0046-8177(93)90194-l. [DOI] [PubMed] [Google Scholar]
- 53.Larbouret C, Robert B, Navarro-Teulon I, Thezenas S, Ladjemi MZ, Morisseau S, Campigna E, Bibeau F, Mach JP, Pelegrin A, Azria D. In vivo therapeutic synergism of anti-epidermal growth factor receptor and anti-HER2 monoclonal antibodies against pancreatic carcinomas. Clin Cancer Res. 2007;13:3356–3362. doi: 10.1158/1078-0432.CCR-06-2302. [DOI] [PubMed] [Google Scholar]
- 54.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 55.Alexis F, Basto P, Levy-Nissenbaum E, Radovic-Moreno AF, Zhang L, Pridgen E, Wang AZ, Marein SL, Westerhof K, Molnar LK, Farokhzad OC. HER-2-targeted nanoparticle-affibody bioconjugates for cancer therapy. Chem Med Chem. 2008;3:1839–1843. doi: 10.1002/cmdc.200800122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, DePinho RA, Mahmood U, Weissleder R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. Plos Med. 2008;5:657–668. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonnenberg A, Liem RKH. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 58.Andra K, Nikolic B, Stocher M, Drenckhahn D, Wiche G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Gene Dev. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bausch D, Thomas S, Mino-Kenudson M, Fernandez-del Castillo C, Bauer TW, Williams M, Warshaw AL, Thayer SP, Kelly KA. Plectin-1 as a Novel Biomarker for Pancreatic Cancer. Clin Cancer Res. 2011;17:302–309. doi: 10.1158/1078-0432.CCR-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konkalmatt PR, Deng D, Thomas S, Wu MT, Logsdon CD, French BA, Kelly KA. Plectin-1 Targeted AAV Vector for the Molecular Imaging of Pancreatic Cancer. Front Oncol. 2013;3:84. doi: 10.3389/fonc.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore A, Medarova Z, Potthast A, Dai GP. In vivo targeting of underglycosylated MUC-1 tumor antigen using a multimodal imaging probe. Cancer Research. 2004;64:1821–1827. doi: 10.1158/0008-5472.can-03-3230. [DOI] [PubMed] [Google Scholar]
- 63.Medarova Z, Pham W, Kim Y, Dai GP, Moore A. In vivo imaging of tumor response to therapy using a dual-modality imaging strategy. Int J Cancer. 2006;118:2796–2802. doi: 10.1002/ijc.21672. [DOI] [PubMed] [Google Scholar]
- 64.Yallapu MM, Ebeling MC, Khan S, Sundram V, Chauhan N, Gupta BK, Puumala SE, Jaggi M, Chauhan SC. Novel Curcumin-Loaded Magnetic Nanoparticles for Pancreatic Cancer Treatment. Molecular Cancer Therapeutics. 2013;12:1471–1480. doi: 10.1158/1535-7163.MCT-12-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logsdon CD, Simeone DM, Binkley C, Arumugam T, Greenson JK, Giordano TJ, Misek DE, Hanash S. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 66.Li M, Zhang YQ, Liu ZJ, Bharadwaj U, Wang H, Wang XW, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen CY, Yao QZ. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. P Natl Acad Sci USA. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu C, Wallace MB, Yang J, Jiang L, Zhai Q, Zhang Y, Hong C, Chen Y, Frank TS, Stauffer JA, Asbun HJ, Raimondo M, Woodward TA, Li Z, Guha S, Zheng L, Li M. ZIP4 is a Novel Diagnostic and Prognostic Marker in Human Pancreatic Cancer: A Systemic Comparison Between EUS-FNA and Surgical Specimens. Curr Mol Med. 2014;14:309–315. doi: 10.2174/1566524013666131217112921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li M, Zhang YQ, Bharadwaj U, Zhai QH, Ahern CH, Fisher WE, Brunicardi FC, Logsdon CD, Chen CY, Yao QZ. Down-regulation of ZIP4 by RNA Interference Inhibits Pancreatic Cancer Growth and Increases the Survival of Nude Mice with Pancreatic Cancer Xenografts. Clin Cancer Res. 2009;15:5993–6001. doi: 10.1158/1078-0432.CCR-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tangutoori S, Spring BQ, Mai ZM, Palanisami A, Mensah LB, Hasan T. Simultaneous delivery of cytotoxic and biologic therapeutics using nanophotoactivatable liposomes enhances treatment efficacy in a mouse model of pancreatic cancer. Nanomed-Nanotechnol. 2016;12:223–234. doi: 10.1016/j.nano.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H, Kim HJ, Nishiyama N, Miyata K, Kataoka K. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J Control Release. 2014;178:18–24. doi: 10.1016/j.jconrel.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 72.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25–37. doi: 10.1007/978-1-60761-609-2_3. [DOI] [PubMed] [Google Scholar]
- 73.Bullock AJ, Hingorani SR, Wu XW, Jiang P, Chondros D, Khelifa S, et al. Final analysis of stage 1 data from a randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients (pts), utilizing Ventana companion diagnostic assay. J. Clin. Oncol. 2016;34 (suppl; abstr 4, 104) [Google Scholar]
- 74.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Gemcitabine Plus nab-Paclitaxel Is an Active Regimen in Patients With Advanced Pancreatic Cancer: A Phase I/II Trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alvarez R, Musteanu M, Garcia-Garcia E, Lopez-Casas PP, Megias D, Guerra C, Munoz M, Quijano Y, Cubillo A, Rodriguez-Pascual J, Plaza C, de Vicente E, Prados S, Tabernero S, Barbacid M, Lopez-Rios F, Hidalgo M. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Brit J Cancer. 2013;109:926–933. doi: 10.1038/bjc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ji SR, Xu J, Zhang B, Yao WT, Xu WY, Wu WZ, Xu YF, Wang H, Ni QX, Hou HM, Yu XJ. RGD-conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy. Cancer Biol Ther. 2012;13 doi: 10.4161/cbt.13.4.18692. [DOI] [PubMed] [Google Scholar]
