Abstract
Psychophysical and neurobiological evidence suggests that central and peripheral vision are specialized for different functions. This specialization of function might be expected to lead to differences in the large-scale functional interactions of early cortical areas that represent central and peripheral visual space. Here, we characterize differences in whole-brain functional connectivity among sectors in primary visual cortex (V1) corresponding to central, near-peripheral, and far-peripheral vision during resting fixation. Importantly, our analyses reveal that eccentricity sectors in V1 have different functional connectivity with non-visual areas associated with large-scale brain networks. Regions associated with the fronto-parietal control network are most strongly connected with central sectors of V1, regions associated with the cingulo-opercular control network are most strongly connected with near-peripheral sectors of V1, and regions associated with the default mode and auditory networks are most strongly connected with far-peripheral sectors of V1. Additional analyses suggest that similar patterns are present during eyes-closed rest. These results suggest that different types of visual information may be prioritized by large-scale brain networks with distinct functional profiles, and provide insights into how the small-scale functional specialization within early visual regions such as V1 relates to the large-scale organization of functionally distinct whole-brain networks.
Keywords: attention, eccentricity, fMRI, primary visual cortex, top-down control
Introduction
In humans, primary visual cortex (Brodmann area 17; V1) contains representations of different visual eccentricities that gradually progress from central representations to far-peripheral representations from posterior to anterior portions of the calcarine sulcus (Inouye 1909; Fox et al., 1987; Engel et al., 1997). Central and peripheral vision, as well as their corresponding cortical representations in early visual areas like V1, V2, V3 and hV4, differ in many ways. Some of these differences arise simply from differences in retinal anatomy and physiology. For example, the central retina has a higher density of sensory receptors and ganglion cells than other portions of the retina, and therefore central vision has higher spatial acuity than peripheral vision (Wandell 1995; Anstis 1998). Central vision also depends on inputs from cones that respond to bright light only, whereas peripheral vision depends on inputs from rods that respond to bright and dim light (Aguilar and Stiles, 1954).
Following this low level distinction, central sectors of V1 respond only under bright viewing conditions, whereas peripheral sectors of V1 respond to a broader range of light levels (Hadjikhani and Tootell 2000). Importantly, some hierarchically higher visual areas such as V8 and MT+ show similar light-level dependent responses to stimuli, suggesting that retinal distinctions in cone and rod-mediated visual information remain embedded throughout the visual processing hierarchy (Hadjikhani and Tootell 2000). Biases for processing detailed visual objects (e.g. faces, words, and letters) in central vision, as well as biases for processing more integrated visual objects such as buildings in peripheral vision (Levy et al. 2001; Hasson et al. 2002), further suggest that such early distinctions may have led to specializations for processing different types of information in central vs. peripheral vision.
Higher-order cognitive processes such as attention show similar retinotopic distinctions. For example, attention to the receptive fields of central V1 neurons decreases spatial summation, whereas attention to the receptive fields of peripheral V1 neurons increases spatial summation (Roberts et al., 2007). This suggests that while attention may enhance the fine-grained analysis of detailed visual features in central vision, it may facilitate more integrated and/or holistic processing in the periphery. Evidence that attention to local features enhances activity in central portions of early visual areas (e.g. Sasaki et al., 2002) suggests that these effects are preserved at later stages of visual processing. This central-peripheral difference in attentional effects is corroborated by the observation that central visual attention enhances activity throughout the ventral visual processing stream, whereas peripheral visual attention enhances activity throughout the dorsal visual processing stream (Bressler et al., 2013). Central visual biases have also been documented for cognitive operations such as visual working memory (Yoo and Chong, 2012), spatial prioritization (Linnell and Humphreys, 2004), and distractor inhibition (Chen and Treismann, 2008). Conversely, peripheral visual biases have been documented for cognitive operations such as cross-modal processing (Eckert et al., 2008; Cate et al., 2009; Gleiss and Kayser, 2013; Griffis et al., 2015a) and threat detection (Bayle et al., 2009; Gomez et al., 2011). Thus, basic differences between central and peripheral vision (and their cortical representations) appear to be reflected in higher cognitive operations.
On the basis of these effects, one might expect that central and peripheral representations in early visual areas (such as V1) would interact differently with large-scale brain networks involved in the control of attention and other higher-order cognitive functions. Recent studies using functional connectivity, a measurement of correlated fluctuations in hemodynamic or neural activity, have carefully described networks of functionally connected brain regions that play distinct roles in cognitive control (Fox et al. 2005; Dosenbach et al. 2006, 2007). The fronto-parietal network includes regions such as the inferior parietal lobule (IPL), frontal eye fields (FEF), and dorsolateral prefrontal cortex (dlPFC), and is involved in moment-to-moment adaptations of attentional control (Dosenbach et al. 2008; Vincent et al. 2008; Ptak 2012; Hellyer et al. 2014). The cingulo-opercular (or salience) network includes regions such as the anterior insula and dorsal anterior cingulate cortex (dACC), and is thought to be involved in tonic aspects of cognitive control such as maintaining task goals over time (Dosenbach et al. 2008; Dubis et al. 2014). The task-negative (or default mode) network includes regions such as the ventro-medial prefrontal cortex (vmPFC) and posterior cingulate cortex (PCC). Activity in this network is often suppressed during the performance of difficult tasks, and it may be involved in functions such as regulating emotional responses and monitoring external and internal environments when attention is not being actively directed towards a specific goal (Raichle et al. 2001; Dosenbach et al., 2007). Regions that form the foundations of these networks also interact with regions that are primarily associated with other networks, including early visual areas, to accomplish task goals (Ruff et al. 2006; Dosenbach et al. 2007; Spreng et al. 2010; Simpson et al. 2011; Ebisch et al. 2013; Pooresmaeili et al. 2014; Griffis et al. 2015a).
Studies using functional connectivity to investigate the functional architecture of the early visual system indicate that functional connectivity within (Buckner and Yeo 2013) and among (Heinzle et al. 2011; Raemaekers et al. 2014; Arcaro et al. 2015; Genç et al. 2015; Striem-Amit et al. 2015) early visual areas follows retinotopic boundaries. Indeed, retinotopic patterns of functional connectivity in the early visual system have been documented in the absence of visual input (Heinzle et al. 2011; Raemaekers et al. 2014; Arcaro et al. 2015), during periods of fixation on static stimuli (Arcaro et al. 2015; Genç et al. 2015), during active visual stimulation (Arcaro et al. 2015; Genç et al. 2015), and even in congenitally blind individuals (Striem-Amit et al. 2015), indicating that ongoing signaling among hierarchical visual areas is strongly influenced by the topographic organization of early visual areas. However, it is unclear how the retinotopic organization of early visual areas influences their interactions with areas outside of the visual system.
The aim of the current study was to assess whether there are retinotopic effects on the functional connections between early visual cortex and areas outside of the visual system. Our rationale was that by characterizing differences in how regions that are specialized for different aspects of early visual processing (i.e. central vs. peripheral vision) interact with regions associated with other functional brain networks, we might better understand the types of information that are processed/prioritized by these networks. Ultimately, this may allow for a better understanding of the specific roles these networks play in cognition. Thus, in this study, we used BOLD fMRI data that were acquired during periods of resting fixation to examine whether portions of V1 that have distinct functions (i.e. represent different visual eccentricities) differ in their functional connectivity with the rest of the brain during alert, eyes-open rest when no task is being performed and visual stimulation is fixed.
Methods
Participants
All participants were recruited through campus-wide advertisements. All protocols adhered to ethical standards as set and reviewed by the IRB at the University of Alabama at Birmingham. All participants provided a written consent prior to admission to the study. The dataset analyzed for this study consisted of fMRI data collected from periods of eyes-open resting fixation that were interleaved between blocks of visual and auditory attention tasks (Fair et al. 2007). This dataset allowed for the analysis of functional connectivity during eyes-open resting fixation, thus ensuring constant and controlled visual inputs while also acting as a constraint on cognitive state. Data used for the primary analyses were collected from twenty healthy right-handed participants (8 males, 12 females; mean age = 26). Data used for a supplemental analysis (Supplementary Material) on data from eyes closed rest were collected from 13 healthy right-handed participants (6 males, 7 females; mean age = 23). All participants had normal hearing and normal or corrected-to-normal vision.
MRI Data Acquisition
All BOLD fMRI data were collected in a 3T Siemens Allegra fMRI scanner. For all participants, whole-brain BOLD-weighted images were obtained with a TR of 2s, TE of 30 ms, and a voxel size of 3.75×3.75.×4 mm3. An anatomical MPRAGE scan of each participant's brain was acquired at the beginning of each session, producing an image with a voxel size of 1.0×1.0×1.1mm3. Fixation period data were obtained from rest block periods that were interleaved with visual and auditory attention task blocks in a mixed blocked/event-related design (Visscher et al. 2003; Petersen and Dubis 2011). Scan sessions consisted of eight runs. Each run included 5 task blocks and 6 rest blocks. Task blocks lasted 70s each. Rest blocks occurred at the beginning of each scan and following each task block, and lasted 24s each.
Task and stimulus details have been described in detail elsewhere (Elkhetali et al. 2015; Griffis et al. 2015b), and will only be briefly described here, as current analyses focus on the rest periods only. Task blocks consisted of unimodal and bimodal visual and auditory attention tasks. Visual stimuli consisted of centrally presented gray-scale horizontal gratings that varied sinusoidally in luminance over space (Gabor patches). Auditory stimuli consisted of tones that varied sinusoidally over time and in tone (ripple sounds) that were presented binaurally. During the rest blocks, participants fixated on a centrally located fixation cross.
To account for task effects, a general linear model (GLM) was applied to the data (Friston et al. 1995). Task block onsets and offsets were modeled using FIR regressors corresponding to 14s (7 TRs) at the onset and offset of each block. Individual trials were modeled using FIR regressors corresponding to 24 s (12 TRs). Sustained block effects were modeled with a single boxcar regressor that started immediately after the task onset regressor and that ended at the end of the block. The data used in the functional connectivity analyses were obtained following the removal of task effects from the BOLD time-series using a General Linear Model (GLM) (Fair et al. 2007), and are described more fully in the below sections. Participants’ eyes were monitored throughout each scan using an Eyelink 1000 fMRI eye tracking system (SR Research Ontario, Canada). Eye position was calibrated at the beginning of each scan, and was monitored throughout the course of the scan to ensure compliance with instructions.
General fMRI Preprocessing
All BOLD fMRI data were preprocessed using MATLAB scripts implementing functions provided in SPM8 (Statistical Parametric Mapping, The Wellcome Department of Imaging Neuroscience, University College London). The images were slice-time corrected, realigned and re-sliced, normalized to an EPI template using an affine transformation, re-sampled to 2mm isotropic resolution, and then smoothed using a 5mm Gaussian kernel. Motion-correction was applied using MATLAB scripts to minimize artifacts caused by movement: images in which a participant moved more than 0.5mm in one TR (2 s) were replaced with an interpolated image from adjacent images. Runs were excluded if mean movement across the run was greater than 3mm in any direction.
The residual BOLD time-courses from the rest blocks were extracted after fitting the GLM to avoid the introduction of task-related effects into the functional connectivity analyses (Fair et al. 2007). Along with the preprocessing steps already described, additional preprocessing steps were performed on the residual BOLD data to reduce spurious variance not associated with neural activity. These steps were implemented using MATLAB scripts to perform temporal band pass filtering (0.009 < f < 0.08 Hz), regression of motion parameters obtained during motion-artifact correction, rejection of volumes containing greater than 0.5mm of motion per TR, and the regression of principal components of white matter and CSF signals. MATLAB scripts were also used to implement a motion-scrubbing algorithm developed by Power and colleagues (Power et al. 2012) according to recommendations for optimal motion scrubbing (Carp 2013) in order to control for additional potential movement-related confounds. Following these steps, the data acquired during the rest blocks were extracted and concatenated into a single 4D volume. After these steps, participants had at least 11.2 minutes of rest block data, with an average of 16.47 minutes and a standard deviation of 2.20 minutes. It is worth noting that one of the original studies to define several of the intrinsic connectivity networks described in the introduction (i.e. cingulo-opercular, fronto-parietal, and task-negative networks) used a similar design to the current study (rest period concatenation), and so the use of this approach in the current study is consistent with the prior literature. In the Dosenbach et al. study, for 64 of 74 participants concatenated periods of resting fixation were taken from different interleaved task designs and were pre-processed using similar spatial smoothing (6mm FWHM) to the current study (5mm FWHM) (Dosenbach et al. 2007).
V1 Eccentricity Sector Definitions
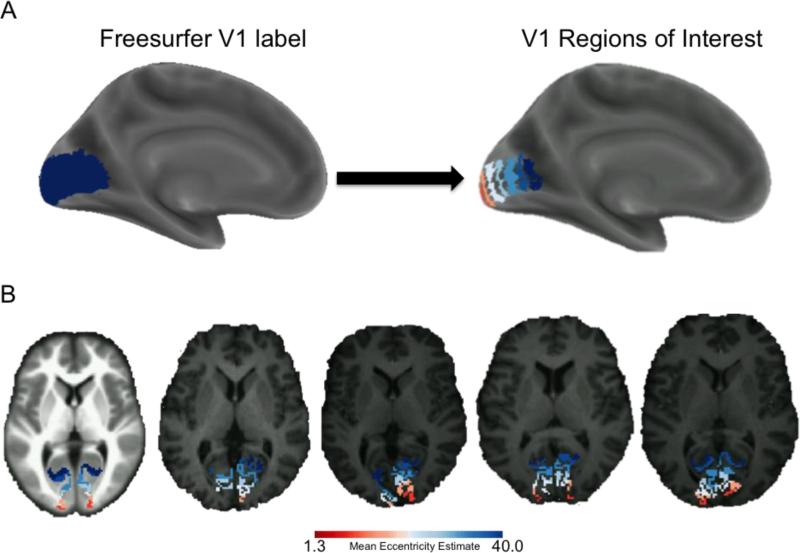
A total of 9 V1 eccentricity sector segments were hand drawn along the calcarine sulcus within the boundary of the Freesurfer fsaverage V1 label file as described in our previous publications (Griffis et al. 2015a; Burge et al. 2016), and the full segmentations are shown in detail in Supplementary Figure 1. Each of the segmentations were constrained to a width of approximately 10mm as calculated with the plot_curv function in the Freesurfer tool tksurfer. Segmentations were created accordingly for both the left and right hemisphere and then transformed from the fsaverage space to each subject's Freesurfer anatomical space, giving subject-specific segmentations that are customized to fit each subject's calcarine sulcus. To avoid the potential for edge-of-brain artifacts at the occipital pole and to reduce the potential for signal contamination from regions adjacent to the anterior calcarine sulcus such as the posterior cingulate, the most posterior (1st) and most anterior (9th) segments were not included in further analyses. This resulted in seven seed regions that each spanned ~10mm anterior-posterior (Figure 1A) as described in our previous publication (Griffis et al. 2015a). The subject-specific labels were then transformed to the subject's 3-D space, and then finally transformed to MNI atlas space (Figure 1B). Homologous eccentricity sectors in the left and right hemispheres were combined to create a single V1 ROI for each eccentricity sector that spanned both hemispheres. This choice was motivated by evidence for strong inter-hemispheric correlations between homotopic visual areas (Arcaro et al. 2009; Heinzle et al. 2011; Raemaekers et al. 2014; Genç et al. 2015), and because we did not have any hypothesis regarding hemispheric contributions to the effect of eccentricity on the functional connectivity of V1.
Figure 1.
V1 Eccentricity Sectors. A. The Freesurfer fsaverage V1 label is shown in dark blue overlaid on an inflated cortical surface (left), and 7 colored V1 eccentricity sectors obtained from the fsaverage brain are shown overlaid on an inflated cortical surface template (right). Note that the 7 eccentricity sectors were obtained after discarding the most anterior and most posterior segments from the Freesurfer V1 label. Mean eccentricity estimates for each sector are 1.3°, 2.2°, 4.1°, 7.3°, 14.0°, 25.5°, and 40.0°. Red colors correspond to more central sectors, and blue colors correspond to more far-peripheral sectors. B. Color-coded eccentricity sectors obtained from the fsaverage brain are shown overlaid on an axial slice from an anatomical template brain (far left), and color-coded eccentricity sectors from 4 example subjects are shown overlaid on axial slices from the corresponding skull-stripped normalized T1-weighted scans. This figure shows that our eccentricity sectors map cleanly onto individual-subject anatomy. Note: all 9 segmentations are shown on the flattened fsaverage surface in Supplementary Figure 1.
To facilitate interpretation, the average eccentricity of each segment was estimated from the probabilistic retinotopy template developed by Benson and colleagues (Benson et al. 2012). Mean eccentricity estimates for each sector were 1.3°, 2.2°, 4.1°, 7.3°, 14.1°, 25.5°, and 40.0° degrees visual angle (Figure 1). It is worth noting that although these eccentricity estimates are based on a probabilistic template, previously published validation of the template against real retinotopy suggests that the template provides similar accuracy to 10-25 minutes of functional retinotopic mapping (Benson et al. 2012), and this is consistent with previous studies showing that the cortical anatomy is a reliable predictor of the location and retinotopic organization of V1 (Hinds et al. 2008, 2009). However, our main interpretations do not depend on these eccentricity values and rest only on the well accepted idea that there is a retinotopic map in V1 where more polar portions represent more central visual space.