- 77.Min SY, Byeon HJ, Lee C, Seo J, Lee ES, Shin BS, Choi HG, Lee KC, Youn YS. Facile one-pot formulation of TRAIL-embedded paclitaxel-bound albumin nanoparticles for the treatment of pancreatic cancer. Int J Pharmaceut. 2015;494:506–515. doi: 10.1016/j.ijpharm.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 78.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Madiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, VanHoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 80.Licciardi M, Paolino D, Celia C, Giammona G, Cavallaro G, Fresta M. Folate-targeted supramolecular vesicular aggregates based on polyaspartyl-hydrazide copolymers for the selective delivery of antitumoral drugs. Biomaterials. 2010;31:7340–7354. doi: 10.1016/j.biomaterials.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 81.Meng H, Wang MY, Liu HY, Liu XS, Situ A, Wu B, Ji ZX, Chang CH, Nel AE. Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice. Acs Nano. 2015;9:3540–3557. doi: 10.1021/acsnano.5b00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arya G, Vandana M, Acharya S, Sahoo SK. Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy (vol 7, pg 859, 2011) Nanomed-Nanotechnol. 2013;9:580–580. doi: 10.1016/j.nano.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 83.Rapoport N, Payne A, Dillon C, Shea J, Scaife C, Gupta R. Focused ultrasound-mediated drug delivery to pancreatic cancer in a mouse model. J Ther Ultrasound. 2013;1:11. doi: 10.1186/2050-5736-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou HY, Qian WP, Uckun FM, Wang LY, Wang YA, Chen HY, Kooby D, Yu Q, Lipowska M, Staley CA, Mao H, Yang L. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. Acs Nano. 2015;9:7976–7991. doi: 10.1021/acsnano.5b01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wiegel T, Runkel N, Frommhold H, Rube C, Hinkelbein W. [Radiotherapeutic strategies in the multimodal therapy of resectable and nonresectable pancreatic carcinoma] Strahlenther Onkol. 2000;176:299–306. doi: 10.1007/s000660050011. [DOI] [PubMed] [Google Scholar]
- 86.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 87.Pittella F, Miyata K, Maeda Y, Suma T, Watanabe S, Chen Q, Christie RJ, Osada K, Nishiyama N, Kataoka K. Pancreatic cancer therapy by systemic administration of VEGF siRNA contained in calcium phosphate/charge-conversional polymer hybrid nanoparticles. Journal of Controlled Release. 2012;161:868–874. doi: 10.1016/j.jconrel.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Anderson T, Hu R, Yang CB, Yoon HS, Yong KT. Pancreatic cancer gene therapy using an siRNA-functionalized single walled carbon nanotubes (SWNTs) nanoplex. Biomater Sci-Uk. 2014;2:1244–1253. doi: 10.1039/c4bm00019f. [DOI] [PubMed] [Google Scholar]
- 89.Hata T, Furukawa T, Sunamura M, Egawa S, Motoi F, Ohmura N, Marumoto T, Saya H, Horii A. RNA interference targeting aurora kinase A suppresses tumor growth and enhances the taxane chemosensitivity in human pancreatic cancer cells. Cancer Research. 2005;65:2899–2905. doi: 10.1158/0008-5472.CAN-04-3981. [DOI] [PubMed] [Google Scholar]
- 90.de la Fuente M, Jones MC, Santander-Ortega MJ, Mirenska A, Marimuthu P, Uchegbu I, Schatzlein A. A nano-enabled cancer-specific ITCH RNAi chemotherapy booster for pancreatic cancer. Nanomed-Nanotechnol. 2015;11:369–377. doi: 10.1016/j.nano.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 91.Wang G, Zhang F, Tian R, Zhang L, Fu G, Yang L, Zhu L. Nanotubes-Embedded Indocyanine Green-Hyaluronic Acid Nanoparticles for Photoacoustic-Imaging-Guided Phototherapy. ACS Appl Mater Interfaces. 2016;8:5608–5617. doi: 10.1021/acsami.5b12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao S, Wang G, Qin Z, Wang X, Zhao G, Ma Q, Zhu L. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials. 2016;112:324–335. doi: 10.1016/j.biomaterials.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 93.Wang JJ, Zhang LW, Chen ML, Gao S, Zhu L. Activatable Ferritin Nanocomplex for Real-Time Monitoring of Caspase-3 Activation during Photodynamic Therapy. Acs Appl Mater Inter. 2015;7:23248–23256. doi: 10.1021/acsami.5b07316. [DOI] [PubMed] [Google Scholar]
- 94.Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, Jones L, Wyld P, Gillams A, Hatfield AW. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, Kent E, Bown SG, Hasan T, Pogue BW, Pereira SP. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br J Cancer. 2014;110:1698–1704. doi: 10.1038/bjc.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu Z, Li HJ, Zhang LM, Zhu ZH, Yang LQ. Enhancement of phototoxicity against human pancreatic cancer cells with photosensitizer-encapsulated amphiphilic sodium alginate derivative nanoparticles. Int J Pharmaceut. 2014;473:501–509. doi: 10.1016/j.ijpharm.2014.07.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.