While retinotopic localizers were performed during the first scan session as described in our previous publications (Elkhetali et al. 2015; Griffis et al. 2015b), they were not used to define the primary V1 regions of interest in the current study for several reasons. Primarily, the retinotopic localizers obtained for these participants only mapped to approximately 8° visual angle, whereas using the Freesurfer anatomical template enables the inclusion of the entirety of V1. Secondly, the use of the Freesurfer segmentation coupled with the retinotopic template allows for greater generalizability as it can be employed consistently across studies even when retinotopy is not acquired. Lastly, the inferences drawn from our analyses do not require the precise estimation of eccentricity values, as our inferences could equally be applied to specific anatomical sectors of V1 (e.g. the sector of the V1 label spanning the cortex ~10mm-20mm anterior to the posterior-most vertex) rather than to the corresponding eccentricity sectors (e.g. the sector of the V1 label with an estimated eccentricity of 1.3° visual angle according to the probabilistic template) without loss of generalizability. Nonetheless, because retinotopic mapping was performed on these participants, supplemental analyses were performed using retinotopically defined calcarine sulcus regions of interest that were described in our previous publication (Griffis et al. 2015b) in order to provide a more complete characterization of the data and to demonstrate that our results do not differ substantially when alternate ROI definitions are employed.
Functional Connectivity Analyses
Subject-level correlation maps were obtained for each eccentricity sector by computing the Pearson's (linear) correlation between the mean BOLD time series extracted from each eccentricity sector seed region and the BOLD time series extracted from each voxel in the brain. The resulting correlation coefficient maps were converted to z-score maps using Fischer's r-to-z transform. In order to test the general hypothesis that mean levels of functional connectivity vary according to eccentricity sector, these subject-level correlation maps were entered into a one-way repeated measures analysis of variance (ANOVA) with a factor of eccentricity (7 levels; 1 for each eccentricity sector). The resulting F-contrast was intensity thresholded at a voxel-level p<0.001 (uncorrected) and cluster-thresholded at p<0.05 to correct for multiple comparisons across voxels using Gaussian random field theory correction as implemented in SPM8 (kcrit = 47 contiguous voxels to achieve FWE-correction at p<0.05 for the whole brain).
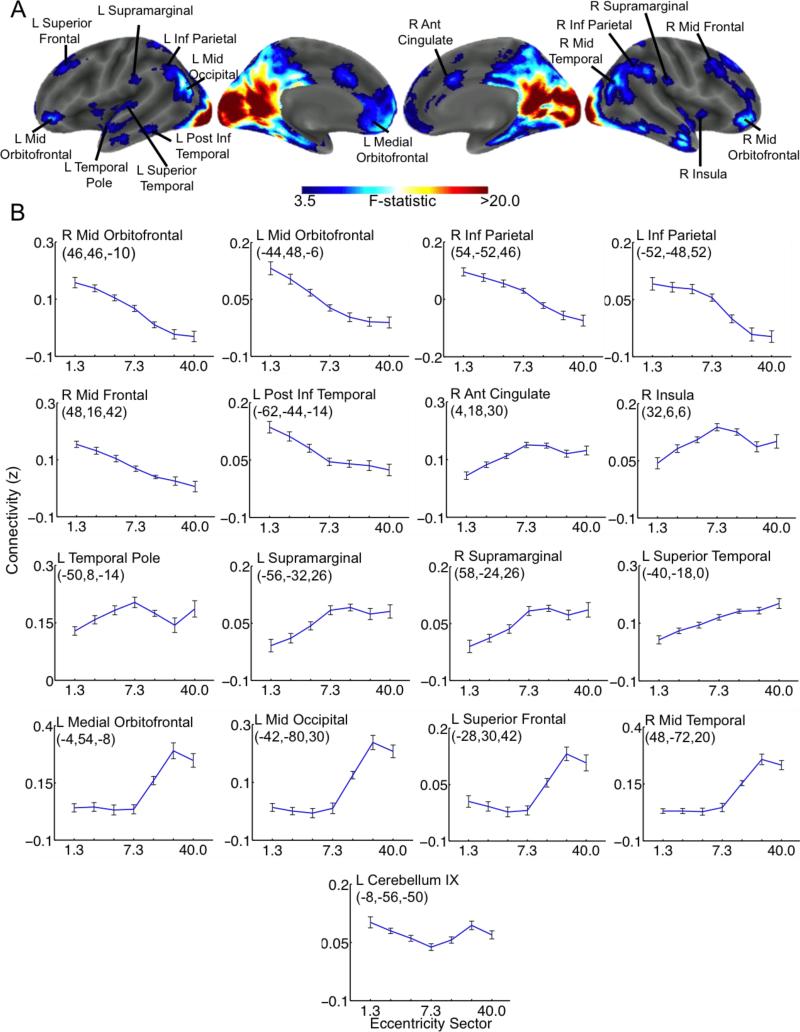
In order to create descriptive graphs illustrating the effects of eccentricity for each of the surviving clusters (i.e. Figure 2B), a maximum of 3 ROIs (per surviving cluster) were defined as 5mm radius spheres centered on peak co-ordinates within each surviving cluster that were at least 30mm apart (some clusters had less than 3 ROIs because they did not contain 3 peaks with at least 30mm distance between them). Note that these criteria were used because they are the default criteria for reporting cluster peaks in the bspmview toolbox used for visualization (http://www.bobspunt.com/bspmview/). The resulting ROIs were masked using the thresholded F statistic image, and the mean connectivity values between each ROI and each V1 eccentricity sector were extracted from the subject-level correlation maps using the marsbar toolbox for SPM (www.marsbar.sourceforge.net). These values were used to create group-level descriptive plots illustrating the main effect of eccentricity on mean functional connectivity between V1 and the peaks of significant clusters identified by the ANOVA that are provided to show how connectivity with each region varies as a function of eccentricity sector.
Figure 2.
ANOVA Results. A. Regions showing significant effects of eccentricity on functional connectivity with V1 are shown on an inflated cortical surface reconstruction. B. Plots illustrating the mean and within-subjects standard error of functional connectivity strength (Fischer z-score units) between cluster peaks and each V1 eccentricity sector. Statistical maps were intensity thresholded at p<0.001 (uncorrected) and cluster thresholded at 47 contiguous voxels to control the FWE rate at 0.05. Peak coordinates are given in MNI space. ROIs correspond to 5mm radius spheres centered on cluster peaks obtained from the ANOVA. ROIs were created for a maximum of 3 peaks with minimum distances of 30mm for each cluster. Plots are ordered according to the general pattern of V1 connectivity in that ROI.
Follow-up tests were performed using dependent samples t-contrasts to compare whole-brain functional connectivity profiles between all pairs of eccentricity sectors. Bonferroni correction at the cluster level was used to correct for multiple comparisons across all contrasts. Each t-statistic image was intensity thresholded at p<0.001 (uncorrected) and cluster-thresholded at p<0.001 (kcrit = 91 contiguous voxels, FWE-corrected for the whole brain to control the FWEr at 0.05, adjusted by Bonferroni correction for all 21 two-tailed t-contrasts). Because changes in functional connectivity were found to be consistently graded, with the most pronounced effects occurring at the estimated 1.3°, 7.3°, and 40.0° eccentricity sectors, as well as to enable more straight-forward reporting of the results, follow-up test results reported in the text were restricted to the comparisons among these sectors. Follow-up test results for the other eccentricity sectors are provided in Supplementary Figures 2-7. All overlay images were created using the bspmview toolbox for SPM (http://www.bobspunt.com/bspmview/).
Results
The whole-brain repeated measures ANOVA revealed significant effects of eccentricity on functional connectivity between V1 and clusters that spanned a diverse set of brain regions, as shown in Figure 2. Importantly, the effects of eccentricity on the functional connectivity of V1 were found to extend well beyond the early visual system. The plots of mean functional connectivity strengths between each V1 eccentricity sector and each cluster peak ROI illustrate several distinct eccentricity-dependent patterns of functional connectivity exhibited by V1 (Figure 2B). Remarkably, a nearly linear decrease in functional connectivity strength from the 1.3° eccentricity sector to the 40.0° eccentricity sectors was observed between V1 and a set of clusters with peaks in bilateral IPL, bilateral lateral orbitofrontal gyri, the left posterior inferior temporal gyrus, and the right dorsal middle frontal gyrus (Figure 2B). Clusters with peaks in the right insula, right dACC, and left temporal pole showed increasing functional connectivity from the 1.3° eccentricity sector to the 7.3° eccentricity sector that decreased again in more far-peripheral sectors (Figure 2B). Clusters with peaks in the left superior temporal gyrus and bilateral supramarginal gyri showed nearly linear increases in functional connectivity from the 1.3° eccentricity sector to the 40.0° eccentricity sector (Figure 2B). Clusters with peaks in the right middle temporal gyrus, left middle occipital gyrus, left medial orbitofrontal gyrus, and left superior frontal gyrus showed little effect of eccentricity on functional connectivity between the 1.3° to 7.3° eccentricity sectors, but sharply increased in the 14.0° and more far-peripheral eccentricity sectors (Figure 2B). These results support our hypothesis that the functional interactions of V1 and brain regions not associated with the early visual system depend on eccentricity, even when no task is being performed and visual inputs are constant.
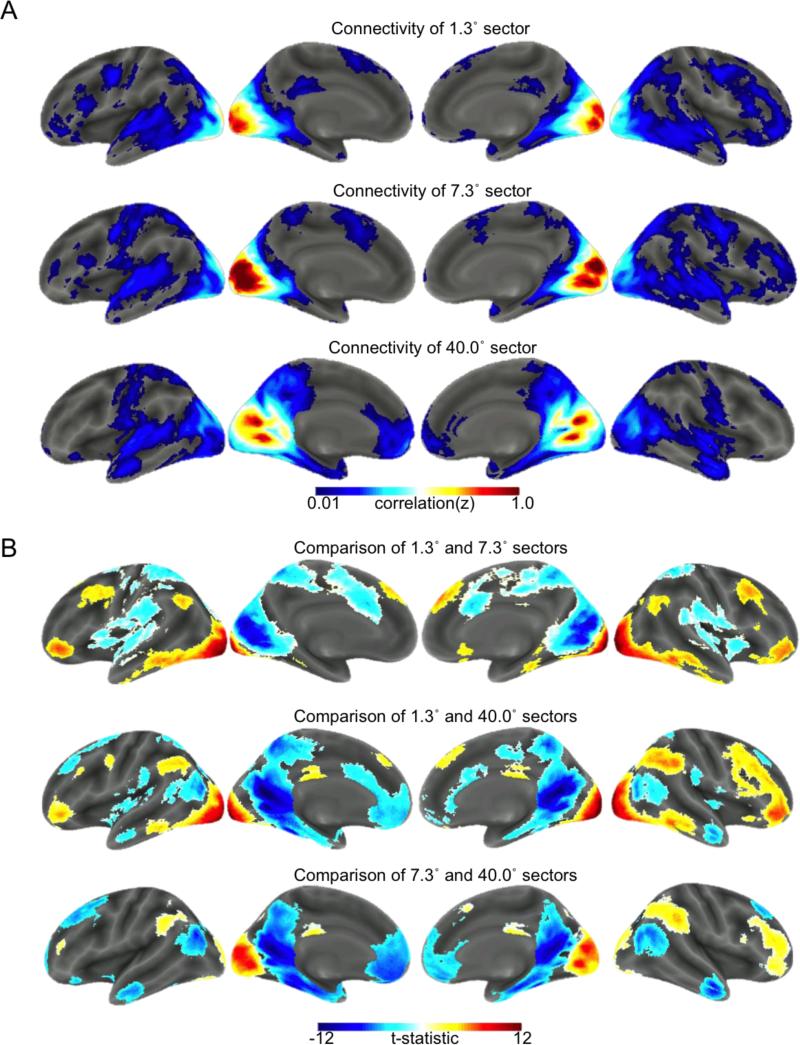
To follow up on the repeated measures ANOVA, we compared the patterns of whole-brain functional connectivity between each pair of eccentricity sectors. As mentioned in the Methods section, only results for comparisons between the 1.3°, 7.3°, and 40.0° eccentricity sectors are reported here. Results from the comparisons of whole-brain functional connectivity between the 1.3°, 7.3°, and 40.0° eccentricity sectors are shown in Figure 3 along with the whole-brain functional connectivity patterns observed for each sector, and the results from the other comparisons can be found in Supplementary Figures 2-7. Cluster peak locations and statistics are presented in Supplementary Tables 1-3 for each of the reported comparisons. The results of identical supplemental analyses using ROIs defined on functional retinotopy showed remarkably similar effects, although comparisons to the far-periphery were not possible due to the limits of our retinotopic localizer (Supplemental Figure 8). Finally, additional supplemental analyses of data obtained from an independent sample of participants who were scanned during 10 minutes of eyes-closed rest suggest that, while much less robust, traces of many of the differences observed in the fixation data are also apparent during eyes-closed rest (Supplementary Figure 9). It is worth noting that some discrepancies between eyes-open fixation and eyes-closed rest are expected based on previously documented differences in the spontaneous activity and functional connectivity of visual cortex and other brain areas between eyes-open and eyes-closed resting states (Bianciardi et al., 2009; Jao et al., 2013; McAvoy et al., 2012; Nir et al., 2006; Zou et al., 2009).
Figure 3.
Functional connectivity profiles and follow-up test results for central, near-peripheral, and far-peripheral eccentricity sectors. A. Z-transformed correlation maps are displayed for central (top), near-peripheral (middle), and far-peripheral (bottom) eccentricity sectors. To allow for clear visualization, correlation maps are thresholded to include only clusters of at least 100 voxels with correlations that survive voxel-wise False Discovery Rate correction at 0.001. Note that statistical inferences are not being drawn from these maps, and they are simply intended to illustrate the overall patterns of functional connectivity for each ROI assessed in the follow-up tests. B. Follow-up test showing regions with stronger connectivity for central (warm colors) vs. near-peripheral (cool colors) eccentricity sectors (top), regions with stronger connectivity for central (warm colors) vs. far-peripheral (cool colors) eccentricity sectors (middle), and regions with stronger connectivity for near-peripheral (warm colors) vs. far-peripheral (cool colors) eccentricity sectors (bottom). Statistical maps shown in (B) were intensity thresholded at p<0.001 (uncorrected) and cluster thresholded at 91 contiguous voxels to control the FWE rate across all pair-wise comparisons at p<0.05. Inferences regarding differences in functional connectivity are based on the maps shown in (B).
Discussion
The functional connections of a given brain region describe how it shares information with the rest of the brain. Thus, regional patterns of connectivity are intricately tied to regional function (Felleman and Van Essen 1991). The findings reported here show that in V1, functional zones that represent different visual eccentricities show distinct functional connectivity patterns with regions not classically considered to be “visual” areas, including regions that are implicated in different aspects of higher-order cognitive control processes. Our results are relevant to the understanding of the neurobiological bases for differences in the function of central and peripheral vision, and provide important insights into how the functional specializations within regions of the brain involved in low-level information processing relate to the functional specializations of large-scale networks.
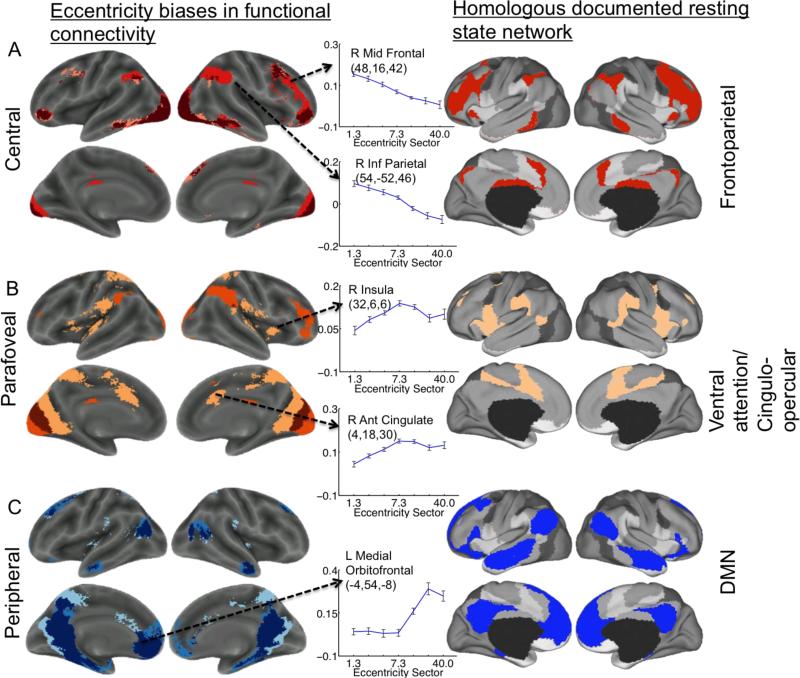
Our approach compared functional connections among anatomically defined sectors within V1. Functional connectivity, while distinct from structural connectivity, provides insight into how information is shared among brain regions and has played important roles in elucidating the organization of large-scale brain networks (Bartels and Zeki 2005; Power et al. 2011; Yeo et al. 2011; Park and Friston 2013) and in delineating distinct functional zones within brain regions (Johansen-Berg et al. 2004; O'Reilly et al. 2010; Uddin et al. 2010; Deen et al. 2011; Dobromyslin et al. 2012; Mars et al. 2012; Buckner and Yeo 2013). Analyses of sub-areal differences in functional connectivity profiles have been used to investigate functional sub-divisions of diverse cortical regions such as the insula (Deen et al. 2011), anterior cingulate cortex (Margulies et al. 2007), and inferior parietal cortex (Vincent et al. 2008; Uddin et al. 2010). As noted in the introduction, several recent studies have found eccentricity-dependent patterns of functional connectivity among early visual areas under various stimulus and rest conditions (Buckner and Yeo 2013;Heinzle et al. 2011; Raemaekers et al. 2014; Arcaro et al. 2015; Genç et al. 2015), as well as in the congenitally blind (Striem-Amit et al. 2015). A previous study by Yeo and colleagues (2011) reported relatively clean delineations of central and peripheral V1 during eyes-open fixation using a 17-network parcellation scheme. In subsequent region of interest analyses, Yeo and colleagues (2011) also found that peripheral V1 showed stronger connectivity than central V1 to the dorsal posterior middle temporal/occipital cortex, which our analyses corroborate (Figure 2;Figure 3). In addition to corroborating the findings of Yeo and colleagues, our findings add to the literature by demonstrating that central and peripheral V1 differ in functional connectivity to a diverse set of brain regions. Specifically, our results show that regions associated with previously documented control networks show distinct eccentricity-dependent patterns of functional connectivity with V1 during alert, resting fixation (Figure 4). The implications of these findings and future directions are discussed below.
Figure 4.
Summary of eccentricity-dependent functional connectivity patterns and their similarity to control networks. The left-hand column shows regions with connectivity patterns selective for central, near-peripheral and far-peripheral V1 (taken from Figure 3). Plots illustrate eccentricity-dependent connectivity patterns observed for core regions associated with the fronto-parietal, cingulo-opercular, and task-negative/default mode networks (taken from Figure 2). The right-hand column illustrates previously documented spatial patterns for the fronto-parietal, cingulo opercular, and task-negative/default mode networks (figure modified from (Yeo et al. 2011)). Note that the regions showing preferential connectivity to central V1 resemble the fronto-parietal network, regions showing preferential connectivity to near-peripheral V1 resemble the cingulo-opercular network, and regions showing preferential connectivity to far-peripheral V1 resemble the task negative/default mode network. A. Regions showing stronger functional connectivity to the 1.3° (central) eccentricity sector than to the other sectors are color-coded in shades of red. Darkest red indicates regions that showed stronger connectivity to the 1.3° sector than to both the 7.3° and 40.0° sectors. Lighter red indicates regions that showed stronger connectivity to the 1.3° compared to the 40.0° sector, but did not differ between the 1.3° and 7.3° sectors. Lightest red indicates regions that showed stronger connectivity to the 1.3° sector compared to the 7.3° sector, but did not differ between the 1.3° and 40.0° sectors. B. Regions showing stronger functional connectivity to the 7.3° (near-peripheral) eccentricity sector than to the other sectors. Darkest orange indicates regions that showed stronger connectivity to the 7.3° sector than to both the 1.3° and 40.0° sectors. Lighter orange indicates regions that showed stronger connectivity to the 7.3° sector compared to the 40.0° sector, but that did not differ between the 7.3° and 1.3° sectors. Lightest orange indicates regions that showed stronger connectivity to the 7.3° sector compared to the 1.3° sector, but did not differ between the 7.3° sector and the 40.0° sector. C. Regions showing stronger functional connectivity to the 40.0° (far-peripheral) eccentricity sector than to the other sectors. Darkest blue indicates regions that showed stronger connectivity to the 40.0° sector compared to both the 1.3° and 7.3° sectors. Lighter blue indicates regions that showed stronger connectivity to the 40.0° sector than to the 7.3° sector, but did not differ between the 40.0° sector and the 1.3° sector. Lightest blue indicates regions that showed stronger connectivity to the 40.0° sector compared to the 1.3° sector, but that did not differ between the 40.0° sector and the 7.3° sector. Note that this figure is intended to provide a qualitative illustration of the spatial similarities between the results of our quantitative analysis of eccentricity-dependent differences in functional connectivity and previously described control networks, and does not include quantitative information beyond that included in Figures 2 and 3.
Regions with preferential connectivity to central V1
From a network-level perspective, many of the regions that showed preferential functional connectivity to central sectors in V1 (warm colors in Figure 3B, top row; Figure 4A, left column) correspond to elements of a “task-positive” system that is comprised of several overlapping sub-systems (Serences et al. 2005; Dosenbach et al. 2006, 2007; Fair et al. 2007; Vincent et al. 2008; Mantini et al. 2009) that are involved in the control of different aspects of externally-oriented attention and goal-directed behavior. Specifically, central sectors were most tightly coupled to a set of regions that strongly resembled the putative fronto-parietal control network (Dosenbach et al., 2007, 2008; Vincent et al., 2008; Leech et al., 2011; Power et al., 2011b, Yeo, 2013). Broadly, the fronto-parietal network includes regions in frontal and parietal cortex that are involved in initiating task-related processing, processing performance feedback, and adjusting sensory processing throughout task performance (Dosenbach et al. 2008). Fronto-parietal regions play a key role in goal-directed cognitive functions such as visuo-spatial planning (Spreng et al., 2010), visual working memory (Rowe et al., 2005; Gazzaley et al., 2007; Bollinger et al., 2010), and selective visuo-spatial attention (Giesbrecht et al. 2003; Hahn et al. 2006; Szczepanski et al. 2010; Liu et al. 2014).
While speculative, it is possible that intrinsic central visual advantages in visual processing, distracter inhibition, and detailed object recognition (Linnell and Humphreys 2004; Chen and Treisman 2008; Yoo and Chong 2012) might be related in part to an intrinsic bias of this network towards the fovea. This explanation is in line with a recent proposal regarding the roles of central and far-peripheral V1 in attentional control (Zhaoping, 2014). According to this proposal, far-peripheral vision is primarily involved in visual selection, whereas central vision is primarily involved in visual decoding. Thus, far-peripheral V1 may help to guide exogenous attention to a salient far-peripheral object in order to better recognize the object using central vision (Li, 2002). Behavioral work has shown that the influence of prior expectation on recognition is stronger in central than far-peripheral vision (Zhaoping, 2014), and that interindividual variability in visual search speed is related to differences in the size of central/near-peripheral portions of V1 (Verghese et al. 2014). Our data (Figure 4) leads to the suggestion that such effects might be due to a bias of fronto-parietal regions to information represented by central compared to far-peripheral portions of V1.
It is worth noting that our supplementary analysis of independent data collected during eyes-closed rest suggests that the DLPFC, IPL, and several other fronto-parietal regions show preferential connectivity to central V1 even in the absence of retinal input (Supplementary Figure 9). This effect persisted despite the fact that overall functional connectivity between visual cortex and the rest of cortex was weaker during eyes closed rest. This suggests that the observed effects may reflect intrinsic biases of fronto-parietal connections toward central over peripheral V1.
This leads to a question of whether the observed retinotopic variations in connection strength may change with the participant's state. The brain's network architecture is strikingly similar during rest and during a range of tasks (Cole et al, 2014). However, changes in task state cause small but reliable deviations from the resting network structure (Cole et al, 2014). This phenomenon holds for visual cortical networks. A recent study of the task data from these participants examined background functional connectivity -- a measure of connectivity based on stimulus-independent BOLD fluctuations during active task performance -- in V1 during different attention conditions that featured identical stimuli (Griffis et al., 2015a). In that study, we compared the strength of background connectivity between different eccentricity sectors in V1 and 37 regions associated with the fronto-parietal, cingulo-opercular, and task-negative/default mode networks during two task conditions with identical stimuli: (1) attention to a centrally presented visual stimulus paired with a task-irrelevant auditory stimulus vs. (2) attention to an auditory stimulus paired with a task-irrelevant central visual stimulus. When attention was directed to the central visual stimulus, the left FEF and bilateral IPL showed a pronounced increase in the gradient of background connectivity with V1, such that connectivity decreased from central to far-peripheral eccentricity sectors. Importantly, we found that this gradient was weak-to-absent during attention to the auditory stimulus, despite the presence of central visual stimulation in both conditions. This indicates that some fronto-parietal control regions modulate their ongoing interactions with different eccentricity sectors in V1 depending on whether the visual information represented by them is being actively attended. In the current work, we find that additional regions associated with the fronto-parietal network show selective connectivity with central eccentricity sectors in V1 when no task is being performed, suggesting that some regions associated with this network have a baseline bias towards central visual information. To determine the extent to which this bias reflects the prioritization of visual information at the locus of (passive or active) attention, future studies might examine how retinotopic patterns of fronto-parietal connectivity with V1 are affected by the covert direction of attention to peripheral vision.
Regions with stronger connectivity to near-peripheral V1
When compared to central eccentricity sectors, near-peripheral eccentricity sectors showed stronger functional connectivity with a set of regions that have been described as part of the cingulo-opercular (Dosenbach and Fair 2007) and salience networks (Fox et al. 2006; Cauda et al. 2011) (Figure 4). The potential reasons for preferential functional connectivity between near-peripheral V1 and the cingulo-opercular network are not clear, but previous observations suggest several possibilities. First, we note that this finding is consistent with the results of one of the first studies to characterize the functional connectivity of the cingulo-opercular network (Dosenbach et al., 2007). Using ROIs based on task-evoked activations, Dosenbach and colleagues (2007) found that a portion of V1 that partially overlaps with our near-peripheral ROI clustered into the cingulo-opercular network based on its functional connections (Dosenbach et al. 2007). Other studies have reported increases in functional connectivity between the anterior insula and near peripheral portions of the calcarine sulcus during visualization tasks (Ebisch et al. 2013). This suggests that top-down signals from the cingulo-opercular network may target near peripheral representations in V1 during certain task conditions. Similar implications are suggested by findings that non-perceptual task events evoke responses in near peripheral representations in V1 (Jack et al., 2006), because robust responses are also observed in many cingulo-opercular regions during task transitions (Fox et al., 2005).
Second, clues relating the function of the cingulo-opercular network to near-peripheral vision may come from the literature examining the temporal patterns of control signals. Sustained signals from the cingulo-opercular network are related to the maintenance of task sets (Dosenbach et al. 2008; Nelson et al., 2010), though their precise function remains unclear (Dubis et al. 2014). Recent work by our lab has demonstrated that sustained task-driven responses occur in early visual areas including V1 (Elkhetali et al. 2015), but do not show the spatial specificity of cue-driven and stimulus-driven responses (Griffis et al. 2015b). While we have interpreted these sustained V1 responses as reflecting ongoing task maintenance signals that are likely to have originated in the cingulo-opercular network, it is unclear why this network would show preferential connectivity to the near-periphery of V1 during fixation. It is worth noting that our previous analysis of how background connectivity between V1 and control regions is modulated by task demands did not find evidence for task-driven changes in retinotopic connectivity patterns between V1 and cingulo-opercular regions (Griffis et al., 2015a), suggesting that attentional factors may not strongly influence the retinotopic biases of this network.
Third, some regions associated with the cingulo-opercular network play a role in the control of eye-movements, providing further clues as to why this network might show a bias in connectivity to near-peripheral sectors in V1. For example, the dACC and supplementary motor area (SMA) have been implicated in stop-signal inhibition related to over-riding automatic eye-movements and facilitating goal-directed eye-movements (Isoda and Hikosaka 2007). The dACC/SMA may increase inhibitory drive to visual areas during tasks requiring inhibitory over-rides of automatic visual processing (Duann et al. 2009), which may occur via via modulations of local GABAergic signaling (Zhang et al. 2014). Similarly, ventral attention regions in temporo-parietal cortex have been implicated in attentional re-orienting and in the detection of salient stimuli (Geng and Mangun 2011; Beauchamp et al. 2012; Daselaar et al. 2013). Thus, it is possible that preferential functional connectivity between near-peripheral V1 and these regions may be related to the ecological value of re-orienting attention and making eye movements to stimuli and locations just outside of the zone of fixation (Zhaoping 2014).
Regions with preferential connectivity to far-peripheral V1
Far-peripheral eccentricity sectors showed stronger functional connectivity with many regions associated with the “default mode” network compared to central and near-peripheral eccentricity sectors (Figures 2-4). Notably, these regions often show deactivations during tasks that require active attention (Shulman et al. 1997; Mayer et al. 2010; Lin et al. 2011). Along these lines, occipital alpha power as measured with EEG relates to suppression of visual information (Foxe and Snyder 2011), and is also positively correlated with activity in regions of the default mode network (Mo et al. 2013). This suggests that the suppression of irrelevant visual information may be related to signals originating in “task negative” regions. It is therefore possible that our finding of preferential functional connectivity between far-peripheral V1 and these regions during periods of central fixation may reflect a general suppression of far-peripheral visual processing during fixation on central locations. This explanation is consistent with evidence that the “task-negative” network shows increased interactions with nodes of other networks (including far-peripheral V1, see Figure 1B in de Pasquale et al., 2012) during periods of low within-network connectivity (de Pasquale et al. 2012), and suggests that information processed by far-peripheral representations may potentially be suppressed from awareness during central fixation.
Alternatively, rather than being ignored entirely, far-peripheral visual information may be transmitted to cortical areas that are specialized for the analysis of low-resolution visual information in order to enable ongoing environmental monitoring when attention is directed elsewhere. Studies from non-human primates indicate that homologues of the posterior cingulate cortex (areas 23 and 23v), a primary task-negative region and part of the default mode network, show anatomical connections specifically with far-peripheral representations in dorsal visual areas specialized for the processing of visual motion (MT+ and MST) (Palmer and Rosa 2006). Accordingly, functional MRI studies of human subjects have reported activity in the PCC in response to visual motion (Antal et al. 2008; Fischer et al. 2012). Far-peripheral visual areas also show distinct anatomical (Clavagnier et al. 2004) and functional (Eckert et al. 2008) connectivity with auditory cortex (also shown in Figure 3), suggesting that far-peripheral vision may interact with other early sensory regions to monitor the environment for salient events. Consistent with a specialized role for far-peripheral vision in environmental monitoring and threat detection, threatening stimuli in far-peripheral vision are processed very quickly (~80ms) by medial PFC and the amygdala, although this effect is not observed for central stimulus presentations (Bayle et al. 2009). It is thus possible that strong connectivity between far-peripheral V1 and areas involved in fast alerting (auditory cortex, parahippocampal areas, medial frontal cortex; see Figures 2 and 3) may allow for the rapid localization and evaluation of threatening stimuli in the periphery and the execution of reactive motor responses.
Limitations and Future Directions
Our study has several limitations that should be discussed. First, the data utilized in our primary analyses are limited to eyes-open resting fixation periods that were taken from interleaved task data. While this approach has been shown to provide similar estimates of functional connectivity to purely eyes-open fixation data (Fair et al. 2007), and has been employed in influential studies that helped identify the fronto-parietal, cingulo-opercular, and task-negative intrinsic connectivity networks (e.g. Dosenbach et al. 2007), the possibility of residual task effects cannot be definitively excluded. However, the results of our supplementary analysis of independent eyes-closed resting state data indicate that retinotopic differences in functional connectivity between V1 and many of the regions identified in our primary analysis are present during eyes-closed rest (Supplementary Figure 9). This suggests that the observed effects during resting fixation could not be entirely driven by residual task effects, central fixation, or visual inputs. Nonetheless, future studies should (using within-subjects measures) explicitly address whether eccentricity-dependent patterns of functional connectivity change between eyes-open and eyes-closed resting states, as this question was beyond the scope of the current study.
In addition, because our previous study showed that background functional connectivity between V1 and several fronto-parietal control regions is modulated in an eccentricity-dependent manner by visual attention (Griffis et al. 2015a), it is possible that similar effects might occur for fixation compared to other eyes-open conditions such as covert attention to the periphery, but this cannot be addressed by the current study. Thus, future studies should also address whether eccentricity-dependent patterns of functional connectivity differ between eyes-open contexts without explicit stimulation, such as between periods of central fixation and periods of covert attention to the periphery.
Lastly, it is worth noting that there is the possibility that the functional connectivity of far peripheral V1 is influenced by its proximity to task-negative regions such as the posterior cingulate. We consider this unlikely primarily due to our use of a conservative smoothing kernel (5mm FWHM) and our discarding of the most anterior V1 label segment to reduce the likelihood of this effect. We also consider this unlikely because much of the posterior cingulate actually showed stronger connectivity to the 25.0° sector than to the 40.0° sector, which is more anterior and closer to the posterior cingulate (Supplementary Figure 7). In addition, our supplementary analyses using retinotopically defined ROIs provide support for our general conclusion that functional connectivity between V1 and large-scale networks depends on eccentricity during resting fixation (Supplementary Figure 8), although they only extended to roughly 8° visual angle, and thus could not corroborate the effects for far-peripheral V1.
Conclusions
In summary, our data support the notion of functional specialization within early visual areas. Our findings highlight important distinctions in the long-range interactions of different eccentricity sectors in V1, and provide insights about the relationship between functional specializations at small and large scales in the brain. This approach serves as a model for using functional connectivity of regions with known functions to help delineate and identify the functional role of interactions between higher-order brain networks and sensory regions.
Supplementary Material
Highlights.
Functional connectivity of human V1 varies among eccentricity sectors.
Central sectors are more strongly connected to fronto-parietal networks.
Early peripheral sectors are more strongly connected to cingulo-opercular networks.
Far peripheral sectors are more strongly connected to task negative networks.
Results provide evidence for distinct connectivity profiles within V1.
Acknowledgements
This work was supported by the Vision Science Research Center (P30 EY003039)
Civitan International Research Center
The McKnight Brain Research Foundation
Compumedics Neuroscan, Inc.
Rodolphe Nenert for discussions about technical issues.
Zhaoping Li for discussions about interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Aguilar M, Stiles WS. Saturation of the rod mechanism of the retina at high levels of simulation. Optica Acta. 1954;1:59–65. [Google Scholar]
- Anstis S. Picturing peripheral acuity. Perception. 1998;27:817–25. doi: 10.1068/p270817. [DOI] [PubMed] [Google Scholar]
- Antal A, Baudewig J, Paulus W, Dechent P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis. Neurosci. 2008;25:17–26. doi: 10.1017/S0952523808080024. doi:10.1017/S0952523808080024. [DOI] [PubMed] [Google Scholar]
- Arcaro MJ, Honey CJ, Mruczek REB, Kastner S. Widespread correlation patterns of fMRI signal across visual cortex reflect eccentricity organization. Elife. 2015;1–28 doi: 10.7554/eLife.03952. doi:10.7554/eLife.03952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro MJ, McMains SA, Singer BD, Kastner S. Retinotopic organization of human ventral visual cortex. J. Neurosci. 2009;29:10638–52. doi: 10.1523/JNEUROSCI.2807-09.2009. doi:10.1523/JNEUROSCI.2807-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the cerebral cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2005;360:733–50. doi: 10.1098/rstb.2005.1627. doi:10.1098/rstb.2005.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle DJ, Henaff M-A, Krolak-Salmon P. Unconsciously perceived fear in peripheral vision alerts the limbic system: a MEG study. PLoS One. 2009;4:e8207. doi: 10.1371/journal.pone.0008207. doi:10.1371/journal.pone.0008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Sun P, Baum SH, Tolias AS, Yoshor D. Electrocorticography links human temporoparietal junction to visual perception. Nat. Neurosci. 2012;15:957–9. doi: 10.1038/nn.3131. doi:10.1038/nn.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson NC, Butt OH, Datta R, Radoeva PD, Brainard DH, Aguirre GK. The retinotopic organization of striate cortex is well predicted by surface topology. Curr. Biol. 2012;22:2081–5. doi: 10.1016/j.cub.2012.09.014. doi:10.1016/j.cub.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage. 2009;45:160–8. doi: 10.1016/j.neuroimage.2008.10.034. doi:10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger J, Rubens MT, Zanto TP, Gazzaley A. Expectation-driven changes in cortical functional connectivity influence working memory and long-term memory performance. J. Neurosci. 2010;30:14399–410. doi: 10.1523/JNEUROSCI.1547-10.2010. doi:10.1523/JNEUROSCI.1547-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler DW, Fortenbaugh FC, Robertson LC, Silver M. a. Visual spatial attention enhances the amplitude of positive and negative fMRI responses to visual stimulation in an eccentricity-dependent manner. Vision Res. 2013;85:104–112. doi: 10.1016/j.visres.2013.03.009. doi:10.1016/j.visres.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Yeo BT. Borders, map clusters, and supra-areal organization in visual cortex. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.12.036. doi:10.1016/j.neuroimage.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. Optimizing the order of operations for movement scrubbing: Comment on Power et al. Neuroimage. 2013;76:436–8. doi: 10.1016/j.neuroimage.2011.12.061. doi:10.1016/j.neuroimage.2011.12.061. [DOI] [PubMed] [Google Scholar]
- Cate AD, Herron TJ, Yund EW, Stecker GC, Rinne T, Kang X, Petkov CI, Disbrow EA, Woods DL. Auditory Attention Activates Peripheral Visual Cortex. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004645. doi:10.1371/journal.pone.0004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. doi:10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chen Z, Treisman A. Distractor inhibition is more effective at a central than at a peripheral location. Percept. Psychophys. 2008;70:1081–1091. doi: 10.3758/pp.70.6.1081. doi:10.3758/PP.70.6.1081. [DOI] [PubMed] [Google Scholar]
- Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn. Affect. Behav. Neurosci. 2004;4:117–26. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and Task-Evoked Network Architectures of the Human Brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. http://doi.org/10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Huijbers W, Eklund K, Moscovitch M, Cabeza R. Resting-state functional connectivity of ventral parietal regions associated with attention reorienting and episodic recollection. Front. Hum. Neurosci. 2013;7:38. doi: 10.3389/fnhum.2013.00038. doi:10.3389/fnhum.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Marzetti L, Pizzella V, Romani GL, Corbetta M. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron. 2012;74:753–64. doi: 10.1016/j.neuron.2012.03.031. doi:10.1016/j.neuron.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex. 2011;21:1498–506. doi: 10.1093/cercor/bhq186. doi:10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobromyslin VI, Salat DH, Fortier CB, Leritz EC, Beckmann CF, Milberg WP, McGlinchey RE. Distinct functional networks within the cerebellum and their relation to cortical systems assessed with independent component analysis. Neuroimage. 2012;60:2073–85. doi: 10.1016/j.neuroimage.2012.01.139. doi:10.1016/j.neuroimage.2012.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11073–8. doi: 10.1073/pnas.0704320104. doi:10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. doi:10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A Core System for the Implementation of Task Sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, Li CR. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. 2009;29:10171–9. doi: 10.1523/JNEUROSCI.1300-09.2009. doi:10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubis JW, Siegel JS, Neta M, Visscher KM, Petersen SE. Tasks Driven by Perceptual Information Do Not Recruit Sustained BOLD Activity in Cingulo-Opercular Regions. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu187. doi:10.1093/cercor/bhu187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJH, Mantini D, Romanelli R, Tommasi M, Perrucci MG, Romani GL, Colom R, Saggino A. Long-range functional interactions of anterior insula and medial frontal cortex are differently modulated by visuospatial and inductive reasoning tasks. Neuroimage. 2013;78:426–38. doi: 10.1016/j.neuroimage.2013.04.058. doi:10.1016/j.neuroimage.2013.04.058. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Kamdar NV, Chang CE, Beckmann CF, Greicius MD, Menon V. A cross-modal system linking primary auditory and visual cortices: Evidence from intrinsic fMRI connectivity analysis. Hum. Brain Mapp. 2008;29:848–857. doi: 10.1002/hbm.20560. doi:10.1002/hbm.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhetali AS, Vaden RJ, Pool SM, Visscher KM. Early visual cortex reflects initiation and maintenance of task set. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2014.11.061. doi:10.1016/j.neuroimage.2014.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb. Cortex. 1997;7:181–92. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. doi:10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H. Anatomical evidence of multimodal integration in primate striate cortex. J. Neurosci. 2002;22:5749–59. doi: 10.1523/JNEUROSCI.22-13-05749.2002. doi:20026562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb. cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fischer E, Bülthoff HH, Logothetis NK, Bartels A. Visual motion responses in the posterior cingulate sulcus: a comparison to V5/MT and MST. Cereb. Cortex. 2012;22:865–76. doi: 10.1093/cercor/bhr154. doi:10.1093/cercor/bhr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard D a, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005;28:956–966. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10046–51. doi: 10.1073/pnas.0604187103. doi:10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. doi:10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Fox PT, Miezin FM, Miezin FM, Allman JM, Allman JM, Van Essen DC, Van Essen DC, Raichle ME, Raichle ME. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J. Neurosci. 1987;7:913–22. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00154. doi:10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995;2:189–210. doi:10.1002/hbm.460020402. [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex. 2007;17(Suppl 1):i125–35. doi: 10.1093/cercor/bhm113. doi:10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç E, Schölvinck ML, Bergmann J, Singer W, Kohler A. Functional Connectivity Patterns of Visual Cortex Reflect its Anatomical Organization. Cereb. Cortex. 2015:bhv175. doi: 10.1093/cercor/bhv175. doi:10.1093/cercor/bhv175. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Mangun GR. Right temporoparietal junction activation by a salient contextual cue facilitates target discrimination. Neuroimage. 2011;54:594–601. doi: 10.1016/j.neuroimage.2010.08.025. doi:10.1016/j.neuroimage.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff M, Song A, Mangun G. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. doi:10.1016/S1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gleiss S, Kayser C. Eccentricity dependent auditory enhancement of visual stimulus detection but not discrimination. Front. Integr. Neurosci. 2013;7:52. doi: 10.3389/fnint.2013.00052. doi:10.3389/fnint.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A, Rothkirch M, Kaul C, Weygandt M, Haynes J-D, Rees G, Sterzer P. Emotion modulates the effects of endogenous attention on retinotopic visual processing. Neuroimage. 2011;57:1542–51. doi: 10.1016/j.neuroimage.2011.05.072. doi:10.1016/j.neuroimage.2011.05.072. [DOI] [PubMed] [Google Scholar]
- Griffis JC, Elkhetali AS, Burge WK, Chen RH, Visscher KM. Retinotopic patterns of background connectivity between V1 and fronto-parietal cortex are modulated by task demands. Front. Hum. Neurosci. 2015a;9:1–14. doi: 10.3389/fnhum.2015.00338. doi:10.3389/fnhum.2015.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis JC, Elkhetali AS, Vaden RJ, Visscher KM. Distinct Effects of Trial-Driven and Task Set-Related Control in Primary Visual Cortex. Neuroimage. 2015b doi: 10.1016/j.neuroimage.2015.07.005. doi:10.1016/j.neuroimage.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Tootell RB. Projection of rods and cones within human visual cortex. Hum. Brain Mapp. 2000;9:55–63. doi: 10.1002/(SICI)1097-0193(2000)9:1<55::AID-HBM6>3.0.CO;2-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein E. a. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32:842–53. doi: 10.1016/j.neuroimage.2006.04.177. doi:10.1016/j.neuroimage.2006.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. doi:10.1016/S0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Heinzle J, Kahnt T, Haynes J-D. Topographically specific functional connectivity between visual field maps in the human brain. Neuroimage. 2011;56:1426–36. doi: 10.1016/j.neuroimage.2011.02.077. doi:10.1016/j.neuroimage.2011.02.077. [DOI] [PubMed] [Google Scholar]
- Hellyer PJ, Shanahan M, Scott G, Wise RJS, Sharp DJ, Leech R. The Control of Global Brain Dynamics: Opposing Actions of Frontoparietal Control and Default Mode Networks on Attention. J. Neurosci. 2014;34:451–461. doi: 10.1523/JNEUROSCI.1853-13.2014. doi:10.1523/JNEUROSCI.1853-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O, Polimeni J, Rajendran N, Balasubramanian M, Amunts K, Zilles K, Schwartz EL, Fischl B, Triantafyllou C. Locating the functional and anatomical boundaries of human primary visual cortex. Neuroimage. 2009;46:915–22. doi: 10.1016/j.neuroimage.2009.03.036. doi:10.1016/j.neuroimage.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds OP, Rajendran N, Polimeni JR, Augustinack JC, Wiggins G, Wald LL, Diana Rosas H, Potthast A, Schwartz EL, Fischl B. Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage. 2008;39:1585–1599. doi: 10.1016/j.neuroimage.2007.10.033. doi:10.1016/j.neuroimage.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T. Die Sehstörungen bei Schussverletzungen der kortikalen Sehsphäre, nach Beobachtungen an Verwundeten der letzten japanischen Kriege. Engelmann; Leipzig: 1909. [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat. Neurosci. 2007;10:240–8. doi: 10.1038/nn1830. doi:10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- Jack AI, Shulman GL, Snyder AZ, McAvoy M, Corbetta M. Separate modulations of human V1 associated with spatial attention and task structure. Neuron. 2006;51:135–147. doi: 10.1016/j.neuron.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Jao T, Vértes PE, Alexander-Bloch AF, Tang I-N, Yu Y-C, Chen J-H, Bullmore ET. Volitional eyes opening perturbs brain dynamics and functional connectivity regardless of light input. Neuroimage. 2013;69:21–34. doi: 10.1016/j.neuroimage.2012.12.007. doi:10.1016/j.neuroimage.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13335–40. doi: 10.1073/pnas.0403743101. doi:10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011;31:3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011. doi:10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat. Neurosci. 2001;4:533–539. doi: 10.1038/87490. doi:10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Li Z. A saliency map in primary Visual Cortex. Trends Cogn. Sci. 2002;6:9–16. doi: 10.1016/s1364-6613(00)01817-9. [DOI] [PubMed] [Google Scholar]
- Lin P, Hasson U, Jovicich J, Robinson S. A neuronal basis for task-negative responses in the human brain. Cereb. Cortex. 2011;21:821–30. doi: 10.1093/cercor/bhq151. doi:10.1093/cercor/bhq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnell KJ, Humphreys GW. Attentional selection of a peripheral ring overrules the central attentional bias. Percept. Psychophys. 2004;66:743–751. doi: 10.3758/bf03194969. doi:10.3758/BF03194969. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bengson J, Huang H, Mangun GR, Ding M. Top-down Modulation of Neural Activity in Anticipatory Visual Attention: Control Mechanisms Revealed by Simultaneous EEG-fMRI. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu204. doi:10.1093/cercor/bhu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. Neuroimage. 2009;44:265–74. doi: 10.1016/j.neuroimage.2008.08.019. doi:10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. doi:10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS. Connectivity-based subdivisions of the human right “temporoparietal junction area”: evidence for different areas participating in different cortical networks. Cereb. Cortex. 2012;22:1894–903. doi: 10.1093/cercor/bhr268. doi:10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Roebroeck A, Maurer K, Linden DEJ. Specialization in the default mode: Task-induced brain deactivations dissociate between visual working memory and attention. Hum. Brain Mapp. 2010;31:126–39. doi: 10.1002/hbm.20850. doi:10.1002/hbm.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J, Liu Y, Huang H, Ding M. Coupling between visual alpha oscillations and default mode activity. Neuroimage. 2013;68:112–8. doi: 10.1016/j.neuroimage.2012.11.058. doi:10.1016/j.neuroimage.2012.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE. Role of the anterior insula in task-level control and focal attention. Brain Structure & Function. 2010;214(5-6):669–680. doi: 10.1007/s00429-010-0260-2. http://doi.org/10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–65. doi: 10.1093/cercor/bhp157. doi:10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SM, Rosa MGP. A distinct anatomical network of cortical areas for analysis of motion in far peripheral vision. Eur. J. Neurosci. 2006;24:2389–405. doi: 10.1111/j.1460-9568.2006.05113.x. doi:10.1111/j.1460-9568.2006.05113.x. [DOI] [PubMed] [Google Scholar]
- Park H-J, Friston KJ. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. doi:10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Dubis JW. The mixed block/event-related design. Neuroimage. 2011;62:1177–1184. doi: 10.1016/j.neuroimage.2011.09.084. doi:10.1016/j.neuroimage.2011.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooresmaeili A, Poort J, Roelfsema PR. Simultaneous selection by object-based attention in visual and frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6467–72. doi: 10.1073/pnas.1316181111. doi:10.1073/pnas.1316181111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes K. a, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018. doi:10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church J. a, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–78. doi: 10.1016/j.neuron.2011.09.006. doi:10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18:502–15. doi: 10.1177/1073858411409051. doi:10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Raemaekers M, Schellekens W, van Wezel RJA, Petridou N, Kristo G, Ramsey NF. Patterns of resting state connectivity in human primary visual cortical areas: a 7T fMRI study. Neuroimage. 2014;84:911–21. doi: 10.1016/j.neuroimage.2013.09.060. doi:10.1016/j.neuroimage.2013.09.060. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. doi:10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Delicato LS, Herrero J, Gieselmann M. a, Thiele A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nat. Neurosci. 2007;10:1483–91. doi: 10.1038/nn1967. doi:10.1038/nn1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RSJ, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb. Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. doi:10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes J-D, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and Psychophysics Reveal Frontal Influences on Human Retinotopic Visual Cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. doi:10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hadjikhani N, Fischl B, Liu AK, Marrett S, Dale AM, Tootell RB. Local and global attention are mapped retinotopically in human occipital cortex. Proceedings of the National Academy of Sciences. 2002;98:2077–2082. doi: 10.1073/pnas.98.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol. Sci. 2005;16:114–22. doi: 10.1111/j.0956-7976.2005.00791.x. doi:10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J. Cogn. Neurosci. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. doi:10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Weber DL, Dale CL, Pantazis D, Bressler SL, Leahy RM, Luks TL. Dynamic activation of frontal, parietal, and sensory regions underlying anticipatory visual spatial attention. J. Neurosci. 2011;31:13880–9. doi: 10.1523/JNEUROSCI.1519-10.2011. doi:10.1523/JNEUROSCI.1519-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–17. doi: 10.1016/j.neuroimage.2010.06.016. doi:10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striem-Amit E, Ovadia-Caro S, Caramazza A, Margulies DS, Villringer A, Amedi A. Functional connectivity of visual cortex in the blind follows retinotopic organization principles. Brain. 2015:1679–1695. doi: 10.1093/brain/awv083. doi:10.1093/brain/awv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J. Neurosci. 2010;30:148–60. doi: 10.1523/JNEUROSCI.3862-09.2010. doi:10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen D. a, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb. Cortex. 2010;20:2636–46. doi: 10.1093/cercor/bhq011. doi:10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese A, Kolbe SC, Anderson AJ, Egan GF, Vidyasagar TR. Functional size of human visual area V1: a neural correlate of top-down attention. Neuroimage 93 Pt. 2014;1:47–52. doi: 10.1016/j.neuroimage.2014.02.023. doi:10.1016/j.neuroimage.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. doi:10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher KKM, Miezin FFM, Kelly JJE, Buckner RL, Donaldson DI, McAvoy MP, Bhalodia VM, Petersen SE. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. Neuroimage. 2003;19:1694–708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- Wandell BA. Foundations of Vision. 1995. 1st ed. Sinauer Associates; 1995. [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. doi:10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-A, Chong S-C. Eccentricity biases of object categories are evident in visual working memory. Vis. cogn. 2012;20:233–243. [Google Scholar]
- Zhang S, Xu M, Kamigaki T, Do J. Long-range and local circuits for top-down modulation of visual cortex processing. Science (80−. ) 2014:4–9. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhaoping L. Understanding Vision: theory, models, and data. Oxford University Press; 2014. [Google Scholar]
- Zou Q, Long X, Zuo X, Yan C, Zhu C, Yang Y, Liu D, He Y, Zang Y. Functional connectivity between the thalamus and visual cortex under eyes closed and eyes open conditions: a resting-state fMRI study. Hum. Brain Mapp. 2009;30:3066–78. doi: 10.1002/hbm.20728. doi:10.1002/hbm.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.